Abstract
Expression of major histocompatibility complex (MHC) class II molecules in human activated T cells is under normal circumstances regulated exclusively by the CIITA-PIII subtype of the class II transactivator (CIITA). In this study, we show that the absence of MHC class II expression in leukemic T cells was due to a lack of expression of CIITA, whereas in T-lymphoma cells, expression of CIITA correlated with expression of MHC class II. Interestingly, activation of a CIITA-promoter (P)III–reporter construct was not affected in leukemic T cells. This revealed that the absence of endogenous CIITA expression was not caused by a lack of transcription factors critical for CIITA-PIII activation but suggests the involvement of an epigenetic silencing mechanism. Subsequent analysis showed that the lack of human leukocyte antigen–DR (HLA-DR) expression correlated with hypermethylation of CIITA-PIII in leukemic T-cell lines and in primary T-cell acute lymphoblastic leukemia (T-ALL) and a T-cell prolymphocytic leukemia (T-PLL). Treatment of leukemic T-cell lines with a demethylation agent showed re-expression of CIITA-PIII and HLA-DRA. Furthermore, in vitro methylation of CIITA-PIII and subsequent assessment of CIITA-PIII activity in Jurkat leukemic T cells resulted in reduction of constitutive and CREB-1 (cyclic adenosine monophosphate [cAMP]–response element binding protein 1)–induced promoter activity. Together, these results argue for an important role of DNA hyper-methylation in the control of CIITA expression in leukemic T cells.
Introduction
Leukemias and lymphomas of T-cell origin are a heterogeneous group of tumor cells that arise from the malignant transformation of normal T cells. This process of transformation can occur at various stages of T-cell differentiation and maturation. Although the molecular basis of T-cell acute lymphoblastic leukemia (T-ALL) is not completely understood, in approximately 30% of T-ALL it has been shown that malignant transformation is due to chromosomal translocations.1 These translocations usually induce aberrant gene expression patterns due to the juxtaposition of strong promoters or enhancers (frequently of the T-cell–receptor [TCR] loci) next to developmentally important genes2 or due to gene truncation.3 Transformations can also be due to a viral protein, which has been reported for the viral oncoprotein Tax of the human T-cell leukemia virus-1 (HTLV-1).4
The prognosis of T-ALL in children and adolescents has improved in recent years due to intensified therapies, with 5-year relapse-free survival rates now in the range of 60% to 75%.5,6 Concerning T-ALL in adults, complete remission rates of 80% to 85% and leukemia-free survival rates of 46% can be achieved.7 However, in a large-scale study it was found that major histocompatibility complex (MHC) class II expression on neoplastic T cells in adults is associated with a poor clinical outcome.8 Standard treatment modalities to eradicate malignant T cells combine different cytotoxic drugs and irradiation, and for patients with relapses these also include allogeneic bone marrow transplantation (BMT). With respect to the graft-versus-leukemia response after allogeneic BMT, a close correlation has been observed in vitro between the proliferative response of alloactivated T lymphocytes and the percentage of MHC class II–expressing T-leukemic cells used as stimulators.9 Notably, this T-lymphocyte proliferation could be blocked with anti–MHC class II monoclonal antibodies (mAbs), revealing the importance of MHC class II expression on malignant T cells in the generation of a graft-versus-leukemia response.
Recently, promising results have been obtained in animal studies with a fully humanized antibody against the MHC class II protein human leukocyte antigen–DR (HLA-DR).10 This antibody exhibited potent in vitro and in vivo tumoricidal activity on several lymphoma and leukemia cell lines, including T-cell lymphoma. The antibody caused no long-lasting hematologic toxicity in primates in vivo, and thereby offers the potential for a novel therapeutic approach to lymphoid malignancies. However, the use of tumoricidal MHC class II antibodies depends on the presence of these molecules on the surface of malignant cells, and it should be noted that only a small percentage (5%-17%) of T-ALL are reported to express HLA-DR.11 Because MHC class II molecules might be a potential target for antibody-based immune therapy and MHC class II is also associated with poor clinical prognosis, we sought to unravel the regulatory mechanisms determining MHC class II expression in T-cell malignancies.
Under normal circumstances, MHC class II molecules play a pivotal role in the induction and regulation of an antigen-specific immune response. They are specialized for the presentation of antigens to CD4+ T lymphocytes. Because of their crucial role in the initiation of the immune response, MHC class II molecules are expressed constitutively on antigen-presenting cells (APCs) of the immune system only. These include dendritic cells (DCs), B lymphocytes, monocytes, macrophages, and thymic epithelial cells. On all other cell types (eg, fibroblast and epithelial cells) their expression can be induced in an environment rich in inflammatory cytokines of which interferon γ (IFNγ) is the most potent. Notably, human T lymphocytes also express MHC class II molecules following activation. Besides their role in presenting antigens,12-20 the T-cell–expressed MHC class II molecules may exert additional functions. In particular, in lymphocytes it has been well documented that MHC class II molecules serve as signal-transducing receptors. MHC class II molecules can transduce signals via their cytoplasmic tails into activated T lymphocytes, which results in protein kinase C (PKC) membrane translocation and inositol phosphate (IP) accumulation. Notably, simultaneous triggering of CD3 and MHC class II molecules leads to an increase in CD3-mediated T-blast proliferation.21 In accordance, cross-linking of HLA-DR molecules of T-cell helper 1 (Th1) cells with solid-phase mAbs are reported to induce proliferation resulting in anergy of T lymphocytes.22 Thus, MHC class II molecules on activated T cells could serve on the one hand as antigen-presentation entities that could affect the activity of bystander T lymphocytes and on the other hand could provide signals that modulate T-cell functioning.
