Abstract
The control of dendritic cell (DC) migration is pivotal for the initiation of cellular immune responses. When activated with inflammatory stimuli, the chemokine receptor CCR7 is up-regulated on DCs. Activated DCs home to lymphoid organs, where the CCR7 ligands CCL19 and CCL21 are expressed. We previously found that human monocyte-derived DCs (MoDCs) exclusively migrated to CCL19 and CCL21 when matured in the presence of prostaglandin (PG) E2. Because PGE2 did not alter CCR7 cell surface expression, we examined whether PGE2 may exert its effect by coupling CCR7 to signal transduction modules. Indeed, stimulation with CCR7 ligands led to enhanced phosphatidylinositol-3-kinase–mediated phosphorylation of protein kinase B when MoDCs were matured in the presence of PGE2. Moreover, CCL19/CCL21-induced intracellular calcium mobilization in MoDCs occurred only when PGE2 was present during maturation. MoDC migration to CCL19 and CCL21 was dependent on phospholipase C and intracellular calcium flux but not on phosphatidylinositol-3 kinase. Hence, our data provide insight into CCL19/CCL21-triggered signal transduction pathways and identify a novel function for PGE2 in controlling the migration of mature MoDCs by facilitating CCR7 signal transduction.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells capable of antigen transport and presentation in secondary lymphoid organs, which is crucial for the initiation and maintenance of T-cell–mediated immune responses.1 They reside in the periphery in an immature state, taking up pathogens through pinocytosis or receptor-mediated endocytosis, leading to the induction of DC maturation. Mature DCs undergo phenotypical and functional changes, including up-regulation of the chemokine receptor CCR7.2 The expression of CCR7 on mature DCs and on naive and central memory T cells is essential for their coordinated migration to the T-cell area of draining lymph nodes because this migration is guided by CCL19 (EBI1-ligand chemokine [ELC], macrophage inflammatory protein [MIP]-3β), and CCL21 (secondary lymphoid-tissue chemokine [SLC], 6-Ckine), the 2 ligands for CCR7. Both chemokines are expressed by stromal cells in the T-cell area of secondary lymphoid organs. The essential role of CCR7 and its ligands for the migration of mature DCs to lymph nodes was demonstrated in CCR7-deficient mice3 and plt/plt mice, which lack the ligands for CCR7.4
Chemokine receptors are transmembrane receptors of the class A rhodopsin-like family, which span the membrane 7-fold and transduce their signals through G proteins, mainly of the Gαi subclass.5 Receptor stimulation leads to the inhibition of adenyl cyclases through Gαi- and Gβγ-mediated activation of phospholipase C (PLC), followed by diacylglycerol (DAG)–mediated activation of protein kinase C (PKC) and the release of calcium from intracellular stores.6 Furthermore, Gβγ subunits that are released from Gαi proteins transiently activate phosphatidylinositol 3-kinases (PI3Ks), leading to the activation of protein kinase B (PKB, Akt) and extracellular signal-regulated kinase-2 (Erk-2).7 However, most data on the signal transduction of chemokine receptors have been obtained with CXCR1, CCR2, CCR5, and CXCR4, but little information is available about CCR7 signal transduction. Recently, Rho-associated kinases have been shown to be required for the CCR7-mediated polarization and chemotaxis of T lymphocytes.8 Another study suggests that CCR7 signaling involves the activation of a Janus kinase (JAK).9 It is still a matter of debate, however, whether the JAK/signal transducer and activator of transcription (JAK/STAT) pathway, which requires chemokine receptor dimerization, contributes to chemokine-mediated signal transduction.10 In addition, most studies have been performed with neutrophils, peritoneal macrophages, and T cells but not with DCs.
Recently, we and others observed that the maturation-induced up-regulation of CCR7 expression on human monocyte-derived DCs (MoDCs) was insufficient to allow MoDC migration to CCL19 and CCL21.11,12 Human MoDCs matured either with soluble CD40L (sCD40L) or with polyI:C markedly enhanced surface expression of CCR7 but were not at all or were only poorly responsive to CCL19 and CCL21. Interestingly, MoDC migration to CCL19 and CCL21 was readily observed on maturation in the presence of the proinflammatory mediator prostaglandin E2 (PGE2), though PGE2 did not change the expression level of CCR7 on mature MoDCs, providing evidence for an alternative effect of PGE2. The importance of PGE2 for DC migration in vivo has recently been shown in Ptger4–/– mice lacking the PGE2 receptor EP4. These mice display impaired migration of Langerhans cells and reduced skin immune responses.13 However, other DC types, such as CD1b/c+ peripheral blood DCs, do not require an additional PGE2 stimulus for effective CCR7-mediated migration.12
In this study we investigated the hypothesis that PGE2 may facilitate CCL19/CCL21-directed migration by coupling the cognate chemokine receptor CCR7 to its signal transduction modules. Indeed, we found that PGE2 was required during sCD40L-stimulated maturation of MoDCs to activate PKB and to mobilize intracellular free calcium as downstream signaling events of CCR7. Moreover, we demonstrate that PLC-mediated cytoplasmic Ca2+ mobilization was a prerequisite for the chemotaxis of MoDCs to CCL19 and CCL21. Our results, hence, provide evidence that PGE2 is required to facilitate the CCR7-mediated signal transduction and migration of MoDCs.
