Abstract
Imerslund-Gräsbeck syndrome (I-GS, megaloblastic anemia 1) is an autosomal recessive disorder characterized by intestinal cobalamin (vitamin B12) malabsorption and proteinuria. I-GS–causing mutations are found in either of 2 genes encoding the epithelial proteins: cubilin and amnionless (AMN). Cubilin recognizes intrinsic factor (IF)–cobalamin and various other proteins to be endocytosed in the intestine and kidney, respectively, whereas the function of AMN is unknown. Here we show that cubilin and AMN colocalize in the endocytic apparatus of polarized epithelial cells and copurify as a tight complex during IF-cobalamin affinity and nondenaturing gel filtration chromatography. In transfected cells expressing either AMN or a truncated IF-cobalamin–binding cubilin construct, neither protein alone conferred ligand endocytosis. In cubilin transfectants, cubilin accumulated in early biosynthetic compartments. However, in cells cotransfected with AMN and the cubilin construct, cubilin trafficked to the cell surface and endosomes, and the cells exhibited IF-cobalamin endocytosis and lysosomal degradation of IF. These data indicate that cubilin and AMN are subunits of a novel cubilin/AMN (cubam) complex, where AMN binds to the amino-terminal third of cubilin and directs subcellular localization and endocytosis of cubilin with its ligand. Therefore, mutations affecting either of the 2 proteins may abrogate function of the cubam complex and cause IG-S.
Introduction
Cobalamin (vitamin B12) is a coenzyme for the enzymes of intermediate metabolism, methionine synthase, and methylmalonyl-CoA mutase, and deficiency of the vitamin leads to potentially lethal manifestations such as megaloblastic anemia and severe combined degeneration of the central nervous system. Cobalamin deficiency, which is one of the most common vitamin-deficiency diseases, is most often due to failure at a step in the complicated and highly specific gastrointestinal uptake mechanisms for dietary cobalamin rather than an insufficient supply from food.1
Intrinsic factor (IF) is a glycoprotein produced in the gastric epithelium. It tightly binds to cobalamin in the gastrointestestinal tract, and in the distal small intestine the IF-cobalamin complex is recognized by cubilin, a multiligand apical membrane protein that participates in endocytosis of the complex.2,3 IF is subsequently degraded in enterocyte lysosomes, and cobalamin is secreted into plasma in complex with transcobalamin-II.4
Cubilin is a large membrane protein (460 kDa) with a unique set of extracellular protein modules comprising 8 tandem epidermal growth factor domains followed by 27 tandem CUB domains (initially found in complement components C1r/C1s, Uegf, and bone morphogenic protein-1) harboring the IF-cobalamin binding site (CUB domains 5-8).5,6 Although cubilin has no apparent transmembrane segment or cytoplasmic tail, several studies have shown that binding of IF-cobalamin to cubilin leads to endocytosis of the ligand and recycling of the receptor.2,3 Besides expression and function in the intestine, cubilin has many-fold higher expression in the apical membrane of kidney proximal tubule and rodent yolk sac epithelial cells.2,3,7,8 Consistent with this pattern of expression, cubilin is involved in reabsorption of several specific nutrient-carrying proteins from renal glomerular filtrate, including albumin,9 transferrin,10 vitamin D–binding protein,11 and apolipoprotein AI,12,13 and cubilin has a crucial but not-yet-defined role in early embryonic development of rodents.14 Evidence to date indicates that the mechanism of cubilin-mediated endocytosis is the same in the various cubilin-expressing epithelia.2 Accordingly, IF-cobalamin is effectively endocytosed in a cubilin-dependent manner in the proximal tubule15 and yolk sac,16 and because these tissues have higher density of cubilin in apical membranes and less luminal proteolytic activity than intestine, they have been the preferred tissues for studying cubilin function.2,8,14-16 However, it should be noted that ligands other than IF-cobalamin are likely to be physiologically more important in the kidney and yolk sac because little or no gastric IF circulates in plasma.
Cobalamin deficiency is most often caused by decreased production or function of IF due to acquired autoimmune disease of the gastric mucosa (pernicious anemia), but selective malabsorption of the vitamin may also be due to inherited defects of the various components in the cobalamin uptake system. Imerslund-Gräsbeck syndrome (I-GS; aka megaloblastic anemia 1, OMIM no. 261 100) is an autosomal recessive disorder characterized by selective malabsorption of cobalamin despite normal production and function of IF.17,18 Proteinuria is often an additional sign of the disorder.17,18 More than 200 human cases have been reported with familial clusters in Finland, Norway, the Middle East, and North Africa.19 The same disorder has also been identified in a dog kindred.20 Genetic and functional studies provide evidence that abnormal function of cubilin causes the disease.21-23 For instance, the majority of the Finnish patients with I-GS are homozygous for a missense mutation21 that reduces IF-cobalamin binding,23 and canine I-GS cases have abnormal processing and deficient apical membrane expression of cubilin.20 However, extensive genetic analyses of Norwegian and Middle Eastern kindreds24 as well as of canine I-GS cases25 have disclosed that the disease displays locus heterogeneity. Very recently, Tanner et al24 demonstrated that mutations in the amnionless (AMN) gene also cause I-GS, and canine I-GS shows highly significant linkage to the same locus.26
AMN was initially identified by random mutagenesis27 as a protein essential for amnion and primitive streak formation in mice. AMN is a transmembrane 45- to 50-kDa protein of polarized epithelia.24,27 The function of AMN is otherwise unknown, but it is highly expressed in cubilin-expressing tissues, including the kidney, intestine, and mouse visceral yolk sac.24,27 Their coincident tissue distribution combined with the observation that inherited abnormalities in AMN and cubilin have similar phenotypic consequences14,20,24,27 suggest a functional linkage between these 2 gene products. In the present study we investigated the possibility that the 2 proteins interact. Here we present evidence that cubilin and AMN form a tightly bound complex early in the biosynthetic pathway that is essential for apical membrane localization and endocytic functions previously ascribed to cubilin alone.