The master regulator of MHC class II gene expression is the class II transactivator (CIITA), which acts as a coactivator by virtue of its ability to interact with components of the MHC class II enhanceosome consisting of regulatory factor X (RFX), CREB/ATF (cyclic adenosine monophosphate [cAMP]–response element binding protein/activating transcription factor), and nuclear factor Y (NFY) bound to the SXY module in MHC class II promoters.23 Coinciding with MHC class II expression, the constitutive expression of CIITAis confined toAPCs only and CIITAexpression can be induced in various other cell types. The transcriptional regulation of human CIITA is controlled by at least 3 independent promoter units (CIITA-PI, -PIII, and -PIV), each transcribing a unique first exon, and that are located within an approximately 14-kb region upstream of the CIITA gene.24 CIITA-PI and CIITA-PIII are used for the constitutive expression in dendritic cells and B lymphocytes, respectively.24 CIITA-PIV has been shown to be the promoter predominantly involved in IFNγ-inducible CIITA expression.25-27 In addition, CIITA-PIII can also be activated by IFNγ through a 4-kb IFNγ regulatory region located 2 kb upstream of the CIITA-PIII core promoter region in human non–B cells, such as fibroblasts and epithelial cells.26-28 We and others29,30 have recently established that normal human activated T cells exclusively employ CIITA-PIII, formerly referred to as the B-cell–specific CIITA promoter, to drive expression of CIITA.
In this report we demonstrate that the lack of MHC class II expression in both cultured and primary malignant T cells results from a lack of CIITA expression following T-cell activation, which is caused by hyper-methylation of CIITA-PIII.
Materials and methods
Cell culture
The following cell lines were purchased from the American Type Culture Collection (Manassas, VA): Jurkat clone E6-1 (TIB-152), HSB-2 (CCL-120.1), CEM (CCL-119), HUT78 (TIB-161), HH (CRL-2105), Molt-4 (CRL-1582), H9 (HTB-176), JEG-3 (HTB-36), and Raji (CCL-86). Karpas-299 (ACC 31) was purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany). PEER and HPB-ALL were obtained through Dr Saskia Cillessen (Vrije Universiteit Medical Center, Amsterdam, the Netherlands). JEG-3, Raji, HUT78, and HSB-2 were cultured in Iscove modified Dulbecco medium (IMDM) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Greiner Labortechnik, Frickenhausen, Germany), 100 U/mL streptomycin, 100 U/mL penicillin. All other cell lines were cultured in RPMI supplemented with 10% heat-inactivated FBS (Greiner Labortechnik), 100 U/mL streptomycin, 100 U/mL penicillin, and 2 mM l-glutamine. Isolation and characterization of primary malignant T cells has been previously described,31-33 and relevant characteristics of these cells are summarized in Table 1.
Characteristics of panel of primary T-cell leukemias
Patient no. . | Tumor type . | HLA-DR surface expression . |
|---|---|---|
| T058 | T-ALL | Absent |
| T003 | T-ALL | Absent |
| T087 | T-ALL | Absent |
| 85-002 | T-PLL | Absent |
| 94-056 | T-LGL/T-CLL | Present |
| 95-016 | T-LGL/T-CLL | Present |
Patient no. . | Tumor type . | HLA-DR surface expression . |
|---|---|---|
| T058 | T-ALL | Absent |
| T003 | T-ALL | Absent |
| T087 | T-ALL | Absent |
| 85-002 | T-PLL | Absent |
| 94-056 | T-LGL/T-CLL | Present |
| 95-016 | T-LGL/T-CLL | Present |
T-ALL indicates T-cell acute lymphoblastic leukemia; T-LGL, T-cell large granular lymphocyte; and T-CLL, T-cell chronic lymphocytic leukemia.
Fluorescent-activated cell sorter analysis (FACS)
Cells were harvested, washed with phosphate-buffered saline (PBS) containing 2% FBS, and stained for direct immunofluorescence detection of CD2, CD3, CD4, CD8, CD25, CD28, CD45RA, CD45RO, HLA-DR, HLA-DQ, and TCRαβ using fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated mAb (BD Pharmingen, San Diego, CA). HLA class I was detected with mAb W6/32 and HLA-DP with the SPV-L3 mAb.34 The acquisition was performed on a FACScan using CELL Quest software (Becton Dickinson, Mountain View, CA) for analysis.
RNA isolation and reverse transcriptase–polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from the human T-cell lines using the RNAzol extraction method (Cinna/Biotecx Laboratories, Houston, TX). RNA samples (2 μg) were transcribed into cDNA using avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI). PCR primers and conditions for glyceraldehyde phosphate dehydrogenase (GAPDH), CIITA-PI, CIITA-PIII, CIITA-PIV, panCIITA (to detect all isoforms), HLA-DRA, interleukin 4 (IL-4), and IFNγ have been described previously.29,35-37 The primer sequence, final MgCl2 concentration, and amplification rounds used in the PCR to detect the components of the RFX complex are indicated in Table 2. All PCR products were visualized on an ethidium bromide–stained agarose gel and colors were reversed for reasons of clarity.