Materials and methods
Tissue culture media and reagents
AIM V was purchased from Invitrogen (Groningen, The Netherlands). Interleukin-4 (IL-4) was obtained from Strathmann (Hamburg, Germany), and granulocyte macrophage–colony-stimulating factor (GM-CSF) was obtained from Novartis (Leukomax, Basel, Switzerland). Soluble CD40L (sCD40L) was kindly provided by Immunex (Seattle, WA). Prostaglandin E2 was purchased from Pharmacia & Upjohn (Prostin E2; Dübendorf, Switzerland). Human CCL19 and CCL21 were provided by R&D Systems (Wiesbaden-Nordenstadt, Germany), and CXCL-12 was purchased from PromoCell (Heidelberg, Germany). Staurosporine, U-73122, H-89, wortmannin, Ly-294002, Y-27632 and 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-tetra(acetomethyl) ester (BAPTA-am) were purchased from Calbiochem (Lucerne, Switzerland). Phorbol-myristate-acetate (PMA) and ionomycin was purchased from Sigma (Buchs, Switzerland).
Antibodies
Horseradish peroxidase (HRP)–conjugated antimouse antibody was obtained from DAKO (Zug, Switzerland), and antirabbit antibody was obtained from Milan (La Roche, Switzerland). Antibodies against Akt/PKB, phospho-Akt/PKB (Ser473), p42/44 mitogen-activated protein (MAP) kinase/Erk-1/2, and phospho-p44/42 MAP kinase/Erk-1/2 (Thr202/Tyr204) were from Cell Signaling Technology (Frankfurt, Germany).
Generation of human MoDCs
MoDCs were generated from human peripheral blood mononuclear cells (PBMCs), as described previously.11 Briefly, PBMCs were separated by density gradient centrifugation on Ficoll-Paque (Pharmacia, Uppsala, Sweden), resuspended at 4 × 106 cells/mL in AIM V medium, and allowed to adhere to plastic for 1 hour at 37°C. Nonadherent cells were removed, and remaining cells were cultured in AIM V medium supplemented with GM-CSF (50 ng/mL) and IL-4 (1000 U/mL). MoDCs derived from the adherence step were only used when the content of remaining B and T cells was below 2%. Alternatively, monocytes were purified from PBMCs by positive selection using anti-CD14–conjugated magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and were cultivated at 1 × 106 cells/mL. On day 6, nonadherent and loosely adherent cells were harvested and recultured (5 × 105 cells/mL) for an additional 48 hours in cytokine-containing medium in the absence or presence of 1 μg/mL sCD40L to induce maturation. Where indicated, PGE2 (1 μg/mL) or H-89 was added during stimulation.
Cell stimulation and Western blot analysis
MoDCs were washed twice with phosphate-buffered saline (PBS) and resuspended in AIM V medium without additives (2 × 107 cells/mL). Aliquots of 2 × 106 cells were incubated for 10 minutes at 37°C and were stimulated with CCL19 or CCL21 (250 ng/mL) for different periods of time. Incubations were terminated by the addition of trichloroacetic acid (TCA) to a final concentration of 10%. Protein pellets were washed twice with ice-cold acetone and dissolved by boiling in 1× Laemmli sodium dodecyl sulphate (SDS) loading buffer containing 5% 2-mercaptoethanol. Proteins were separated on 10% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to nitrocellulose membranes. The membranes were blocked with 5% low-fat dry milk in 1× TBS-buffer and were incubated with the respective antibodies overnight on a rocking plate at 4°C. After washing, HRP-conjugated secondary antibodies were bound and detected using enhanced chemiluminescence (Pierce, Socochim, Lausanne, Switzerland). Thereafter, membranes were stripped with 0.2 M NaOH for 5 minutes and then washed and reprobed with control antibodies.
Chemotaxis assay
Chemotaxis of MoDCs was measured by migration through a polycarbonate filter of 5 μm pore size in 24-well transwell chambers (Corning Costar, Cambridge, MA). AIM V (600 μL) containing indicated doses of CCL19, CCL21, CXCL-12, or medium alone as a control for spontaneous migration was added to the lower chamber; 1 × 105 DCs (100 μL) were added to the upper chamber and were incubated for 3 hours at 37°C. A 500-μL aliquot of the cells that migrated to the bottom chamber was counted by flow cytometry in a FACScan acquiring events for a fixed time period of 60 seconds using CellQuest software (Becton Dickinson, Basel, Switzerland). Each experiment was performed in duplicate. The mean number of spontaneously migrated cells was subtracted from the total number of migrated cells. Values are given as percentage of migrated cells ± SEM. Where indicated, cells were incubated for 30 minutes at 37°C with various drugs and were washed twice before the migration assay.
Intracellular free Ca2+ mobilization
MoDCs (1 × 106/mL) were loaded with 4 μM fluo-3-actetomethylester (fluo-3-am) in the presence of 1 μM pluronic F-127 (Molecular Probes, Leiden, The Netherlands) in loading-buffer (145 mM NaCl, 5 mM KCl, 1 mM Na2HPO4, 1 mM MgCl2, 5 mM glucose, 1 mM CaCl2, and 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.5) for 30 minutes at room temperature. Where indicated, BAPTA-am was loaded simultaneously into MoDCs. Subsequently, cells were washed twice with loading buffer or Ca2+-free loading buffer containing 2 mM EGTA (ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid) and were stored on ice. For aquisition, cells were incubated for 10 minutes at 37°C before stimulation with CCL19, CCL21 (250 ng/mL), or ionomycin (1 μg/mL), and fluorescence was recorded over time by flow cytometry.