Materials and methods
Antibodies
Two anti-AMN peptide antibodies were raised in rabbits by immunization with peptides comprising the human AMN amino acid residues 165 to 179 of the extracellular domain or residues 415 to 430 in the cytoplasmic tail. Both were tested by immunoblot of kidney cortex proteins and were demonstrated to be AMN specific; neither cross reacted with purified cubilin or megalin. A previously described22 rabbit polyclonal antihuman cubilin antiserum demonstrated not to cross react with megalin or AMN was used on immunoblots and immunohistochemical and immunoelectron microscopic examination of kidney proximal tubule cells. Anti-myc antibody purchased from Invitrogen (Taastrup, Denmark) was used for detection of the AMN-myc construct expressed in Chinese hamster ovary (CHO) cells, and a previously described5 rabbit polyclonal antirat cubilin antiserum was used in immunoblots of products from CHO cells expressing a rat cubilin construct. In cases where CHO cells were doubly stained for immunofluorescent microscopy and the available anti-IF sera was of rabbit origin, a previously described mouse monoclonal anticubilin antibody14,28 was used. A sheep antimegalin antibody9 recognizing rodent, canine, and human megalin was used for immunoblotting.
Immunostaining of human kidney cortex
Cryosections of normal human kidney cortex (uninvolved sections from resected renal carcinoma) were incubated with a rabbit anti-AMN peptide (415-430) serum (1:1000 dilution) or polyclonal antihuman cubilin antibody. Sections were subsequently incubated with goat antirabbit immunoglobulin G (IgG) conjugated to horseradish peroxidase for light microscopy or 10-nm gold particles for electron microscopy as previously.15
Biochemical analysis of the cubilin/AMN complex
Purification of the cubilin/AMN complex from human kidney by affinity chromatography was carried out as described previously.22 Briefly, total membrane and supernatant fractions were prepared from homogenates of postmortem kidney cortex. Triton X-100 (1%) was added to solubilized proteins in the membrane fraction. Each fraction was applied to an IF-cobalamin column, and the columns were washed extensively in phosphate-buffered saline (PBS), pH 7.4, containing 1 mM CaCl2 and 0.6% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS). Column binding proteins were eluted in several fractions of PBS, pH 5, containing 5 mM ethylenediamine tetraacetic acid (EDTA) and 0.6% CHAPS. Immunoblots were prepared on polyvinylidene difluoride (PVDF) membrane after sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 4% to 16% or 10% gels and incubated with rabbit antihuman cubilin serum diluted 1:1000 or rabbit anti-AMN extracellular domain peptide serum diluted 1:500. Secondary antibody was antirabbit IgG–alkaline phosphatase conjugate, and proteins were detected with P-nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate. Amino-terminal sequencing of electroblotted AMN was performed as previously described.22
For gel filtration analysis of IF-cobalamin affinity-purified proteins, fractions containing cubilin were combined and concentrated by centrifugation through a 10 000-kDa cutoff membrane. A 25-mL Superose 6 column (Amersham Biosciences, Hillerod, Denmark) was equilibrated, loaded, and eluted variously in pH 7.4 PBS, pH 5 PBS containing 5 mM EDTA, or pH 7.4 PBS containing 0.2% SDS, 2 M urea, or 6 M urea. Proteins in column fractions were separated by SDS-PAGE and immunoblotted as above. AMN oligosaccharide analysis was carried out by endoglycosidase H (endo H; Roche, Mannheim, Germany) and peptide N-glycosidase F (PNGase F; Roche) digestion of IF-cobalamin affinity purified proteins and SDS-PAGE as described previously.20 Gel slices of proteins separated by SDS-PAGE were subjected to in-gel tryptic digestion followed by MALDI mass spectrometric peptide mapping as described.29,30
Establishment and characterization of stable AMN and cubilin transfectant cells
The cDNA encoding amino acids 1 to 1389 of rat cubilin was ligated into the XbaI and HindIII sites of the expression vector pcDNA3.1/Zeo(-) (Invitrogen), and stable, single-transfectant, Zeocin-resistant CHO cell clones were established as described.6 Establishment of stable, double-transfected CHO clones expressing both the truncated cubilin construct and myc-labeled human AMN was carried out by transfection with a previously described cDNA plasmid24 encoding AMN and selection with 1 mg/mL Geneticin (Invitrogen). For establishment of stable, single-transfected CHO clones expressing AMN-myc, the AMN-myc insert was ligated into the HindIII and XhoI sites of the pcDNA3.1/Zeo(+) expression vector (Invitrogen), and CHO cells were processed as above.
Analysis of expression products and IF-cobalamin endocytosis in CHO cells
Expression products of all clones were analyzed by immunoblotting of growth medium and cell lysates. In other experiments, porcine IF-cobalamin coupled to cyanogen bromid (CNBr)–activated Sepharose 4B beads (Amersham Biosciences), as previously described,15 was incubated with cell lysates of single- and double-transfected CHO cells expressing the cubilin construct of amino acid residues 1-1389. Following precipitation of the recombinant cubilin products, the affinity beads were suspended in 50 mM Na phosphate buffer, pH 5.5, containing 0.01% SDS or in 20 mM Na phosphate buffer, pH 7.2, containing 10 mM EDTA and 0.5% Triton X-100 and incubated overnight at 37°C with endo H or at 30°C with PNGase F, respectively. No endogenous cubilin or megalin expression was detected in the nontransfected CHO cells by immunoblotting.