RFX PCR primer sequence and conditions
Primer . | Sequence . | Annealing temperature . | Cycles . |
|---|---|---|---|
| RFX5 sense | 5′-AAGCTGTATCTCTACCTTCAG-3′ | 59°C | 35 |
| RFX5 antisense | 5′-TTTCAGGATCCGCTCTGCCCA-3′ | 59°C | 35 |
| RFXAP sense | 5′-CACCCTGCTAGTGATGCAACCC-3′ | 66°C | 37 |
| RFXAP antisense | 5′-GAGTAGGTCTTGCAGGAGATCC-3′ | 66°C | 37 |
| RFXB sense | 5′-GCCAGATCGCTGAGGGTCCG-3′ | 66°C | 35 |
| RFXB antisense | 5′-CCGGCAGGCGGCCTTCACTC-3′ | 66°C | 35 |
Primer . | Sequence . | Annealing temperature . | Cycles . |
|---|---|---|---|
| RFX5 sense | 5′-AAGCTGTATCTCTACCTTCAG-3′ | 59°C | 35 |
| RFX5 antisense | 5′-TTTCAGGATCCGCTCTGCCCA-3′ | 59°C | 35 |
| RFXAP sense | 5′-CACCCTGCTAGTGATGCAACCC-3′ | 66°C | 37 |
| RFXAP antisense | 5′-GAGTAGGTCTTGCAGGAGATCC-3′ | 66°C | 37 |
| RFXB sense | 5′-GCCAGATCGCTGAGGGTCCG-3′ | 66°C | 35 |
| RFXB antisense | 5′-CCGGCAGGCGGCCTTCACTC-3′ | 66°C | 35 |
The MgCl2 concentration for all primers was 3 mM.
Promoter constructs and transient transfection
The pGL3–CIITA-PIII and the pGL3–CIITA-PIV reporter constructs were previously described.29,35 Human T-cell lines were transfected by electroporation (Genepulser; BioRad Laboratories, Hercules, CA) at 250 V and 960 μF (microfarad) and harvested after 48 hours. Electroporations were performed with 8 × 106 cells and 10 μg of luciferase pGL3-reporter construct together with 0.1 μg of β-actin–Renilla-reporter construct as an internal control. Luciferase and Renilla luciferase activity were measured using the Dual Luciferase Assay kit according to the manufacturer (Promega) and promoter activity was normalized for transfection efficiency with the Renilla luciferase activity.
Southern blot analysis
For Southern blot analysis, total genomic DNA was isolated from 10 × 106 cells using 500 μL lysis buffer (100 mM Tris-HCl; pH, 8.5, 5 mM ethylenediaminetetraacetic acid [EDTA], 0.2% sodium dodecyl sulfate [SDS], 200 mM NaCl, 100 μg/mL proteinase K) and incubated overnight at 56°C. After several phenol/chloroform extractions the DNA was digested with HindIII in combination with HpaII or MspI, fragments were separated in a 1% Tris-borate-EDTA (TBE) agarose gel and subsequently blotted overnight. The blots were hybridized with a [32P]-labeled CIITA-PIII probe containing nucleotides –545 to +123 from the transcription start site, or with a [32P]-labeled CIITA-PIV probe containing nucleotides –355 to +74 from the transcription start site.
5-AZA-2′-deoxycytidine treatment
Jurkat cells were grown at 37°C for 3 days in RMPI/10% fetal calf serum (FCS) without or with different concentrations of 5-AZA-2′-deoxycytidine (Sigma, St Louis, MO), as indicated. After 3 days of culture, cells were harvested and RNA isolation, cDNA synthesis, and PCR analysis were performed as described in “RNA isolation and reverse transcriptase–polymerase chain reaction (RT-PCR) analysis.”
In vitro CIITA promoter methylation
The CIITA-PIII promoter fragment was obtained from the pGL3–CIITA-PIII luciferase reporter construct29 and the SV40 promoter fragment from the pGL3–simian virus 40 (SV40) luciferase reporter construct (Promega). Isolated promoter fragments were either methylated for 16 hours at 37°C using 11 U SssI Methylase and 16 μM S-adenosylmethionine (both from New England BioLabs, Beverly, MA) or not methylated by omitting S-adenosylmethionine from the reaction mix. Methylation of the promoter fragments was confirmed by the complete protection from digestion with methylation-sensitive restriction enzymes. The promoter fragments were then ligated back into the pGL3 vector. Methylated and unmethylated CIITA-PIII constructs were cotransfected with the expression vector pRc-RSV (Invitrogen) containing either no insert or CREB-1.28 Equal amounts of unmethylated and methylated constructs were transfected into Jurkat T cells, and promoter activity was assayed as described above.
Results
MHC class II and T-cell surface markers on human malignant T cells
Expression of MHC class II molecules on malignant T cells was evaluated by FACS analysis with antibodies specific for the MHC class II isotypes (HLA-DR, -DQ, and -DP). These analyses revealed that all acute T-cell leukemia cell lines tested (HSB-2, HPB-ALL, PEER, Molt-4, Jurkat) did not express HLA-DR, -DQ, and -DP at the cell surface (Figure 1). In contrast, all tested T-cell lymphoma cell lines (HH, HUT78, H9, and Karpas-299) abundantly expressed all MHC class II isotypes at the cell surface (Figure 1 and data not shown).