Results
MoDC migration to CCL19 and CCL21 requires activation of protein kinase A and Rho-associated kinases
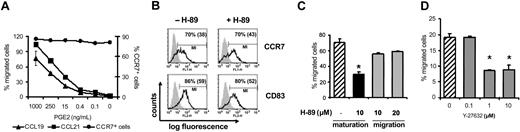
Previously, we and others demonstrated that human MoDCs required PGE2 during maturation to migrate to CCL19 and CCL21 effectively.11,12 Earlier studies showing PGE2-induced migration of MoDCs were performed in the presence of high PGE2 concentrations (in the micromolar range), at concentrations far above the physiologic levels found in extracellular fluids at least under noninflammatory conditions.14 For a better understanding of the effect of PGE2 to enhance MoDC migration, we first performed transwell chemotaxis assays with MoDCs matured with sCD40L and graded concentrations of PGE2. Maturation with CD40L resulted in mature MoDCs with homogeneous surface expression of CCR7 irrespective of the addition of PGE2 (Figure 1A).11 However, CCL19- and CCL21-induced migration of sCD40L-matured MoDCs required PGE2 and were dose dependent and already significant at a PGE2 concentration of 15 ng/mL (Figure 1A). These PGE2 concentrations were expected to occur at sites of inflammation but not in healthy tissues. Furthermore, PGE2 was needed throughout the MoDC maturation process because 1-hour preincubation of mature MoDCs with PGE2 was insufficient to induce MoDC migration in response to CCL19 and CCL21 (data not shown).
PGE2-induced MoDC migration to CCL19 and CCL21 is PKA and Rho kinase dependent. (A) Titration of PGE2; immature MoDCs were stimulated with sCD40L and titrated concentrations of PGE2. Subsequently, MoDCs were tested for CCL19- and CCL21-triggered migration in transwell assays (left axis) and were analyzed by flow cytometry for the expression of CCR7 (right axis). (B) MoDCs were matured with sCD40L and PGE2 in the presence or absence of the PKA inhibitor H-89 for 48 hours. Subsequently, MoDCs were analyzed by flow cytometry for the expression of CD83 and CCR7. The portion of gated positive cells is given as a percentage, with the mean fluorescence intensity given in parentheses. (C) MoDCs were matured with sCD40L and PGE2 in the presence (▪) or absence (▨) of the PKA inhibitor H-89, or MoDCs matured with sCD40L and PGE2 were preincubated with H-89 for 30 minutes (▦) before they were allowed to migrate in response to CCL21. (D) MoDCs matured with sCD40L and PGE2 were left untreated (▨) or were pretreated with the Rho kinase inhibitor Y-27632 (▦) and were analyzed in a migration assay in response to CCL21. Results from 1 of 3 representative experiments are shown. Error bars indicate SEM and asterisks indicate a significant reduction.
PGE2-induced MoDC migration to CCL19 and CCL21 is PKA and Rho kinase dependent. (A) Titration of PGE2; immature MoDCs were stimulated with sCD40L and titrated concentrations of PGE2. Subsequently, MoDCs were tested for CCL19- and CCL21-triggered migration in transwell assays (left axis) and were analyzed by flow cytometry for the expression of CCR7 (right axis). (B) MoDCs were matured with sCD40L and PGE2 in the presence or absence of the PKA inhibitor H-89 for 48 hours. Subsequently, MoDCs were analyzed by flow cytometry for the expression of CD83 and CCR7. The portion of gated positive cells is given as a percentage, with the mean fluorescence intensity given in parentheses. (C) MoDCs were matured with sCD40L and PGE2 in the presence (▪) or absence (▨) of the PKA inhibitor H-89, or MoDCs matured with sCD40L and PGE2 were preincubated with H-89 for 30 minutes (▦) before they were allowed to migrate in response to CCL21. (D) MoDCs matured with sCD40L and PGE2 were left untreated (▨) or were pretreated with the Rho kinase inhibitor Y-27632 (▦) and were analyzed in a migration assay in response to CCL21. Results from 1 of 3 representative experiments are shown. Error bars indicate SEM and asterisks indicate a significant reduction.
PGE2 signaling occurs through the prostaglandin receptors EP2 and EP4, which are expressed on MoDCs and which lead to increased cyclic adenosine monophosphate (cAMP) levels and to the activation of cAMP-dependent protein kinase A (PKA) in the cytosol.11 Therefore, we investigated whether MoDC migration in the presence of PGE2 is dependent on the activation of PKA. To this end, MoDCs were stimulated with sCD40L and PGE2 in the presence of the PKA-specific inhibitor H-89. Treating MoDCs with H-89 at a concentration of 10 μM had no effect on MoDC maturation, CCR7 expression, or viability (Figure 1B and data not shown). However, adding H-89 for the entire period of maturation reduced the migration of MoDCs in response to CCL19 and CCL21 by approximately 50% (Figure 1C). In contrast, blocking activation of PKA during the migration assay had no significant effect on MoDC migration, indicating that the PGE2-induced activation of PKA during MoDC maturation may provide a signal that allows them to migrate.
Recently, the Rho family of GTPases was shown to be involved in CCR7-mediated migration of T lymphocytes.8 To test the involvement of Rho kinase, mature MoDCs were pretreated with graded doses of Y-27632, a specific inhibitor of Rho-associated kinases. Blocking Rho-associated kinases resulted in a significant reduction of CCL21-mediated migration of MoDCs (Figure 1D), confirming the findings observed in T lymphocytes.