Endocytosis of human IF-cobalamin in cubilin-transfected, AMN-myc–transfected, cubilin/AMN-myc–double transfected, and mock-transfected CHO cells was analyzed as described for other ligands.12,31 Briefly, triplicates of cells grown to confluence in 24-well plates (0.08-0.13 mg protein/well) were incubated for 4 hours at 37°C with 125I-labeled human IF-cobalamin with or without added chloroquine and leupeptin. The medium was removed, and soluble fragments of degraded IF in the medium were separated from intact radiolabel by addition of 12.5% trichloroacetic acid (TCA) and centrifugation. The washed cell layers were lysed with 0.5 M NaOH, and radioactivity of all fractions was determined. In this assay, degradation of ligand is represented by the cell-mediated increase in TCA-soluble radioactivity in the medium, and ligand uptake is represented by cell-associated radioactivity plus cell-mediated degradation.
Confocal immunofluorescence and immunoelectron microscopy of CHO cells
Immunofluorescent analyses of CHO cells expressing cubilin, AMN, and cubilin/AMN to determine localization of these proteins were performed on fixed permeabilized cells (fixed 30 minutes at room temperature in 4% formaldehyde in 10 mM PBS and washed in 10 mM PBS, pH 7.4, 0.05% Triton X-100) for intracellular distribution or on living nonpermeabilized cells to visualize proteins on the extracellular surface. Fixed and permeabilized cells were incubated for 1 hour at room temperature variously with polyclonal antirat cubilin serum, monoclonal antihuman cubilin antibody, and the anti-myc antibody each diluted to 10 μg/mL in washing buffer. For surface staining, living cells were incubated for 90 minutes at 4°C with polyclonal antirat cubilin antiserum diluted to 10 μg/mL in growth medium, washed, and then fixed for 1 hour at 4°C. To visualize IF-cobalamin uptake and intracellular distribution of ligand, living cells were incubated for 30 minutes or 1 hour at 37°C with IF-cobalamin diluted to 40 μg/mL in growth medium, then fixed and incubated as above with rabbit polyclonal antiporcine IF serum and mouse monoclonal antihuman cubilin antibody. Finally, the cells were incubated for 1 hour at room temperature with the fluorescence-labeled secondary antibodies Alexa Fluor 488 goat antirabbit IgG and/or Alexa Fluor 594 goat antimouse IgG (Molecular Probes, Eugene, OR), each diluted 1:200. Stained cells were examined by confocal fluorescence microscopy using a laser scanning confocal unit (LSM510; Carl Zeiss, Jena, Germany) attached to an Axiovert (Carl Zeiss) microscope.
For electron microscopy, cells were fixed for 30 minutes with 2% formaldehyde in 0.1 M Na cacodylate buffer, pH 7.4. Cells were removed from the plastic surface with a rubber policeman in the same buffer containing 1% gelatin, pelleted, embedded in 12% gelatin, and finally infiltrated with 2.3 M sucrose in PBS for 30 minutes before freezing in liquid nitrogen. Ultrathin cryosections (70-90 nm) were prepared with an FCS Reichert Ultracut S cryoultramicrotome (Leica, Vienna, Austria) at about –100°C and collected on 200 mesh Ni grids. The sections were incubated overnight with a rabbit polyclonal antirat cubilin antiserum diluted 1:1000, prior to 2 hours of incubation with a 10-nm gold particle–labeled goat antirabbit antibody (BioCell, Cardiff, United Kingdom). All incubations were performed at 4°C. The sections were finally contrasted with methylcellulose containing 0.3% uranyl acetate and studied in a Philips CM100 electron microscope (FEI Denmark, Glostrup, Denmark).