MHC class II surface expression on leukemic and lymphoma T-cell lines. FACS analysis of HLA-DR, -DQ, and -DP surface expression in HSB-2, HPB-ALL, PEER, Molt-4,and Jurkat T-ALL cell lines and of HH and HUT78 T-cell lymphomas. Filled curve is the specific MHC class II staining and open curve represents the isotype control.
MHC class II surface expression on leukemic and lymphoma T-cell lines. FACS analysis of HLA-DR, -DQ, and -DP surface expression in HSB-2, HPB-ALL, PEER, Molt-4,and Jurkat T-ALL cell lines and of HH and HUT78 T-cell lymphomas. Filled curve is the specific MHC class II staining and open curve represents the isotype control.
In addition, we also performed a global characterization of the different acute T-cell leukemia cell lines and 4 T-cell lymphoma cell lines for cell surface expression of several T-cell–associated markers (Table 3). FACS analysis showed that the expression of CD4 and CD8 differed among the leukemic T-cell lines tested. HSB-2 and HPB-ALL lacked expression of both CD4 and CD8, whereas Molt-4 cells displayed expression of CD4 (weak) and CD8. Jurkat and PEER (γδ cells) expressed CD4 to various extents. The leukemic cells also expressed CD45-RA, CD45-RO (except Jurkat), and CD28 (except HSB-2) and all lacked CD25 and MHC class II surface expression. In contrast, all 4 T-lymphoma cell lines expressed CD4, CD25, and MHC class II on their cell surface and lacked both CD8 and CD28. Furthermore, all tested cell lines were found to be MHC class I positive.
Expression of T-cell markers on T-leukemic and T-lymphoma cell lines
Tumor description . | Acute T-cell leukemia . | . | . | . | . | T-cell lymphoma . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker . | HSB-2 . | Molt-4 . | PEER . | HPB ALL . | Jurkat . | HUT 78 . | HH . | H9 . | Karpas-299 . | |||||||
| CD2 | - | + | +/- | + | + | - | + | - | - | |||||||
| CD3 | - | +/- | + | + | + | + | + | + | - | |||||||
| CD4 | - | +/- | +/- | - | + | + | + | + | + | |||||||
| CD8 | - | + | - | - | - | - | - | - | - | |||||||
| CD25 | - | - | - | - | - | + | + | + | + | |||||||
| CD28 | - | + | + | + | + | - | - | - | - | |||||||
| CD45-RA | + | + | + | + | + | + | - | - | + | |||||||
| CD45-RO | + | + | + | + | - | - | + | + | + | |||||||
| TCRαβ | - | - | - | + | + | + | - | + | - | |||||||
| MHC class I | + | + | + | + | + | + | + | + | + | |||||||
| MHC class II | - | - | - | - | - | + | + | + | + | |||||||
Tumor description . | Acute T-cell leukemia . | . | . | . | . | T-cell lymphoma . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker . | HSB-2 . | Molt-4 . | PEER . | HPB ALL . | Jurkat . | HUT 78 . | HH . | H9 . | Karpas-299 . | |||||||
| CD2 | - | + | +/- | + | + | - | + | - | - | |||||||
| CD3 | - | +/- | + | + | + | + | + | + | - | |||||||
| CD4 | - | +/- | +/- | - | + | + | + | + | + | |||||||
| CD8 | - | + | - | - | - | - | - | - | - | |||||||
| CD25 | - | - | - | - | - | + | + | + | + | |||||||
| CD28 | - | + | + | + | + | - | - | - | - | |||||||
| CD45-RA | + | + | + | + | + | + | - | - | + | |||||||
| CD45-RO | + | + | + | + | - | - | + | + | + | |||||||
| TCRαβ | - | - | - | + | + | + | - | + | - | |||||||
| MHC class I | + | + | + | + | + | + | + | + | + | |||||||
| MHC class II | - | - | - | - | - | + | + | + | + | |||||||
- indicates absent; +, clearly present; +/-, weakly present.
Expression of RFX and CIITA
To investigate whether the lack of MHC class II expression in leukemic T cells is due to the absence of an essential transcription factor, we examined the expression levels of CIITA and RFX complex components because these regulatory proteins are crucial for MHC class II gene transcription. Using RT-PCR analysis, we were able to detect mRNA transcripts for RFX5, RFXB, and RFXAP in all leukemic T-cell lines tested to levels comparable to those found in the MHC class II–expressing T-lymphoma cell line HUT78 (Figure 2A). However, we were unable to amplify CIITA cDNA obtained from the leukemic T cells using primers designed to detect all isoforms of CIITA (Figure 2A). To verify that the MHC class II–deficient cells lack expression of any of the CIITA isoforms, RT-PCR analysis was performed with CIITA-PI, -PIII, or -PIV specific primers (Figure 2B). Indeed, none of the MHC class II–deficient T cells contained CIITA-PI, -PIII, or -PIV transcripts, while the MHC class II–positive cell lines H9, Karpas-299, HUT78, and HH (Figure 2B) expressed CIITA-PIII transcripts abundantly. Furthermore, CIITA-PIV transcripts were also detected in various amounts in these lymphoma cell lines.