PGE2 does not affect chemokine-mediated Erk-1/2 activation
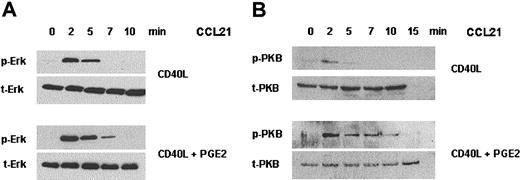
Because PGE2 did not alter the cell surface expression of CCR7 on sCD40L-matured MoDCs, we investigated whether PGE2 might facilitate migration by coupling CCR7 to its signal transduction pathway. Signal transduction of CCR7 in DCs has not been addressed so far. An earlier study on T cells, however, demonstrated that CCL19 stimulation resulted in the activation of the MAP-kinase cascade, leading to a transient activation of Erk-2 by phosphorylation at threonine and tyrosine residues.7 We matured MoDCs with sCD40L in the presence or absence of PGE2, stimulated them with CCL19 for different time periods, and investigated Erk activation using Western blotting. Using an antibody detecting Erk-1 and Erk-2 exclusively when phosphorylated at Thr202 and Tyr204, we found rapid and transient Erk-1/2 activation after 2 to 7 minutes of CCL19 stimulation (Figure 2A). However, there was no significant difference in Erk-1/2 activation or duration between MoDCs matured with sCD40L in the presence or absence of PGE2.
CCL21-mediated activation of Erk-1/2 and PKB in MoDCs. MoDCs were matured in the presence or absence of PGE2 and subsequently were stimulated with CCL21 (250 ng/mL) for the indicated time periods. Whole-cell lysates were separated on SDS-PAGE and transferred to nitrocellulose membranes. (A) Membranes were stained with an antibody against phosphorylated (Thr202/Tyr204) Erk-1/2 (p-Erk) or total Erk-1/2 (t-Erk). (B) Membranes were probed with an antibody against phosphorylated (S473) PKB (p-PKB) or total PKB (t-PKB). Results from 1 of 4 independent representative experiments are shown.
CCL21-mediated activation of Erk-1/2 and PKB in MoDCs. MoDCs were matured in the presence or absence of PGE2 and subsequently were stimulated with CCL21 (250 ng/mL) for the indicated time periods. Whole-cell lysates were separated on SDS-PAGE and transferred to nitrocellulose membranes. (A) Membranes were stained with an antibody against phosphorylated (Thr202/Tyr204) Erk-1/2 (p-Erk) or total Erk-1/2 (t-Erk). (B) Membranes were probed with an antibody against phosphorylated (S473) PKB (p-PKB) or total PKB (t-PKB). Results from 1 of 4 independent representative experiments are shown.
PGE2 enhances CCL19-mediated PKB activation
A second major pathway triggered by chemokines is the stimulation of phosphatidyl-inositol 3-kinase (PI3-kinase) leading to the formation of phosphatidyl-3,4,5,-trisphosphate (PIP3) and the activation of PKB.7 Hence, we investigated the activation of PKB in MoDCs on CCR7 triggering. CCL19 induced rapid and transient phosphorylation of PKB in MoDCs, as demonstrated by Western blotting using phospho Ser473-specific PKB antibodies (Figure 2B). Interestingly, PKB activation was stronger and more prolonged in MoDCs matured in the presence of PGE2 than in MoDCs matured with sCD40L alone. PKB phosphorylation peaked after 2 minutes of CCL19 triggering and remained detectable after 15 minutes in MoDCs matured in the presence of PGE2. In contrast, MoDC maturation without PGE2 allowed only weak phosphorylation of PKB after 2 minutes, which disappeared after 5 minutes of chemokine stimulation (Figure 2B). In some experiments, the activation of PKB in MoDCs matured in the absence of PGE2 was even below the detection limit (data not shown).
Migration of MoDCs to CCL19 and CCL21 is not dependent on PKB activation
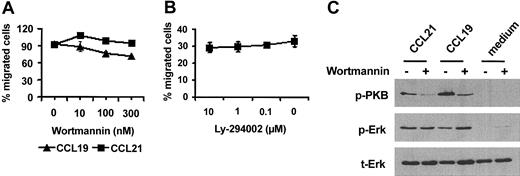
We next analyzed whether inefficient chemokine-mediated activation of PKB in MoDCs matured in the absence of PGE2 was responsible for their reduced migration. Hence, MoDCs matured in the presence of PGE2 were incubated for 10 minutes with the specific PI3-kinase inhibitor wortmannin or Ly-249002, each of which blocks the downstream activation of PKB. Unexpectedly, neither wortmannin nor Ly-249002 had an effect on MoDC migration to CCL19 and CCL21 (Figure 3A-B). To rule out the ineffective inhibition of PKB phosphorylation by wortmannin, MoDC lysates were subjected to Western blot analysis. Preincubating MoDCs with 100 nM wortmannin prevented Ser473 phosphorylation of PKB almost completely, but Erk-1/2 activation was not affected by this treatment (Figure 3C), indicating that wortmannin inhibition was effective.