Results
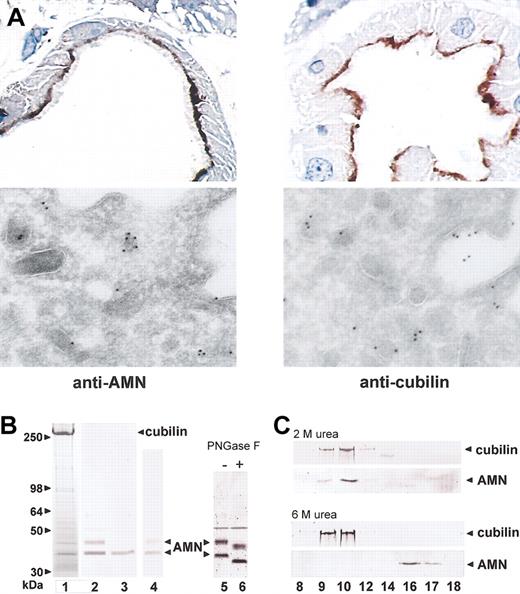
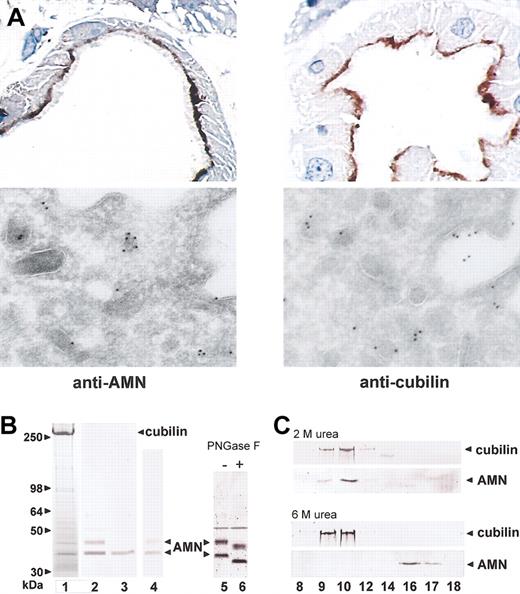
In accordance with the hypothesis that cubilin and AMN interact in polarized epithelia, light and electron microscopy of immunostained human kidney cortex showed indistinguishable localization of the 2 membrane proteins in the apical membrane and endocytic apparatus of renal proximal tubule cells (Figure 1A). To identify possible physical interactions between cubilin and AMN, we analyzed the material isolated from an IF-cobalamin affinity matrix loaded with detergent-solubilized renal cortex membranes. SDS-PAGE of the affinity-purified material showed several bands in addition to the abundant cubilin band (Figure 1B, lane 1). By immunoblotting, AMN was identified in 2 major bands of approximately 35 kDa and approximately 45 kDa (Figure 1B, lane 2), and both had approximately 3 kDa of Asn-linked oligosaccharides as demonstrated by PNGase F digestion (Figure 1B, lanes 5 and 6). MALDI mass spectrometric peptide mapping of in-gel tryptic digests identified AMN peptides 110-119, 120-134, 139-151, 152-162, 172-186, 176-182, and 274-293 in both the 35-kDa and 45-kDa AMN bands and additional peptides 302-323 and 327-347 in the 45-kDa band. Furthermore, mass spectrometry identified minor-intensity AMN bands of 40 kDa, 42 kDa, and 49 kDa as well as IgG, α-actinin, and 2 mitochondrial proteins. Due to their expression patterns, the relationship of these non-AMN proteins to cubilin was not further pursued.
Identification of AMN as a protein colocalizing with and binding to cubilin. (A) Top panels: Immuno-peroxidase staining of AMN (left) and cubilin (right) in human kidney proximal tubules. Bottom panels: Immunoelectron staining with 10-nm gold particles of AMN (left) and cubilin (right) in the dense apical vesicles and plasma membrane of proximal tubules known to function in membrane receptor recycling.32 Original magnification, × 1000 (top panels) and × 46 000 (bottom panels). (B) Detergent-solubilized human kidney cortex membrane proteins were purified by IF-cobalamin affinity chromatography, separated on a 4% to 16% SDS-PAGE gel (lane 1, Coomassie stain), and subjected to anti-AMN immunoblot (lane 2). Also shown are anti-AMN immunoblots of IF-cobalamin–binding proteins from the supernatant fraction of kidney membranes prior to detergent solubilization (lane 3), human urine (lane 4), and kidney cortex membranes before (lane 5) and after (lane 6) PNGase-F digestion. (C) Anticubilin and anti-AMN immunoblots of Superose-6 gel filtration fractions of human kidney cortex IF-cobalamin–binding proteins eluted in PBS, pH 7.4, with 2 M or 6 M urea. Cubilin and AMN coeluted in fractions 9 and 10 in 2 M urea but separated in 6 M urea, where AMN elutes in fractions 16 and 17.
Identification of AMN as a protein colocalizing with and binding to cubilin. (A) Top panels: Immuno-peroxidase staining of AMN (left) and cubilin (right) in human kidney proximal tubules. Bottom panels: Immunoelectron staining with 10-nm gold particles of AMN (left) and cubilin (right) in the dense apical vesicles and plasma membrane of proximal tubules known to function in membrane receptor recycling.32 Original magnification, × 1000 (top panels) and × 46 000 (bottom panels). (B) Detergent-solubilized human kidney cortex membrane proteins were purified by IF-cobalamin affinity chromatography, separated on a 4% to 16% SDS-PAGE gel (lane 1, Coomassie stain), and subjected to anti-AMN immunoblot (lane 2). Also shown are anti-AMN immunoblots of IF-cobalamin–binding proteins from the supernatant fraction of kidney membranes prior to detergent solubilization (lane 3), human urine (lane 4), and kidney cortex membranes before (lane 5) and after (lane 6) PNGase-F digestion. (C) Anticubilin and anti-AMN immunoblots of Superose-6 gel filtration fractions of human kidney cortex IF-cobalamin–binding proteins eluted in PBS, pH 7.4, with 2 M or 6 M urea. Cubilin and AMN coeluted in fractions 9 and 10 in 2 M urea but separated in 6 M urea, where AMN elutes in fractions 16 and 17.
The peptides identified by mass spectrometry in the 35-kDa AMN product (Figure 1B) and identification of the amino-terminal sequence of Val-Ser-Lys-Leu in the same protein indicate that it represents the extracellular domain of AMN cleaved near the membrane. Consistent with being a protein with no membrane anchorage, the same AMN form was also identified as a soluble molecule in the supernatant fraction of human kidney membranes prior to detergent solubilization (Figure 1B, lane 3).
Cubilin and AMN coeluted in proportional amounts from the affinity column, and gel filtration of the eluted material showed that cubilin and AMN coelute as a strongly bound molecular complex that required denaturing conditions (6 M urea or 0.2% SDS) to separate (Figure 1C). Furthermore, in contrast to all other known cubilin ligands,2,3 the binding of AMN and cubilin was not calcium dependent, as indicated by coelution of the proteins in the presence of EDTA. We conclude from this set of analyses that AMN strongly binds cubilin in a region that is not sterically hindered by IF-cobalamin,6 and that cubilin bound to AMN truncated above the transmembrane segment is easily shed from membranes, as was previously observed for cubilin.5 While some of the soluble truncated 35-kDa AMN isolated from postmortem human kidney may be due to cleavage during autolysis, we also identified the 35-kDa AMN form together with cubilin in human urine (Figure 1B, lane 4), indicating that at least some AMN truncation occurs in vivo.
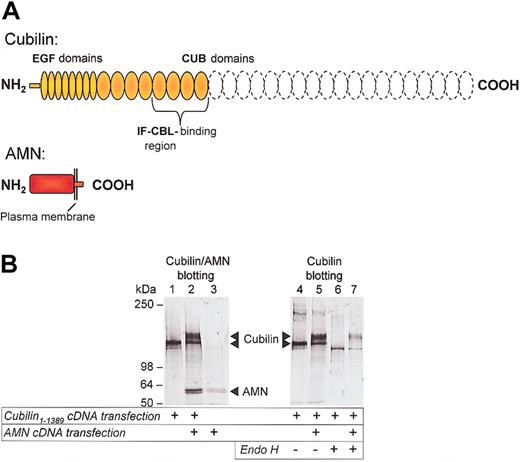
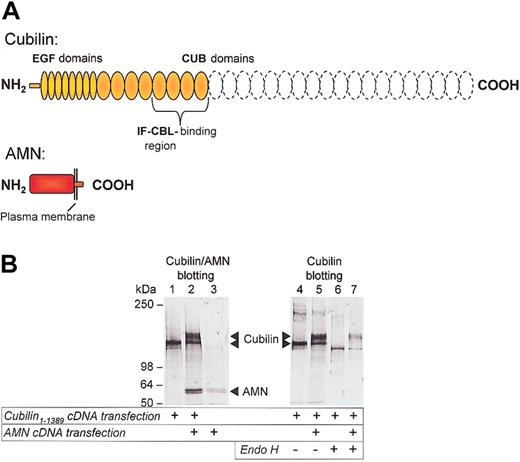
In order to investigate whether AMN is involved in cubilin processing and transport to the plasma membrane and endocytosis of cubilin-ligand complexes, we transfected CHO cells with cubilin and AMN cDNA plasmid constructs. Because we could not obtain a stable transfectant expressing the entire cubilin molecule, we expressed a “mini-cubilin” protein (amino acids 1-1389) from which the 19 carboxy-terminal CUB domains, suggested to be involved in binding other ligands,2,6 had been deleted (Figure 2A). This construct contains the 100-residue amino-terminal region previously demonstrated to be required for cellular retention of cubilin,6 the cluster of 8 epidermal growth factor repeats and CUB domains 1-8 harboring the IF-cobalamin binding site (CUBs 5-8).6 In CHO cells transfected with only the cubilin construct, immunoconfocal and immunoelectron microscopic examination revealed that the cubilin construct accumulated in the Golgi and other vesicular structures close the Golgi (Figure 3A,C, left panels), but no expression was seen in endosome-like structures or on the cell surface (Figure 3B, left panel). Although retained intracellularly, the cubilin construct was capable of binding to an IF-cobalamin Sepharose matrix (Figure 2B, lane 4). However, concordant with absent cell-surface expression of cubilin, these cells did not effectively endocytose and degrade 125I-labeled IF-cobalamin (Figure 4). Additionally, Asn-linked oligosaccharides of the expressed cubilin construct were endo H sensitive, as seen by increased gel mobility (Figure 2B, compare lanes 4 and 6), thus indicating lack of oligosaccharide maturation in Golgi.
Effect of AMN on cubilin expression and processing in CHO cells. (A) Cubilin (amino acids 1-1389) and AMN (full length plus myc epitope) constructs encoded by plasmid cDNA used for stable transfection of CHO cells. The position of the IF-cobalamin (IF-cbl) binding site (CUB domains 5-8)6 is indicated and the truncated CUB domains 9-27 are shown by dashed lines. (B) Immunoblots of cubilin and AMN in cell lysates (lanes 1-3) and endo H digestion of IF-cobalamin affinity-purified cubilin from cells transfected with cubilin, AMN, and cubilin/AMN cDNA. Gel mobility shifts indicate that cubilin oligosaccharides in single transfectants are endo H sensitive (compare lanes 4 and 6), but the bulk of cubilin oligosaccharides in cubilin/AMN double transfectants are endo H resistant (compare lanes 5 and 7).
Effect of AMN on cubilin expression and processing in CHO cells. (A) Cubilin (amino acids 1-1389) and AMN (full length plus myc epitope) constructs encoded by plasmid cDNA used for stable transfection of CHO cells. The position of the IF-cobalamin (IF-cbl) binding site (CUB domains 5-8)6 is indicated and the truncated CUB domains 9-27 are shown by dashed lines. (B) Immunoblots of cubilin and AMN in cell lysates (lanes 1-3) and endo H digestion of IF-cobalamin affinity-purified cubilin from cells transfected with cubilin, AMN, and cubilin/AMN cDNA. Gel mobility shifts indicate that cubilin oligosaccharides in single transfectants are endo H sensitive (compare lanes 4 and 6), but the bulk of cubilin oligosaccharides in cubilin/AMN double transfectants are endo H resistant (compare lanes 5 and 7).
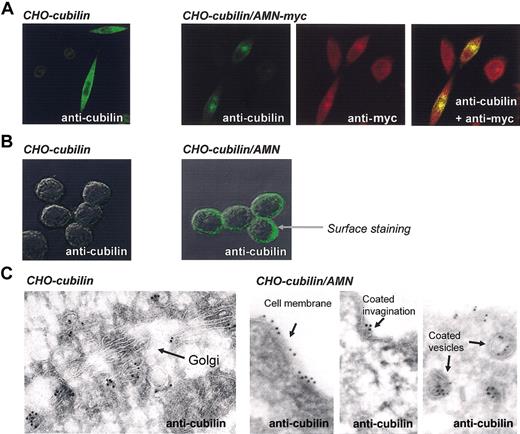
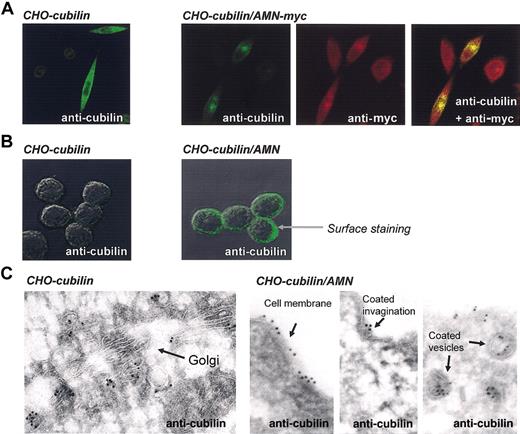
Cubilin and AMN localization in single and doubly transfected CHO cells. (A) Confocal immunofluoresence microscopy of AMN and cubilin in permeabilized cells demonstrating colocalization of AMN and cubilin. Original magnification, × 630. (B) Surface staining of cubilin in cubilin single and cubilin/AMN double transfectants demonstrating absent surface expression in the cubilin single transfectants and punctuate surface expression in cubilin/AMN double transfectants. Original magnification, × 1000. (C) Immunogold staining of cubilin in cubilin single and cubilin/AMN double transfectants examined by electron microscopy demonstrating cubilin in the endocytic apparatus of double transfectants. Original magnification, × 46 000.
Cubilin and AMN localization in single and doubly transfected CHO cells. (A) Confocal immunofluoresence microscopy of AMN and cubilin in permeabilized cells demonstrating colocalization of AMN and cubilin. Original magnification, × 630. (B) Surface staining of cubilin in cubilin single and cubilin/AMN double transfectants demonstrating absent surface expression in the cubilin single transfectants and punctuate surface expression in cubilin/AMN double transfectants. Original magnification, × 1000. (C) Immunogold staining of cubilin in cubilin single and cubilin/AMN double transfectants examined by electron microscopy demonstrating cubilin in the endocytic apparatus of double transfectants. Original magnification, × 46 000.
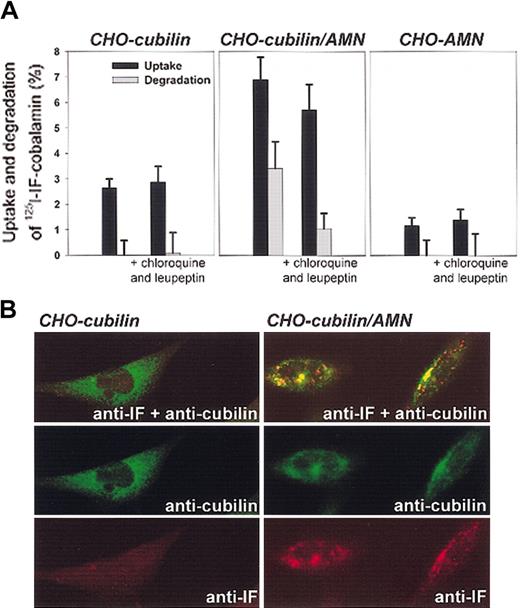
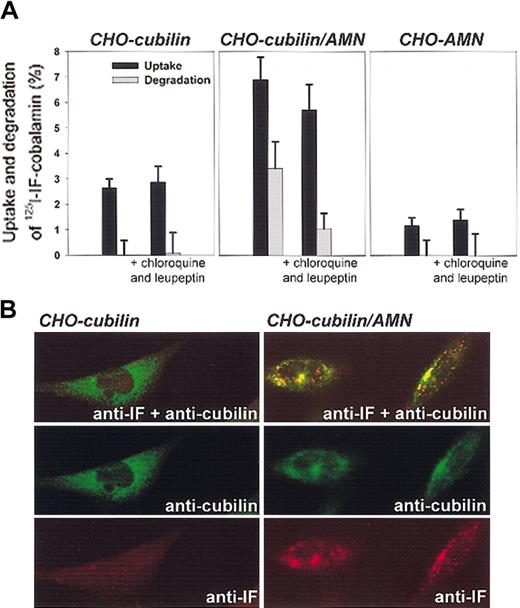
Effect of AMN on endocytosis of IF-cobalamin in cubilin-expressing CHO cells. (A) Endocytosis of 125I–IF-cobalamin after 4 hours at 37°C in cubilin and AMN single transfectants and in cubilin/AMN double transfectants, in the absence and presence of the lysosomal enzyme inhibitors chloroquine and leupeptin. Degradation (▪) represents the cell-mediated increase in TCA-soluble radioactivity in the medium, and uptake (▦) represents cell-associated radioactivity plus cell-mediated degradation. Error bars indicate SD. (B) Confocal immunofluoresence microscopy of the transfected cells incubated with IF-cobalamin at 37°C. Only the double cubilin/AMN transfectants take up IF-cobalamin as seen by vesicular staining of IF. Original magnification, × 630.
Effect of AMN on endocytosis of IF-cobalamin in cubilin-expressing CHO cells. (A) Endocytosis of 125I–IF-cobalamin after 4 hours at 37°C in cubilin and AMN single transfectants and in cubilin/AMN double transfectants, in the absence and presence of the lysosomal enzyme inhibitors chloroquine and leupeptin. Degradation (▪) represents the cell-mediated increase in TCA-soluble radioactivity in the medium, and uptake (▦) represents cell-associated radioactivity plus cell-mediated degradation. Error bars indicate SD. (B) Confocal immunofluoresence microscopy of the transfected cells incubated with IF-cobalamin at 37°C. Only the double cubilin/AMN transfectants take up IF-cobalamin as seen by vesicular staining of IF. Original magnification, × 630.
In distinct contrast, cotransfection of the “mini-cubilin” transfectant cells with cDNA encoding full-length AMN had a striking effect on processing and the endocytic function of the cubilin construct.
First, 2 IF-cobalamin binding forms of cubilin were observed (Figure 2B, lane 5), and most of the cubilin expressed was a higher molecular weight form hardly seen in the cubilin single transfectants. In contrast to the cubilin expressed in cubilin single transfectants, the major cubilin protein in the cubilin/AMN double transfectants showed no gel mobility shift when digested with endo H (Figure 2B, compare lanes 5 and 7), indicating that this larger cubilin form had undergone Golgi-mediated processing of Asnlinked oligosaccharides. PNGase F treatment reduced all forms of cubilin in the single and the double transfectants to the same size as the endo H–treated cubilin from single transfectants, indicating that the different sizes of cubilin in the double transfectants were due to differences of Asn-linked oligosaccharide processing (not shown).
Second, the location of cubilin in cubilin/AMN double transfectants changed from the exclusively intracellular locations observed in the cubilin single transfectants to also include the plasma membrane (Figure 3). Confocal examination of cell-surface immunostained cubilin/AMN double transfectants was seen as a strong dotlike staining, concordant with expression of cubilin in cell-surface clusters (Figure 3B, right panel). Immunoelectron microscopy showed that cubilin in the double transfectants was expressed in coated and noncoated endosome-like vesicles as well as in coated invaginations and noncoated areas of the plasma membrane (Figure 3C, right panels).
Third, the coexpression of AMN in the cubilin transfectants conferred to the cells the ability to endocytose IF-cobalamin. The uptake of 125I-labeled IF-cobalamin by double transfectants was accompanied by degradation of ligand and appearance of TCA-soluble radioactivity in the medium (Figure 4A). Inhibition of lysosomal enzyme activity by concurrent treatment of cells with chloroquine and leupeptin strongly inhibited degradation of the 125I–IF-cobalamin complex in a manner compatible with lysosomal degradation of the endocytosed ligand. Compared with double transfectants, the uptake of 125I–IF-cobalamin was considerably less in the single transfectants where no degradation of the ligand was measured. Endocytosis and accumulation of IF-cobalamin in endosome- and lysosome-like structures was also seen in immunostained double transfectants but not in cubilin single transfectants as examined by confocal microscopy (Figure 4B).
The indication of a novel mechanism by which AMN confers endocytic function to cubilin/AMN complexes was also supported by alignment of the AMN amino acid sequences of human, dog, rat, and mouse (Figure 5) that revealed 2 highly conserved copies of the sequence Phe-X-Asn-Pro-X-Phe in the cytoplasmic domain. This sequence conforms to the well-characterized AP-2 adaptor protein-binding signal (Phe-X-Asn-Pro-X-Tyr/Phe)34 for ligand-independent internalization via clathrin-coated pits that was first recognized in the low-density lipoprotein receptor family of endocytic receptors.35
Multiple species amino acid alignment of the AMN cytoplasmic domain. Alignment of human (GenBank accession no. NP 112205), mouse (accession no. NP 291081), rat (accession no. XP 234547), and dog (accession no. AY 368152) AMN sequences (single-letter amino acid code) was performed by web-based software.33 Residues highlighted in black are identical in all 4 species. Two conserved copies of the internalization signal FXNPXF are underlined.
Multiple species amino acid alignment of the AMN cytoplasmic domain. Alignment of human (GenBank accession no. NP 112205), mouse (accession no. NP 291081), rat (accession no. XP 234547), and dog (accession no. AY 368152) AMN sequences (single-letter amino acid code) was performed by web-based software.33 Residues highlighted in black are identical in all 4 species. Two conserved copies of the internalization signal FXNPXF are underlined.
Discussion
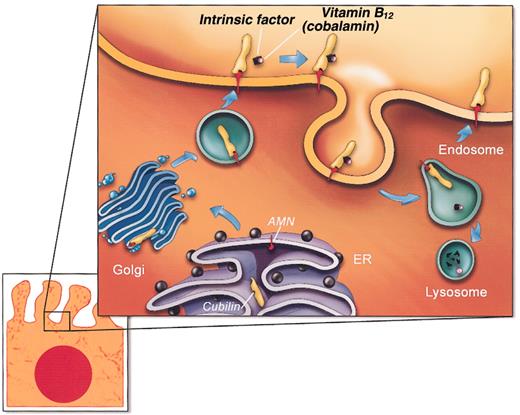
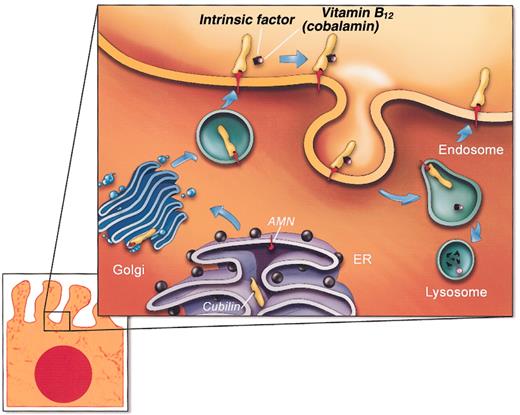
The data presented here suggest a model (Figure 6) of an essential partnership of cubilin with AMN in a functional complex for endocytosis of IF-cobalamin and other ligands. In this model, cubilin contributes ligand-binding regions, and AMN contributes structures required for membrane anchorage, biosynthetic processing, and trafficking to the plasma membrane, as well as putative signal sequences for endocytosis and receptor recycling. The endo H sensitivity of Asn-linked oligosaccharides and apparent Golgi accumulation of a functional “mini-cubilin” construct in AMN-negative cells indicates that the initial cubilin/AMN interaction occurs early in the biosynthetic pathway, most likely in the rough endoplasmic reticulum. Furthermore, the functionality in relation to IF-cobalamin endocytosis of the highly truncated cubilin construct missing CUB domains 9-27 indicates that AMN binds to a cubilin site amino-terminal of the IF-cobalamin site in CUB domain 5-86 (Figure 2A).
A model of AMN and cubilin assembly in the biosynthetic pathway and recycling in the endocytic apparatus of polarized epithelial cells. The depicted model is consistent with data presented here and current paradigms of endocytic receptor expression. This depiction does not show that AMN is sensitive to cleavage close to the transmembrane segment leading to partial shedding of cubilin/AMN to the extracellular fluid.
A model of AMN and cubilin assembly in the biosynthetic pathway and recycling in the endocytic apparatus of polarized epithelial cells. The depicted model is consistent with data presented here and current paradigms of endocytic receptor expression. This depiction does not show that AMN is sensitive to cleavage close to the transmembrane segment leading to partial shedding of cubilin/AMN to the extracellular fluid.
In addition to providing a rationale for the recently discovered I-GS–causing AMN mutations in humans, the present model of cubilin/AMN interdependence is also consistent with previous in vivo observations in dogs with inherited selective intestinal IF-cobalamin malabsorption and proteinuria (canine I-GS). Those studies explicitly demonstrated that I-GS–affected dogs exhibit failed surface expression of cubilin in intestinal and renal proximal tubule epithelial cells and renal cubilin that is retained in an early biosynthetic compartment in an incompletely folded form exhibiting immature glycosylation.7,20 It was further demonstrated that the defects of cubilin expression were not due to a cubilin mutation,25 and recent genetic analysis has mapped canine I-GS to the AMN locus.26
A mechanistic explanation of cubilin endocytic function has been sought ever since transmembrane and cytoplasmic domains were found lacking in the structure of cubilin deduced from cDNA sequence. Previously, it was suggested that megalin, an endocytic receptor of the LDL receptor family, might assist the trafficking of cubilin.5 This suggestion was based on the several observations that megalin strongly colocalizes5 and is coregulated36 with cubilin, that megalin-deficient mice have markedly decreased renal cubilin expression and uptake of cubilin ligands, and that antibodies against megalin reduce the uptake of some cubilin ligands.10,11 However, since the transfected cells of the present study do not express detectable megalin, it seems that the cubilin/AMN complex can function independently of megalin. The observed effects2,3,10,11 of omitting or reducing megalin function in the kidney or in cultured cells may be due to overlap in the cubilin and megalin ligand repertoires9,11 and/or to indirect effects of megalin on cubilin function. The latter is actually indicated by the fact that megalin-deficient kidney proximal tubules have far fewer endocytic vesicles than normal tubules, indicating that the endocytic activity is reduced overall.37 Despite these considerations, our data do not exclude the possibility that megalin interacts with the cubilin/AMN complex or certain cubilin ligands, and further studies are needed to define what may be a functional relationship rather than simple correlations.
In conclusion, we have discovered that AMN controls the trafficking of cubilin, which is a “passive” partner of AMN for binding carriers of vitamins and other nutrients to be endocytosed by polarized epithelial cells. The novel cubilin/AMN complex, which we propose to designate the cubam complex, is the functional receptor essential for cobalamin uptake, renal protein reabsorption, and early rodent embryogenesis. Since cubilin has also been detected in epithelia of other organs such as the lung and genital system,38 the function of the receptor complex may have even wider implications. Furthermore, it is intriguing to speculate that AMN may be involved in the processing and transport of other membrane proteins or endocytosis of directly bound ligands. Finally, the functional interdependence of cubilin and AMN now fully explains why IG-S is caused by genetic defects in either the cubilin or AMN genes.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-08-2852.
Supported in part by grants from the The Danish Medical Research Council, The Novo Nordisk Foundation, National Institutes of Health (DK064161), the National Cancer Institute (CA16058), The Plasmid Foundation, and the Carlsberg Foundation.
J.C.F. and M.M. contributed equally to this work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Our deep thanks go to K. Lassen for her technical assistance and her invaluable input to improve the methods used. Furthermore, we thank G. Biller, I. Kristoffersen, and G. Ratz for technical assistance, E. Nexø for human and porcine IF and anti-IF antibody, P. Verroust for anticubilin antibody, J. Gburek for assistance with microscopy, and J. A. Fyfe for fruitful discussion. The coauthor Mette Madsen has previously published under the name Mette Kristiansen.