RFX and CIITA expression profiles in malignant T cells. (A) The presence of RFX5, RFXAP, RFXB, and CIITA mRNA was detected using RT-PCR analysis on cDNA from several T-ALL cell lines and the T-cell lymphoma HUT78. (B) The usage of CIITA promoters PI, PIII, and PIV was investigated on HLA-DR–negative and HLA-DR–positive malignant T cells using RT-PCR analysis. GAPDH was used as control for equal cDNA loading. DC indicates dendritic cells.
RFX and CIITA expression profiles in malignant T cells. (A) The presence of RFX5, RFXAP, RFXB, and CIITA mRNA was detected using RT-PCR analysis on cDNA from several T-ALL cell lines and the T-cell lymphoma HUT78. (B) The usage of CIITA promoters PI, PIII, and PIV was investigated on HLA-DR–negative and HLA-DR–positive malignant T cells using RT-PCR analysis. GAPDH was used as control for equal cDNA loading. DC indicates dendritic cells.
In normal primary human T cells, expression of CIITA mRNA is detected after 3 to 5 days following stimulation with mitogenic agents such as phorbol myristate acetate (PMA)/ionomycin (Figure 3). In contrast, stimulation of the MHC class II–deficient T-ALL cell lines with PMA/ionomycin for 4 days did not induce CIITA-PIII expression. In the majority of these stimulated leukemic T cells, however, induction of IL-4 and IFNγ was readily detected (Figure 3). Furthermore, PMA/ionomycin stimulation resulted also in an increased cell surface expression of CD69 and CD45RO, both associated with T-cell activation (data not shown). Together, these data indicate that the lack of MHC class II expression in these leukemic T cells is not caused by the disruption of general activation pathways that also control endogenous CIITA expression in normal T cells.
Lack of CIITA expression after stimulation in T-ALL cell lines. T-cell lines were unstimulated or stimulated with 10 ng/mL PMA and 1 μg/mL ionomycin in the medium for 4 days. RT-PCR analysis was then performed with primers specific for CIITA-PIII, IFNγ, or IL-4. GAPDH was used as control for equal cDNA loading. H2O indicates water control; CD4+, CD4+ peripheral human T cells activated with 10 ng/mL PMA and 1 μg/mL ionomycin for 4 days.
Lack of CIITA expression after stimulation in T-ALL cell lines. T-cell lines were unstimulated or stimulated with 10 ng/mL PMA and 1 μg/mL ionomycin in the medium for 4 days. RT-PCR analysis was then performed with primers specific for CIITA-PIII, IFNγ, or IL-4. GAPDH was used as control for equal cDNA loading. H2O indicates water control; CD4+, CD4+ peripheral human T cells activated with 10 ng/mL PMA and 1 μg/mL ionomycin for 4 days.
Regulation of CIITA expression in leukemic T cells
To test whether or not the CIITA-deficient leukemic T cells express all the necessary transcription factors for proper CIITA promoter activation, CIITA-PIII promoter–reporter constructs were transfected into several CIITA-deficient T-cell lines. As shown in Figure 4, exogenous CIITA-PIII is able to drive the constitutive expression of a luciferase-reporter gene in the CIITA-deficient leukemic cell lines Jurkat, Molt-4, HSB-2, and CEM, and which is comparable to the exogenous CIITA-PIII expression observed in the CIITA-expressing cell lines HH, H9, Karpas-299, and HUT78. In contrast, exogenous CIITA-PIV is not active in the leukemic T-cell lines tested, indicating that essential transcription factors necessary for CIITA-PIV activity are not present in T-ALL cell lines. Intriguingly, the lymphoma T-cell lines show variable levels of exogenous CIITA-PIV activity, which is in line with endogenous expression of the CIITA-PIV isoform (Figure 2B).
Exogenous CIITA promoter activity in various T-cell lines. pGL3-CIITA-PIII (PIII) or pGL3-CIITA-PIV (PIV) were transiently transfected in CIITA-deficient Jurkat, Molt-4, CEM, and HSB-2 T-ALL cells and the CIITA-expressing HH, H9, Karpas-299, and HUT78 T-lymphoma cell lines. Promoter activity was determined 48 hours after transfection; pGL3-basic (C), which lacks a promoter preceding the luciferase gene, is used as background control. Promoter activity is measured as relative luciferase units (RLUs) and corrected for transfection efficiency using a β-actin Renilla construct. Error bars represent SD.
Exogenous CIITA promoter activity in various T-cell lines. pGL3-CIITA-PIII (PIII) or pGL3-CIITA-PIV (PIV) were transiently transfected in CIITA-deficient Jurkat, Molt-4, CEM, and HSB-2 T-ALL cells and the CIITA-expressing HH, H9, Karpas-299, and HUT78 T-lymphoma cell lines. Promoter activity was determined 48 hours after transfection; pGL3-basic (C), which lacks a promoter preceding the luciferase gene, is used as background control. Promoter activity is measured as relative luciferase units (RLUs) and corrected for transfection efficiency using a β-actin Renilla construct. Error bars represent SD.
Together, we therefore conclude that the lack of CIITA expression in leukemic T cells is not due to a lack of transcription factors necessary for the activation of CIITA-PIII but is probably due to epigenetic modification of genomic DNA encompassing CIITA regulatory regions.