Activation of PKB is not required for the migration of MoDCs in response to CCL19 and CCL21. MoDCs matured in the presence of PGE2 were incubated for 30 minutes with graded doses of (A) wortmannin or (B) Ly-294002 and were tested for migration to CCL21 and CCL19 in a transwell assay. Error bars indicate SEM. (C) To confirm the effectiveness of wortmannin, MoDCs treated with or without wortmannin were stimulated with CCL19 or CCL21 for 2 minutes. Whole-cell lysates were separated on SDS-PAGE, and activated PKB or Erk-1/2 was detected with an antibody against PKB phosphorylated at S473 (p-PKB) or Erk-1/2 dually phosphorylated at Thr202 and Tyr204 (p-Erk). Membranes were reprobed with an antibody reacting with total Erk-1/2 (t-Erk) to confirm equal protein loading. Results from 1 of 3 representative experiments using different MoDC preparations are shown.
Activation of PKB is not required for the migration of MoDCs in response to CCL19 and CCL21. MoDCs matured in the presence of PGE2 were incubated for 30 minutes with graded doses of (A) wortmannin or (B) Ly-294002 and were tested for migration to CCL21 and CCL19 in a transwell assay. Error bars indicate SEM. (C) To confirm the effectiveness of wortmannin, MoDCs treated with or without wortmannin were stimulated with CCL19 or CCL21 for 2 minutes. Whole-cell lysates were separated on SDS-PAGE, and activated PKB or Erk-1/2 was detected with an antibody against PKB phosphorylated at S473 (p-PKB) or Erk-1/2 dually phosphorylated at Thr202 and Tyr204 (p-Erk). Membranes were reprobed with an antibody reacting with total Erk-1/2 (t-Erk) to confirm equal protein loading. Results from 1 of 3 representative experiments using different MoDC preparations are shown.
PGE2 is required for CCL19/CCL21-mediated Ca2+ mobilization
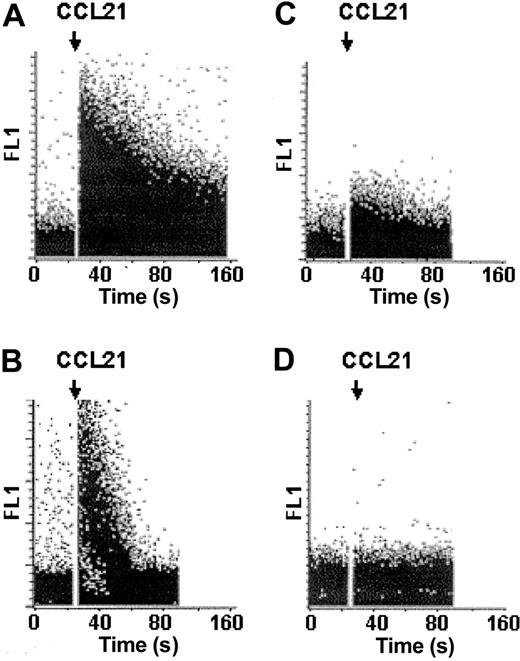
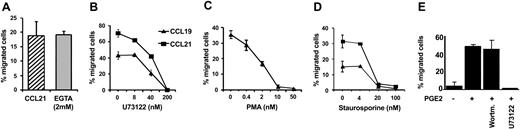
Another consequence of chemokine receptor stimulation is the activation of G-protein–sensitive PLC isoforms, resulting in the generation of DAG and inositol 3,4,5-triphosphate, which leads to the release of Ca2+ from intracellular stores. Hence, we analyzed CCL19/CCL21-induced Ca2+ mobilization in MoDCs matured in the presence or absence of PGE2 by flow cytometry. Triggering MoDCs with CCL19 or CCL21 resulted in a rapid rise in intracellular free Ca2+ concentrations only on maturation in the presence (Figure 4C-D), but not in the absence, of PGE2 (Figure 4A-B). Ca2+ mobilization was substantially reduced when PGE2-treated MoDCs were preincubated with the specific PLC inhibitor U73122 (Figure 5C). In contrast, increases in cytosolic free Ca2+ concentrations were less affected by the chelation of extracellular Ca2+ concentrations by 2 mM EGTA (Figure 5B). The combination of both—the inhibition of PLC with U73122 and the presence of 2 mM EGTA—abrogated the Ca2+ signal completely (Figure 5D), indicating that intracellular and extracellular calcium stores contributed to the cytoplasmic calcium flux. MoDC migration in response to CCL21 was not reduced in the presence of 2 mM EGTA in the assay buffer (Figure 6A), which suggests that calcium mobilization from intracellular stores suffices to maintain the migratory capacity.
Chemokine-induced Ca2+ mobilization differs in MoDCs matured in the presence or absence of PGE2. MoDCs stimulated with sCD40L in the absence (A-B) or presence (C-D) of PGE2 were loaded with fluo-3-AM, and chemokine-induced Ca2+ mobilization was analyzed by flow cytometry. Baseline was established for 30 seconds before chemokines (250 ng/mL) were added, as indicated by the arrow. Ionomycin (1 μg/mL) was added (A-B) to ensure proper fluo-3-AM loading of MoDCs. Representative results obtained from MoDCs of a single donor are shown. At least 5 independent experiments with MoDCs from different donors yielded similar results.