Hyper-methylation of CIITA-PIII in leukemic T cells
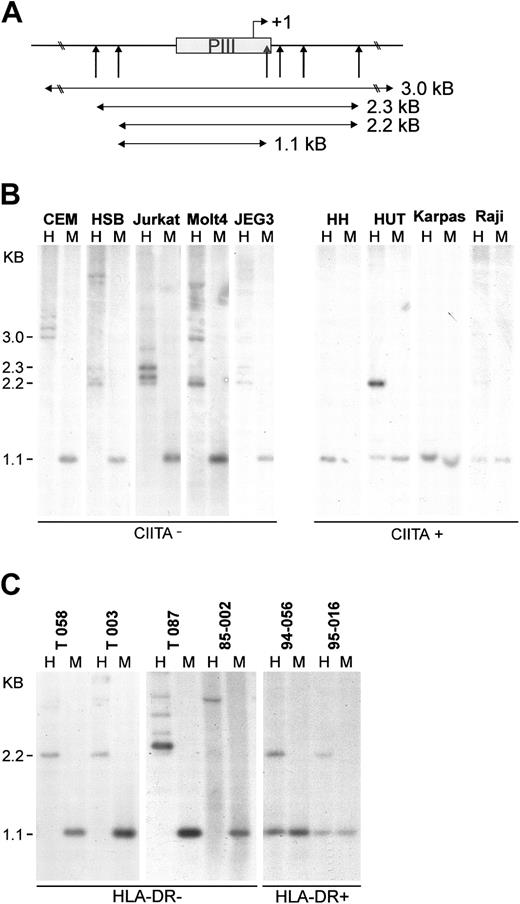
A well-known repression mechanism of gene expression is methylation-based silencing through cytosine methylation at CpG dinucleotides. Early analysis of the role of methylation using a large range of tissue-specific genes introduced into mammalian cells led to the general consensus that DNA methylation results in the formation of nuclease-resistant chromatin and subsequent repression of gene activity.38 To evaluate if the lack of CIITA expression in leukemic T cells is caused by DNA hyper-methylation, the methylation status of CIITA-PIII was determined through digestion of genomic DNA with methylation-sensitive and methylation-tolerant restriction enzymes followed by Southern blot analysis (Figure 5A). The Epstein-Barr virus (EBV)–transformed B-cell line Raji and the trophoblast cell line JEG-3 were used as controls for unmethylated and methylated CIITA-PIII, respectively. Unmethylated DNA will be cleaved by both HpaII and MspI generating a CIITA-PIII fragment of 1.1 kb. Using the methylation-tolerant restriction enzyme MspI and its methylation-sensitive isoschizomer HpaII, we found that several HpaII sites in and around CIITA-PIII were methylated in all the MHC class II–deficient T-cell lines tested (Figure 5B). In addition, it appeared that in CEM a larger promoter region of approximately 3.5 kb is methylated. Multiple bands with equal intensity are observed in the HpaII lanes, probably indicating allelic difference in the length of the methylated promoter region and/or polymorphisms in restriction sites. Also complete methylation (Jurkat, CEM, HSB) or partial methylation (Molt-4) of CIITA-PIV was found in the leukemic T-cell lines (data not shown). In contrast, the CIITA-expressing T-cell lines have unmethylated (HH, Karpas-299) or contain partially methylated (HUT78) CIITA-PIII, which is comparable to the methylation status of CIITA-positive Raji B cells. Furthermore, also CIITA-PIV was found to be unmethylated in HH and HUT78 (data not shown).
Genomic CIITA-PIII DNA from leukemic T cells is methylated. (A) Restriction sites around CIITA-PIII of the methylation-sensitive enzyme HpaII and its methylation-tolerant isoschizomer MspI are indicated with vertical arrows. The probe used for the Southern blot analysis is indicated as a gray box. Detected fragment sizes are indicated. (B) Southern blot analysis on HindIII/HpaII (H) and HindIII/MspI (M) digested DNA of CIITA-deficient or CIITA-expressing T-cell lines. JEG-3 DNA was used as a control for methylated CIITA-PIII,39 and Raji B-cell DNA was used as a control for unmethylated CIITA-PIII. (C) The methylation status of CIITA-PIII in primary T-cell leukemia and lymphoma as determined by Southern blot analysis on HindIII/HpaII (H) and HindIII/MspI (M) digested DNA. The HLA-DR surface expression of the panel of primary malignant T cells has been determined previously (Table 1; Van Dongen et al31 and Langerak et al32,33 ).
Genomic CIITA-PIII DNA from leukemic T cells is methylated. (A) Restriction sites around CIITA-PIII of the methylation-sensitive enzyme HpaII and its methylation-tolerant isoschizomer MspI are indicated with vertical arrows. The probe used for the Southern blot analysis is indicated as a gray box. Detected fragment sizes are indicated. (B) Southern blot analysis on HindIII/HpaII (H) and HindIII/MspI (M) digested DNA of CIITA-deficient or CIITA-expressing T-cell lines. JEG-3 DNA was used as a control for methylated CIITA-PIII,39 and Raji B-cell DNA was used as a control for unmethylated CIITA-PIII. (C) The methylation status of CIITA-PIII in primary T-cell leukemia and lymphoma as determined by Southern blot analysis on HindIII/HpaII (H) and HindIII/MspI (M) digested DNA. The HLA-DR surface expression of the panel of primary malignant T cells has been determined previously (Table 1; Van Dongen et al31 and Langerak et al32,33 ).
To determine if a correlation could also be found in primary leukemic T cells between the lack of MHC class II expression and CIITA-PIII hyper-methylation, we determined the methylation status of genomic CIITA-PIII from a panel of HLA-DR–negative and HLA-DR–positive T-cell leukemias (Table 1). Southern blot analysis of genomic DNA from the HLA-DR–negative malignant T cells clearly showed that CIITA-PIII in these cells is hypermethylated (Figure 5C). Moreover, CIITA-PIII was partially methylated in 2 HLA-DR–positive primary leukemias. Together, these observations show that epigenetic modification of CIITA-PIII also control MHC class II expression in primary malignant T cells.
Modifying CIITA-PIII methylation in vivo and in vitro affects CIITA and MHC class II expression
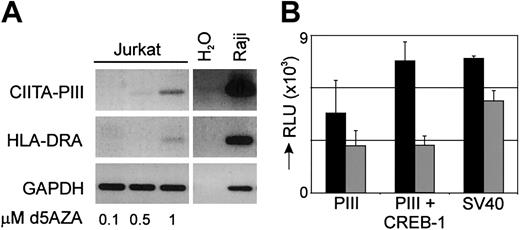
The cytosine nucleoside analog, 5-AZA-2′-deoxycytidine is a potent inhibitor of DNA methylation that can activate tumor suppressor genes in leukemic cells that have been silenced by aberrant methylation. To establish in vivo that the lack of CIITA and congruent MHC class II expression in CIITA-deficient T-ALL is indeed due to hyper-methylation of CIITA-PIII, we incubated Jurkat cells with different concentrations of 5-AZA-2′-deoxycytidine for 3 days. A clear induction of CIITA-PIII and congruent HLA-DRA mRNA could be detected in cells treated with a 1-μM dose of 5-AZA-2′-deoxycytidine, revealing that hyper-methylation of CIITA-PIII is responsible for the lack of MHC class II expression in the leukemic T-cell lines (Figure 6A). In contrast, the 5-AZA-2′-deoxycytidine–treated cells did not re-express CIITA-PIV transcripts (data not shown).
Modification of CIITA-PIII affects CIITA and MHC class II expression. (A) Jurkat T-ALL cells were treated with 0.1, 0.5, or 1 μM of the demethylation agent 5-AZA-2′-deoxycytidine for 3 days and mRNA was used for the detection of CIITA-PIII and HLA-DRA expression by RT-PCR analysis. Raji cDNA is used as a control for CIITA-PIII and HLA-DRA amplification. (B) Isolated CIITA-PIII and SV40 promoter fragments were either unmethylated (▪) or in vitro methylated (▦) , ligated back into the pGL3-vector, and transfected in Jurkat T cells. Unmethylated and methylated CIITA-PIII was also cotransfected with a CREB-1 expression vector.28 Promoter activity was measured 2 days after transfection and was normalized with a β-actin–Renilla construct. Promoter activity is indicated as relative luciferase units (RLUs). Error bars represent SD.
Modification of CIITA-PIII affects CIITA and MHC class II expression. (A) Jurkat T-ALL cells were treated with 0.1, 0.5, or 1 μM of the demethylation agent 5-AZA-2′-deoxycytidine for 3 days and mRNA was used for the detection of CIITA-PIII and HLA-DRA expression by RT-PCR analysis. Raji cDNA is used as a control for CIITA-PIII and HLA-DRA amplification. (B) Isolated CIITA-PIII and SV40 promoter fragments were either unmethylated (▪) or in vitro methylated (▦) , ligated back into the pGL3-vector, and transfected in Jurkat T cells. Unmethylated and methylated CIITA-PIII was also cotransfected with a CREB-1 expression vector.28 Promoter activity was measured 2 days after transfection and was normalized with a β-actin–Renilla construct. Promoter activity is indicated as relative luciferase units (RLUs). Error bars represent SD.
In addition, to further confirm that DNA hyper-methylation is regulating CIITA-PIII promoter activity, the CIITA-PIII promoter region was removed from the pGL3–CIITA-PIII promoter luciferase reporter construct, treated with or without the DNA methylating substrate S-adenosylmethionine and SssI methylase, and ligated back into the pGL3 vector. Transient transfection showed that methylation of CIITA-PIII resulted in a decrease in promoter activity to 60% of the activity of the unmethylated promoter construct (Figure 6B). Likewise, in vitro methylation of the viral promoter SV40 caused a decrease in promoter activity to 70%. Furthermore, we analyzed also the activity of the methylated and unmethylated CIITA-PIII promoters after induction with the transcription factor CREB-1. The promoter activity of unmethylated CIITA-PIII was found to be up-regulated about 2-fold, while methylation of CIITA-PIII caused a complete abolishment of CREB-1–mediated induction.
Discussion
Under normal circumstances, MHC class II molecules are expressed on human T cells following activation. Cell surface expression of MHC class II in in vivo–activated T cells with αCD3 and αCD28 or phytohemagglutinin (PHA) is congruent with expression of CIITA exclusively driven by its promoter III (CIITA-PIII), whereas unstimulated T cells lack CIITA expression.29,30 Here we have shown that the lack of MHC class II expression in a panel of T-ALL cell lines coincides with lack of CIITA expression through promoter I, III, or IV. Furthermore, stimulation of the HLA-DR–negative T-ALL cell lines with well-known T-cell activation agents did not result in the induction of CIITA and MHC class II expression. Because other T-cell activation markers, such as IFNγ, IL-4, CD69, and CD45RO, were induced in the stimulated T-ALL cells, the possibility that general T-cell activation pathways in these malignant T cells were corrupted was eliminated. In addition, we showed in a transient promoter-reporter assay that CIITA-PIII was activated in T-leukemic cells to levels similar to MHC class II–expressing T-lymphoma cells, revealing that all essential transcription factors for CIITA-PIII activation are present in the leukemic cells.