Chemokine-induced Ca2+ mobilization differs in MoDCs matured in the presence or absence of PGE2. MoDCs stimulated with sCD40L in the absence (A-B) or presence (C-D) of PGE2 were loaded with fluo-3-AM, and chemokine-induced Ca2+ mobilization was analyzed by flow cytometry. Baseline was established for 30 seconds before chemokines (250 ng/mL) were added, as indicated by the arrow. Ionomycin (1 μg/mL) was added (A-B) to ensure proper fluo-3-AM loading of MoDCs. Representative results obtained from MoDCs of a single donor are shown. At least 5 independent experiments with MoDCs from different donors yielded similar results.
CCL21 mediates Ca2+ mobilization from intracellular stores. MoDCs stimulated with sCD40L and PGE2 were loaded with fluo-3-AM, and chemokine-induced Ca2+ mobilization was analyzed using flow cytometry (A). Experiments (B-D) were performed in Ca2+-free buffer in the presence of 2 mM EGTA. (C-D) The release of Ca2+ from intracellular stores was inhibited by the addition of the PLC inhibitor U73122 (10 μM). Baseline was established for 30 seconds before CCL21 (250 ng/mL) was added, as indicated by the arrow. Representative results obtained from MoDCs of a single donor are shown. At least 3 independent experiments with MoDCs from different donors gave similar results.
CCL21 mediates Ca2+ mobilization from intracellular stores. MoDCs stimulated with sCD40L and PGE2 were loaded with fluo-3-AM, and chemokine-induced Ca2+ mobilization was analyzed using flow cytometry (A). Experiments (B-D) were performed in Ca2+-free buffer in the presence of 2 mM EGTA. (C-D) The release of Ca2+ from intracellular stores was inhibited by the addition of the PLC inhibitor U73122 (10 μM). Baseline was established for 30 seconds before CCL21 (250 ng/mL) was added, as indicated by the arrow. Representative results obtained from MoDCs of a single donor are shown. At least 3 independent experiments with MoDCs from different donors gave similar results.
PLC pathway is involved in MoDC migration to CCL19, CCL21, and CXCL12. (A) MoDCs matured with sCD40L and PGE2 were analyzed in a migration assay in response to CCL21 in the absence (▨) or presence (▦) of 2 mM EGTA in the assay buffer. MoDCs matured with sCD40L and PGE2 were incubated with titrated concentrations of (B) the PLC inhibitor U73122, (C) the PKC activator PMA, or (D) the PKC inhibitor staurosporine for 30 minutes at 37°C before migration to CCL19 and CCL21was analyzed in a transwell chemotaxis assay. (E) Migration to CXCL12 of MoDCs matured in the presence or absence of PGE2. The PI3K inhibitor wortmannin was used at 100 nM, and U73122 was used at 10 μM. Results from representative experiments of at least 3 different MoDC preparations are shown. Error bars indicate SEM.
PLC pathway is involved in MoDC migration to CCL19, CCL21, and CXCL12. (A) MoDCs matured with sCD40L and PGE2 were analyzed in a migration assay in response to CCL21 in the absence (▨) or presence (▦) of 2 mM EGTA in the assay buffer. MoDCs matured with sCD40L and PGE2 were incubated with titrated concentrations of (B) the PLC inhibitor U73122, (C) the PKC activator PMA, or (D) the PKC inhibitor staurosporine for 30 minutes at 37°C before migration to CCL19 and CCL21was analyzed in a transwell chemotaxis assay. (E) Migration to CXCL12 of MoDCs matured in the presence or absence of PGE2. The PI3K inhibitor wortmannin was used at 100 nM, and U73122 was used at 10 μM. Results from representative experiments of at least 3 different MoDC preparations are shown. Error bars indicate SEM.
PLC activation is required for MoDC migration to CCL19, CCL21, and CXCL-12
To assess whether the activation of PLC is required for migration, MoDCs were incubated with increasing amounts of the PLC inhibitor U73122. As depicted in Figure 6B, MoDC migration in response to CCL19 and CCL21 was completely blocked after treatment with 200 nM U73122. Half-maximal inhibition was observed at 50 nM U73122.
Ca2+ activates, in conjunction with DAG, various isoforms of PKC. Additionally, PKC can be activated through signal transduction events initiated by the stimulation of the PI3-kinase pathway. The contribution of PKC to MoDC migration was investigated using either the PKC inhibitor staurosporine or the PKC activator PMA. Staurosporine is an unspecific protein kinase inhibitor that inhibits PKC (IC50, 3 nM), PKA (IC50, 7 nM), and Ca2+/calmodulin-dependent (CaM) kinase 2 (IC50, 20 nM).15,16 Pretreatment of MoDCs matured in the presence of PGE2 with staurosporine at effective concentrations to block PKC (4 nM) did not affect MoDC migration to CCL19 and CCL21. However, staurosporine significantly reduced the migration of MoDCs in response to CCL19 and CCL21 at concentrations that interfered with the activation of other protein kinases such as CaM kinases (Figure 6D). Interestingly, the migration of MoDCs to CCL19 and CCL21 was abrogated after pretreatment with graded concentrations of PMA, implicating that the heterologous activation of PKC impedes chemokine-induced signaling (Figure 6C).
To investigate whether the dependence of MoDC migration on PGE2 was valid for another chemokine receptor of MoDCs, we examined the CXCR4-directed migration of MoDCs to SDF-1/CXCL12 (Figure 6E). Remarkably, the migration of MoDCs to CXCL12 in transwell assays occurred only when MoDCs were matured in the presence of PGE2, in agreement with a recent report by Luft et al.12 In addition, migration to CXCL12 was inhibited with the PLC inhibitor U73122 but not with the PI3K inhibitor wortmannin.
Intracellular Ca2+ flux is required for MoDC migration
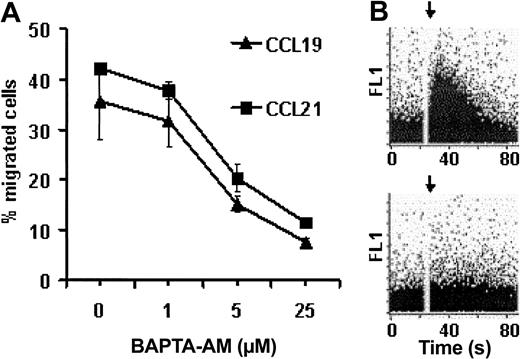
To test whether Ca2+ mobilization is essential for migration, the cell-permeable Ca2+ chelator BAPTA-am was used to trap liberated Ca2+ in the cytoplasm after chemokine stimulation of PGE2-treated MoDCs (Figure 7B). Chelation of intracellular free Ca2+ by BAPTA-am inhibited the migration of MoDCs to CCL19 and CCL21 in a dose-dependent manner (Figure 7A), thus indicating that Ca2+ mobilization is required for MoDC migration. Taken together, MoDCs require PGE2 during maturation for CCL19/CCL21-induced activation of PKB and Ca2+ mobilization. In contrast to the PI3-kinase–mediated activation of PKB, activating the PLC pathway leading to the liberation of intracellular free Ca2+ is essential for MoDC migration.
Chemokine-induced Ca2+ mobilization is required for the migration of MoDCs to CCL19 and CCL21. (A) sCD40L- and PGE2-stimulated MoDCs were incubated with graded doses of BAPTA-AM for 30 minutes at room temperature before migration to CCL19 and CCL21 was analyzed in a transwell chemotaxis assay. Error bars indicate SEM. (B) sCD40L- and PGE2-stimulated MoDCs were loaded with fluo-3-AM alone (top) or with BAPTA-AM (bottom). Chemokine-induced Ca2+ mobilization was analyzed by flow cytometry. Baseline was established for 30 seconds before CCL21 was added, as indicated by the arrow. Representative results obtained from MoDCs of a single donor are shown. Three independent experiments with MoDCs from different donors yielded similar results.
Chemokine-induced Ca2+ mobilization is required for the migration of MoDCs to CCL19 and CCL21. (A) sCD40L- and PGE2-stimulated MoDCs were incubated with graded doses of BAPTA-AM for 30 minutes at room temperature before migration to CCL19 and CCL21 was analyzed in a transwell chemotaxis assay. Error bars indicate SEM. (B) sCD40L- and PGE2-stimulated MoDCs were loaded with fluo-3-AM alone (top) or with BAPTA-AM (bottom). Chemokine-induced Ca2+ mobilization was analyzed by flow cytometry. Baseline was established for 30 seconds before CCL21 was added, as indicated by the arrow. Representative results obtained from MoDCs of a single donor are shown. Three independent experiments with MoDCs from different donors yielded similar results.
Discussion
Up-regulation of the chemokine receptor CCR7 on DCs is crucial for their homing to secondary lymphoid organs, where the CCR7 ligands CCL19 and CCL21 are expressed. Two recent studies demonstrated that CCR7, though present on mature MoDCs, failed under certain conditions to mediate migration to CCL19 and CCL21, suggesting that CCR7 can exist on the cell surface in an inactive form.11,12 One of the factors required for MoDC migration to lymph node–derived chemokines was identified as the inflammatory mediator PGE2, which transmits its effect through the elevation of intracellular cAMP. As we have demonstrated, the amount of PGE2 and the duration of PGE2 stimulation during maturation seemed to be critical for CCR7-mediated MoDC migration. PGE2 concentrations at inflammatory sites were reported to be between 0.2 nM and 1.69 μM.14,17,18 Here, we demonstrate that at a physiologic concentration of PGE2, MoDCs are capable of migrating in response to CCL19 and CCL21. It is also likely that at sites of inflammation, the DC microenvironment contains high levels of PGE2 because it is surrounded by other immune cells, such as monocytes, that produce large amounts of this mediator once they are stimulated.19
Increased cytosolic cAMP levels after PGE2 stimulation and subsequent activation of the cAMP-dependent PKA seem to play a key role in activating the migratory capacity of MoDCs. We showed that inhibiting cAMP-dependent PKA dramatically reduces MoDC chemokine-directed migration. Furthermore, a recent study shows that stimulating MoDCs with adenosine triphosphate (ATP), which also leads to increased intracellular cAMP levels, enhances the migration of MoDCs to CCR7 ligands.20
CCL19/CCL21-induced migration of MoDCs is pertussis toxin sensitive, indicating that the responding chemokine receptor, CCR7, is coupled to G proteins of the Gαi type.11 In this study we show that CCR7 stimulation with CCL19 and CCL21 involves the activation of at least 3 main pathways in PGE2-treated MoDCs, including PI3-kinase and PKB phosphorylation, PLC activation and calcium release, and the MAP-kinase pathway. The requirement for PI3-kinase and PLC activation in chemokine-induced migration is still a matter of debate and seems to vary with the cell type and with the kind of chemokine receptor analyzed. Although the migration of human neutrophils to formyl peptides, complement components such as C5a or C3a, and CXCL8 (IL-8) was independent of PI3-kinase activation, studies in mice lacking the PI3-kinase-γ isoform showed reduced neutrophil migration to these chemoattractants.21-23 In addition it has been reported that, in contrast to monocytes, the migration of human T lymphocytes to CCL5 was sensitive to the specific PI3-kinase inhibitor wortmannin.24 We show that MoDC chemotaxis to CCL19 and CCL21 is independent of the activation of PI3-kinase because migration was insensitive to the PI3-kinase inhibitors wortmannin and Ly294002. This is in accordance with a recent report that treating freshly isolated mouse lymphocytes with wortmannin or Ly294002 had only minor effects on lymphocyte chemotaxis to CCL21.9
The requirement of PLC activation for chemotaxis was mainly reported for murine neutrophils. Neutrophils from PLC-β2– and PLC-β3–deficient mice were normal with respect to chemotaxis to formyl peptides or CXCL8,21 indicating that the Ca2+ signal is not elementary for chemotaxis in these cells. MoDC migration to CCL19 and CCL21, in contrast, was strictly dependent on the activation of PLC and the liberation of intracellular free Ca2+, as shown in our experiments with the PLC-specific inhibitor U73122 and by chelating intracellular Ca2+. One possible role of chemokine-induced Ca2+ mobilization in MoDCs could be the activation of Ca2+/calmodulin-dependent kinases (CaMKs), as reported for CXCL8-mediated migration of human neutrophils.25 Consistent with this notion, treating MoDCs with staurosporine at concentrations that affect CaMK activation significantly reduced the migration of MoDCs to CCL19 and CCL21 (Figure 6D).
Interestingly, chemokine-induced activation of Erk-1/2 was similar in MoDCs matured in the presence or absence of PGE2, suggesting that MAP-kinase activation does not mediate CCL19 or CCL21 migration of MoDCs. Indeed, there is not much evidence that Erk activation is required for cell migration, as shown for neutrophils and monocytes.26,27 Although Erk activation relies on G proteins, the chemokine receptor or cell type determines whether Erk-1/2 phosphorylation occurs directly through G-protein–activated ras or an indirect pathway involving the activation of PI3-kinase and the subsequent stimulation of ras through PKC. The involvement of tyrosine kinase in CCR7-mediated chemotaxis has been shown by Stein et al9 for primary lymphocytes using the JAK inhibitor tyrphostin (AG490). We also observed partial inhibition of MoDC migration to CCL21 using the same concentration (100 μM) of AG490, but the outcome of these experiments was too variable to make a definitive statement (data not shown).
Our results clearly show that PGE2 is essential for CCR7-mediated Ca2+ mobilization and for the subsequent migration of MoDCs. Thus, we provide evidence that CCR7 can act as a functional decoy receptor on MoDCs in the absence of PGE2. The expression of chemokine receptors that fail to generate active intracellular signaling after ligand engagement has been reported in several cell types. For instance, inflammatory chemokine receptors on monocytes and MoDCs treated with IL-10 in combination with an inflammatory stimulus are not down-regulated, but they fail to respond to their respective ligands.28 Moreover, germinal center B cells are unable to activate chemotaxis in response to CXCL12 in spite of their surface expression of CXCR4.29
Chemokine receptor inactivation can occur through phosphorylation of the receptor itself or the downstream effector PLC-β.30 Accordingly, receptor inactivation was mediated by heterologous activation of PKC after ligand binding to other chemoattractant receptors; hence, this mechanism is referred to as cross-desensitization. The fact that MoDC migration to CCL19 and CCL21 can be inhibited through heterologous activation of PKC by PMA (Figure 6C) or ATP20 suggests that CCR7 may be inactivated by cross-desensitization. Correspondingly, CXCR4 inactivation and monocyte migration failure in response to CCL3 (MIP-1α), CCL5, formyl peptides, and CCL2 (monocyte chemoattractant protein [MCP]-1) were reported after PMA treatment.30 However, other mechanisms for chemokine receptor inactivation, such as up-regulation of the suppressors of cytokine signaling proteins that were shown to bind to CXCR4 and to block JAK/STAT and Gαi pathways, have been described.31
In conclusion, we show that MoDCs require environmental instruction by PGE2 to couple CCR7 expression to signal transduction pathways and migration in vitro. Given also that CXCR4-mediated migration of MoDCs to CXCL-12 depended on the presence of PGE2 during maturation, this phenomenon of receptor coupling may be valid for other chemokine receptors (Figure 6E).12 This puts a new focus onto PGE2 and its role in the regulation of immune responses.13 We provide evidence that CCL19- and CCL21-induced migration of MoDCs require activation of the PLC pathway and liberation of intracellular free Ca2+ but are independent of PI3-kinase activation and subsequent phosphorylation of PKB. These findings could open up therapeutic opportunities for immune diseases and transplantation medicine and for the development of MoDC-based vaccines.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-05-1643.
Supported by the Cancer League St Gallen-Appenzell, the Swiss Cancer League, the Foundation Propter Homines Vaduz Liechtenstein, the Cancer Research Institute, the CaPCURE Foundation, the Roche Research Foundation, Rentenanstalt Jubiläumsstiftung, and the German Research Foundation (DFG, TR-SFB 11).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Immunex Corporation for providing sCD40L and Wolfhart Seelentag, Hans Schiefer, and Markus Arn for the irradiation of cells. We thank Dr Markus Fopp and the personnel of St Gallen blood bank for supplying blood products and Edith Uetz von Allmen for help with some of the experiments.