Further analyses showed that the CIITA deficiency in T-ALL correlates with hyper-methylation of CIITA-PIII and also CIITA-PIV (not shown) in these cells. Subsequent demethylation of DNA with 5-AZA-2′-deoxycytidine resulted in re-expression of CIITA-PIII, but not CIITA-PIV (not shown), and expression of HLA-DRA in these leukemic T cells. Unfortunately, we were not able to evaluate MHC class II surface expression in 5-AZA-2′-deoxycytidine–treated cells. However, Jurkat T-ALL cells re-express MHC class II on the cell surface following introduction and expression of exogenous CIITA (Saufuddin et al40 ; T.M.H., E.S., and P.J.v.d.E., unpublished results, 2002). Moreover, we also showed that in primary leukemic T cells the absence of HLA-DR surface staining correlates with hyper-methylation of CIITA-PIII. Therefore, the defect in CIITA expression in leukemic T cells is due to hypermethylation, which specifically blocks factor assembly on CIITA-PIII and its subsequent activation. In this way expression of CIITA is prohibited, while at the same time the expression profiles of other genes that fulfill essential T-cell functions are not affected.
In contrast to the HLA-DR–deficient T-ALL cell lines, the MHC class II–positive T-lymphoma cell lines express both CIITA-PIII and CIITA-PIV transcripts. Expression of both CIITA transcripts in these T-lymphoma cell lines is congruent with the finding that the CIITA-PIII and CIITA-PIV reporter constructs are active (Figure 4) and that both CIITA-PIII and CIITA-PIV are unmethylated (Figure 5 and data not shown) in these cells. Our data therefore indicates that in the tested T-lymphoma cells the essential transcription factors for both CIITA-PIII and CIITA-PIV activation are present and that the promoters are accessible for transcription factor binding, resulting in CIITA-PIII and CIITA-PIV transcription. The precise regulatory mechanisms and factors that activate CIITA promoters, in particular CIITA-PIV, in malignant MHC class II–expressing T cells, however, are still unclear and remain to be investigated.
In the immune system, epigenetic mechanisms have been shown to be involved in the peripheral differentiation of T cells. For instance, differentiation of naive uncommitted T cells into cytokine-producing Th1 and Th2 cells relies on chromatin remodeling and alterations in DNA methylation patterns of effector genes.41,42 In the case of CIITA, epigenetic modifications of CIITA promoters have been described previously for the IFNγ-induced MHC class II expression. The lack of MHC class II–mediated antigen-presenting functions of trophoblast-derived choriocarcinoma cells has been linked to absence of factor assembly resulting from hypermethylation of the IFNγ-responsive CIITA promoters, in particular of CIITA-PIV.35,43 Hyper-methylation of the IFNγ-responsive promoters was also shown to be at the heart of the CIITA deficiency in a panel of developmental tumors that lacked MHC class II expression following IFNγ treatment.39 Down-regulation of gene expression through DNA hyper-methylation represents a common mechanism of gene silencing that occurs in normal cells during early embryonic development and differentiation.44-46 Because the tumor cells currently investigated represent different stages of early T-cell development, the results of these former studies could also infer that lack of MHC class II expression accomplished through CIITA promoter hyper-methylation is associated with normal T-cell development and that tight control of MHC class II expression might be very important during the development of various T-cell types.
However, malignant transformation of cells has been reported to induce differences in epigenetic modifications of genomic DNA.47,48 For instance, it has been shown that the hematopoietic cell–specific protein-tyrosine phosphatase SHP1 (Src homology 2 domain–containing tyrosine phosphatase 1), a mediator of T-cell apoptosis Galectin-1, the proto-oncogene HOX11, and the cyclin-dependent kinase inhibitors p15INK4b, p16INK4a, and p14ARF are all silenced by promoter methylation in various kinds of leukemias and lymphomas.49-52 As mentioned above, the T-ALL cells reported in this paper are not able to express CIITA due to hyper-methylation of CIITA-PIII. Whether the silencing of CIITA through promoter hyper-methylation is a normal feature of T-cell development in the thymus, or is associated with malignant transformation, remains to be investigated.
In conclusion, in this study we show that the lack of MHC class II expression on leukemic T cells is caused by hyper-methylation of CIITA-PIII resulting in lack of MHC class II expression. Our findings therefore ultimately could infer that application of a DNA demethylating agent to induce CIITA and resulting MHC class II expression on otherwise MHC class II–deficient T-ALL might be beneficial in the treatment of T-ALL by taking advantage of the tumoricidal activity of novel MHC class II antibodies.
Prepublished online as Blood First Edition Paper, October 16, 2003; DOI 10.1182/blood-2003-05-1491.
Supported by the Dutch Cancer Society (grant 98-1732) and a Leiden University Medical Center Research Development Grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We want to thank Prof Dr Frans Claas and Dr Ferry Ossendorp for critical reading of the manuscript.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal