Abstract
For reasons that are not apparent, it has been difficult to isolate and expand the adult stem cells referred to as mesenchymal stem cells or marrow stromal cells (MSCs) from murine bone marrow. We developed a protocol that provides rapidly expanding MSCs from 5 strains of inbred mice. The MSCs obtained from 5 different strains of mice were similar to human and rat MSCs in that they expanded more rapidly if plated at very low density, formed single-cell–derived colonies, and readily differentiated into either adipocytes, chondrocytes, or mineralizing cells. However, the cells from the 5 strains differed in their media requirements for optimal growth, rates of propagation, and presence of the surface epitopes CD34, stem cell antigen-1 (Sca-1), and vascular cell adhesion molecule 1 (VCAM-1). The protocol should make it possible to undertake a large number of experiments with MSCs in transgenic mice that have previously not been possible. The differences among MSCs from different strains may explain some of the conflicting data recently published on the engraftment of mouse MSCs or other bone marrow cells into nonhematopoietic tissues.
Introduction
There has been continuing interest in both the biology and potential therapeutic applications of the adult stemlike cells from bone marrow,1-17 referred to as either mesenchymal stem cells or marrow stromal cells (MSCs). MSCs from human and rat bone marrow have been the most extensively characterized, in part because they are relatively easy to isolate by their adherence to plastic and can be extensively expanded in culture. Also, the human and rat MSCs can differentiate into multiple cell phenotypes, including bone, fat, cartilage, muscle, epithelium, and early neural progenitors.
Murine MSCs are far more difficult both to isolate from bone marrow and to expand in culture than human or rat MSCs.10 In contrast to human and rat MSCs, the cultures of murine MSCs (mMSCs) are frequently contaminated by hematopoietic progenitors that overgrow the cultures. Immunodepletion can be used to remove hematopoietic precursors,10 but, even after immunodepletion, the cultures expand poorly, apparently because they accumulate large amounts of extracellular matrix. These features of mMSCs greatly limited the ability to test the cells in the large number of interesting genetic models that are now available as transgenic mice.
In experiments performed here, we have developed a new protocol of the isolation and expansion of mMSCs. The isolated mMSCs were expanded extensively and showed multipotentiality for differentiation to both adipocytes and mineralizing cells. The isolated mMSCs from 5 inbred strains shared a number of characteristics, such as rapid growth from single-cell–derived colonies if plated at low densities. However, the growth rates of mMSCs from the 5 strains varied by as much as 7-fold, and the cells differed in the media requirements for optimal cell growth. In addition, the mMSCs varied in their profiles of surface epitopes.
Materials and methods
Isolation and expansion of mMSCs
mMSCs were obtained from 4 inbred strains of mice (Jackson Labs, Bar Harbor, ME): C57Bl/6J (Bl/6), BALB/c, FVB/N, and DBA1. In addition, mMSCs were also obtained from the inbred transgenic strain C57Bl/6-TgN(ACTbEGFP)1Osb (GFPtg) that ubiquitously expressed enhanced green fluorescent protein (Jackson Labs). Cells were obtained from at least 2 mice of each strain. Female and male mice 8 to 10 weeks old were used, and they were individually killed by CO2. The femurs and tibiae were removed, cleaned of all connective tissue, and placed on ice in 2 mL complete isolation media (CIM). CIM consisted of RPMI-1640 (Invitrogen, Carlsbad, CA) supplemented with 9% fetal bovine serum (FBS; Atlanta Biologicals, Atlanta, GA), 9% horse serum (HS; Hyclone Laboratories, Logan, UT), 100 U/mL penicillin (Invitrogen), 100 μg/mL streptomycin (Invitrogen), and 12 μM l-glutamine (Invitrogen).
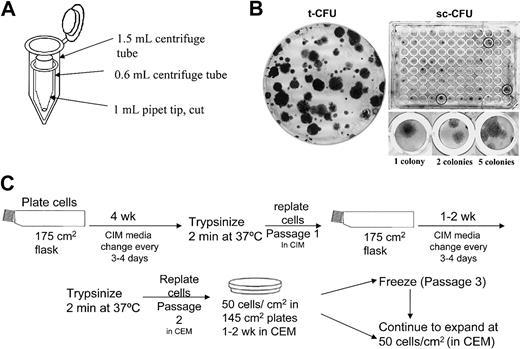
As described by Dobson et al,18 the ends of each tibia and femur were clipped to expose the marrow. The bones were inserted into adapted centrifuge tubes (Figure 1A) and centrifuged for 1 minute at 400g to collect the marrow. The pellet was resuspended in 3 mL CIM with a 21-gauge syringe followed by filtration through a 70-μm nylon mesh filter. The cells from each long bone were plated in 40 mL CIM in a 175-cm2 flask (Figure 1C). After 24 hours, nonadherent cells were removed by washing with phosphate-buffered saline (PBS), and 30 mL fresh CIM was added. The adherent cells (passage 0) were washed, and fresh CIM was added every 3 to 4 days for a period of 4 weeks. After 4 weeks, the cells were washed with PBS and lifted by incubation in 4 mL 0.25% trypsin/1 mM ethylenediaminetetraacetic acid (EDTA; Invitrogen) for 2 minutes at 37°C. The cells that did not lift in 2 minutes were discarded. The trypsin was neutralized by the addition of 12 mL CIM, and all the cells (passage 1) from 1 flask were replated in 30 mL CIM in a 175-cm2 flask. The CIM was replaced every 3 to 4 days. After 1 to 2 weeks, the cells were lifted by incubating with trypsin/EDTA for 2 minutes at 37°C. The cells (passage 2) were then expanded by plating at 50 cells/cm2 in complete expansion media (CEM) consisting of Iscove modified Dulbecco medium (IMDM; Invitrogen) supplemented with 9% FCS, 9% HS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 12 μM l-glutamine. The CEM was replaced every 3 to 4 days. After 1 to 2 weeks, the cells (passage 3) were lifted by incubation with trypsin/EDTA for 2 minutes at 37°C. The passage 3 cells were either frozen or expanded further by plating at 50 cells/cm2 and incubation in CEM. For freezing, the cells were resuspended at 1 × 106 cells/mL in 5% dimethyl sulfoxide (DMSO) and 30% FBS in IMDM, frozen by placement in a –80°C freezer for 24 hours, and then stored in liquid nitrogen. To recover frozen cells, the vials were quickly thawed to 37°C, plated on a 145-cm2 dish in CEM, and incubated for 24 hours. After 24 hours, the plates were washed with PBS, the cells were lifted by incubation with trypsin/EDTA for 2 minutes at 37°C, plated at 50 cells/cm2 in CEM, and incubated for 12 days with a change in medium every 3 to 4 days. The same conditions were used for subsequent passages (passages 4-7).
Schematic for the isolation of mMSCs. (A) The ends of each tibia or femur were removed, and the bones were placed in a pipet tip within a centrifuge tube. The cells were pelleted by centrifugation for 1 to 2 minutes at 400g. (B) Representative t-CFU and sc-CFU analysis. (C) The culturing steps for isolation and expansion of mMSCs.
Schematic for the isolation of mMSCs. (A) The ends of each tibia or femur were removed, and the bones were placed in a pipet tip within a centrifuge tube. The cells were pelleted by centrifugation for 1 to 2 minutes at 400g. (B) Representative t-CFU and sc-CFU analysis. (C) The culturing steps for isolation and expansion of mMSCs.
Effect of plating density on population growth dynamics
Passage 7 mMSCs were plated in triplicate at 5 to 1000 cells/cm2 in CEM. The media was changed every 3 days for a total of 12 days. The cells were lifted with trypsin/EDTA, and the cells from each plate were counted in duplicate with a hemocytometer. The cells were replated to determine the colony-forming efficiency (CFU assay) as a guide to the growth potential of the cultures (Figure 1B). For the traditional colony-forming unit assay (t-CFU), 100 cells were plated in triplicate on 58-cm2 plates in CEM. For the single-cell CFU assay (sc-CFU assay), a single cell was deposited in each well of a 96-well plate by using the Cloncyte program and fluorescence-activated cell sorting (FACS) instrument (FACSVantage; Becton Dickinson, San Jose, CA). Samples were incubated for 21 days in CEM, at which time the plates were stained with 3% Crystal Violet in methanol at room temperature (RT) for 20 minutes. All visible colonies were counted.
Selection of culture media and serum concentrations
Passage 7 mMSCs were plated at 50 cells/cm2 on 58-cm2 tissue-culture dishes in 1 of 4 basal media (Invitrogen): α-modified minimum essential medium (αMEM), Dulbecco modified Eagle medium (DMEM), IMDM, RPMI-1640, or StemPro34 (Invitrogen), a defined serum-free media for human hematopoietic stem cells. Each medium was supplemented with 9% FBS, 9% HS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 12 mM l-glutamine, except the StemPro34 which did not require serum. The media was changed every 3 days. After expansion for 12 days, cell yields were determined by hemacytometer, and the cells were assayed for t-CFUs. The same conditions were used to assay the effects of 5%, 10%, or 20% serum (1:1 HS/FBS).
Differentiation
Cells were plated at 50 cells/cm2 in 58-cm2 dishes and incubated in CEM for 10 days. For osteogenesis, the cultures were then incubated in DMEM (DBA1) or IMDM (all other strains) that was supplemented with 10% FCS, 10% HS, 100 U/mL penicillin, 100 μg/mL streptomycin, 12 mM l-glutamine, 20 mM β-glycerol phosphate (Sigma, St Louis, MO), 50 ng/mL thyroxine (Sigma), 1 nM dexamethasone (Sigma), and 0.5 μM ascorbate 2-phosphate (Sigma). The media was changed 2 times per week for 3 weeks. The cells were fixed with 10% formalin for 20 minutes at RT and stained with Alizarin Red, pH 4.1 (Sigma) for 20 minutes at RT.
For adipogenesis, the cultures were incubated in DMEM (DBA1) or IMDM (all other strains) that was supplemented with 10% FCS, 10% HS, 100 U/mL penicillin, 100 μg/mL streptomycin, 12 mM l-glutamine, 5 μg/mL insulin (Sigma), 50 μM indomethacin (Sigma), 1 × 10–6 M dexamethasone, and 0.5 μM 3-isobutyl-1-methylxanthine (IBMX; Sigma). The medium was changed 2 times per week for 3 weeks. The cells were fixed with 10% formalin for 20 minutes at RT and stained with 0.5% Oil Red O (Sigma) in methanol (Sigma) for 20 minutes at RT.
For chondrocyte differentiation, a pellet culture system was used. Approximately 200 000 mMSCs (passage 7) were placed in a 15-mL polypropylene tube (Falcon, Bedford, MA), and centrifuged to pellet. The pellet was cultured at 37°C with 5% CO2 in 500 μL chondrogenic media that contained 500 ng/mL bone morphogenetic protein-6 (BMP-6; R&D Systems, Minneapolis, MN) in addition to high-glucose DMEM supplemented with 10 ng/mL transforming growth factor β3 (TGF-β3), 10–7 M dexamethasone, 50 μg/mL ascorbate-2-phosphate, 40 μg/mL proline, 100 μg/mL pyruvate, and 50 mg/mL ITS + Premix (Becton Dickinson, Bedford, MA; 6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 ng/mL selenious acid, 1.25 mg/mL bovine serum albumin, and 5.35 mg/mL linoleic acid). The medium was replaced every 3 to 4 days for 21 days. For microscopy, the pellets were embedded in paraffin, cut into 5-μm sections and stained with Toluidine blue Sodium Borate.
Immunostaining and FACS analysis
The following antibodies were obtained from Becton Dickinson: CD11b-FITC (fluorescein isothiocyanate), CD34-PE (phycoerythrin), CD45-FITC, Ter-119-PE, CD45R/B220-FITC, Ly6G-FITC and Ly-6C-FITC, CD3e-FITC, FLK1-PE (vascular endothelial growth factor receptor 2 [VEGF-R2]), CD31-FITC (platelet endothelial cell adhesion molecule [PECAM]), CD90-PE (Thy1), CD117-APC (allo-phycocyanin) (c-kit), CD106-FITC (vascular cell adhesion molecule-1 [VCAM-1]), and Ly-6A/E-FITC (stem cell antigen-1 [Sca-1]). mMSCs cultured in CEM were lifted with trypsin/EDTA and counted. About 200 000 cells were divided into aliquots in amber-tinted 1.5-mL centrifuge tubes and pelleted by centrifugation for 1 minute at 450g. The cells were resuspended in 1 mL PBS. FITC-conjugated antibodies were added at a concentration of 2 μg/mL, and PE- or APC-conjugated antibodies at 2.5 μg/mL. The samples were incubated with gentle shaking at room temperature for 20 minutes. The cells were pelleted, washed twice with PBS, and analyzed by flow cytometry (Cytomics FC 500 FACS; Beckman Coulter, Miami, FL).
Results
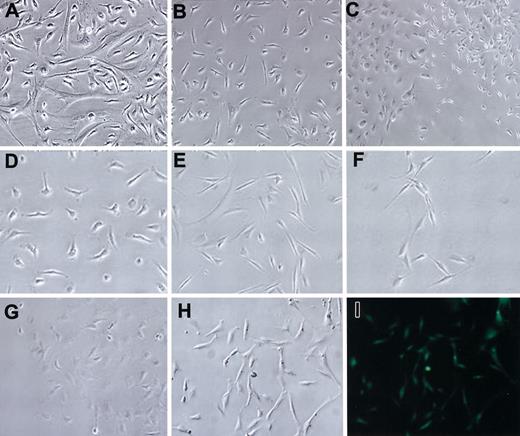
Marrow was isolated from the long bones18 of mice by centrifugation (Figure 1A) and plated in tissue-culture dishes. The use of the centrifuge columns allowed multiple bones to be processed simultaneously, with increased sterility. Previous studies19-21 and initial experiments carried out here indicated that RPMI medium inhibited the growth of hematopoietic cells in cultures. Therefore, it was used for the initial medium (CIM) to isolate mMSCs (Figure 2A). After plating of whole marrow cells, removal of nonadherent cells, and incubation for 4 weeks in CIM, many of the cells detached. Cells (passage 1) with a spindle-shape characteristic of MSCs appeared to predominate in the cultures (Figure 2B). After 4 weeks, all the cells from each tibia or femur were lifted with trypsin/EDTA for 2 minutes at 37°C, replated in CIM on a 175-cm2 flask, and incubated for 1 to 2 weeks. At this stage, most of the cells (passage 2) were spindle shaped (Figure 2B). The passage 2 mMSCs were then expanded by plating at a low density of 50 cells/cm2 and incubated in CEM medium, conditions under which the cells expanded rapidly as single-cell–derived colonies. At passage 7, cells from all 5 strains had similar morphologies (Figure 2D-H). mMSCs from GFPtg mice were fluorescent when examined through an FITC filter (Figure 2I).
Morphologies of mMSCs. mMSCs from Bl/6 mice: (A) passage 0, (B) passage 1, and (C) passage 5. Low-density plating to show the morphology of passage 7 mMSCs from the mouse strains: (D) Bl/6, (E) BALB/c, (F) FVB/N, (G) DBA1, and passage 4 GFPtg, phase contrast (H) and epifluorescence (I). Original magnification, × 10.
Morphologies of mMSCs. mMSCs from Bl/6 mice: (A) passage 0, (B) passage 1, and (C) passage 5. Low-density plating to show the morphology of passage 7 mMSCs from the mouse strains: (D) Bl/6, (E) BALB/c, (F) FVB/N, (G) DBA1, and passage 4 GFPtg, phase contrast (H) and epifluorescence (I). Original magnification, × 10.
Effect of plating density
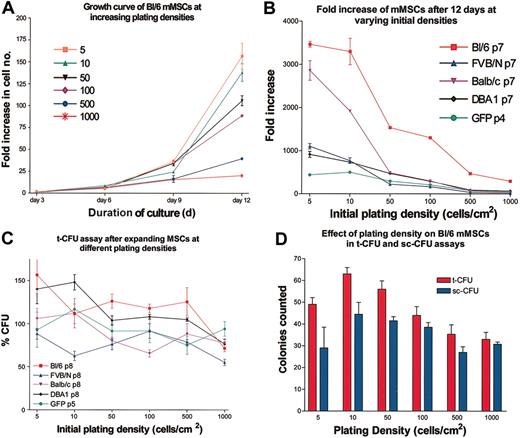
Passage 3 mMSCs from Bl/6 mice were plated at varying densities in CEM on 145-cm2 plates, and cell numbers were assayed every 3 days for 12 days (Figure 3A). As seen previously with human and rat MSCs,9,14-16 the cultures showed a lag period with little expansion in the first 3 days. Thereafter the cells expanded rapidly. The rates of expansion of the cultures were extremely sensitive to the initial plating densities. All the strains showed decreases in growth rates as the initial plating density was increased, with 5 to 10 cells/cm2 having the highest fold increase in cell number (Figure 3B). Therefore, the cells were as sensitive to plating density as both human MSCs9,14,16 or the rat MSCs.15 Surprisingly, incubation of the mMSCs at the higher densities did not decrease the t-CFU efficiency in the next passage (Figure 3C-D). Therefore, the constraints on expansion imposed by high-density plating were reversible. The same reversibility was observed with rat MSCs15 but not with human MSCs.9
Dependence of expansion on initial plating density. (A) Expansion as a function of plating density for passage 3 mMSCs from Bl/6 mice. Cultures were counted every 3 days. Fold increase of cells was determined by total cell number recovered divided by the initial cell number plated. (B) The fold increase in cell number after 12 days of culture for mMSC from 5 different mouse strains. (C) t-CFU assays of the 12-day cultures after initial plating at increasing densities. (D) t-CFU and sc-CFU assays of Bl/6 mMSCs. Data are expressed as the mean ± SE, n = 3to5.
Dependence of expansion on initial plating density. (A) Expansion as a function of plating density for passage 3 mMSCs from Bl/6 mice. Cultures were counted every 3 days. Fold increase of cells was determined by total cell number recovered divided by the initial cell number plated. (B) The fold increase in cell number after 12 days of culture for mMSC from 5 different mouse strains. (C) t-CFU assays of the 12-day cultures after initial plating at increasing densities. (D) t-CFU and sc-CFU assays of Bl/6 mMSCs. Data are expressed as the mean ± SE, n = 3to5.
Selection of optimal media and serum concentrations for the expansion of mMSCs
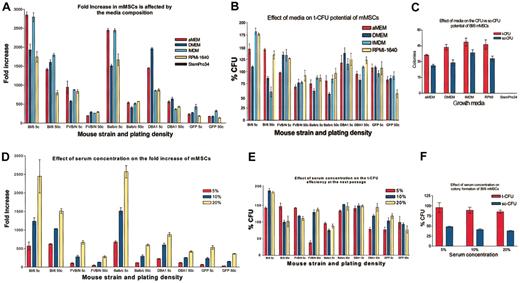
To define the optimal media for expansion of the cells, passage 7 mMSCs were plated at either 5 or 50 cells/cm2 in 145-cm2 plates and incubated either in CEM or in CEM in which the IMDM was replaced with α-MEM, DMEM, RPMI-1640, or StemPro34. The effects of the media compositions were more apparent when the cells were plated at the very low density plating of 5 cells/cm2 instead of at 50 cells/cm2 (Figure 4A). mMSCs from Bl/6 and BALB/c mice expanded at least 2500-fold in 12 days in either the IMDM or αMEM. The relative growth rates for Bl/6 and BALB/c cells in the different media were IMDM and αMEM > RPMI or DMEM. mMSCs from FVB/N mice grew well in IMDM, RPMI, or αMEM but did not proliferate as well in DMEM. For mMSCs from DBA1 mice, the relative growth rates were DMEM > αMEM > IMDM or RPMI. mMSCs from GFPtg that were derived from the Bl/6 strain grew poorly under all the conditions tested. The cells had the highest rate of proliferation in IMDM. None of the mMSCs survived in the StemPro34 media. In experiments in which the ratio of the FCS to HS was maintained at 1:1, the cells in a basal medium of IMDM expanded to a 5-fold greater extent with a total serum concentration of 20% than 5% (Figure 4D).
Medium requirements for mMSCs. (A) mMSCs were plated at 5 or 50 cells per cm2 (5 c or 50 c, respectively) and counted after 12 days of culture. (B) t-CFU assays were performed after the 12 days' incubation in the medium indicated. (C) t-CFU assays and sc-CFU assays were performed on Bl/6 mMSCs expanded in the different media. (D) Serum requirements (HS:FCS = 1:1) were determined for the 5 strains of mMSCs. The mMSCs were plated at 5 or 50 cells per cm2 (5 c or 50 c, respectively) and counted after 12 days. (E) t-CFU assays after the 12-day expansion in varying serum concentrations. (F) t-CFU and sc-CFU assays performed with mMSCs from Bl/6 mice. All strains were passage 7, except GFPtg, which was passage 4. Data are expressed as the mean ± SE, n = 3 to 5.
Medium requirements for mMSCs. (A) mMSCs were plated at 5 or 50 cells per cm2 (5 c or 50 c, respectively) and counted after 12 days of culture. (B) t-CFU assays were performed after the 12 days' incubation in the medium indicated. (C) t-CFU assays and sc-CFU assays were performed on Bl/6 mMSCs expanded in the different media. (D) Serum requirements (HS:FCS = 1:1) were determined for the 5 strains of mMSCs. The mMSCs were plated at 5 or 50 cells per cm2 (5 c or 50 c, respectively) and counted after 12 days. (E) t-CFU assays after the 12-day expansion in varying serum concentrations. (F) t-CFU and sc-CFU assays performed with mMSCs from Bl/6 mice. All strains were passage 7, except GFPtg, which was passage 4. Data are expressed as the mean ± SE, n = 3 to 5.
Assay for colony-forming units
Assays for t-CFUs provide a convenient means of assessing the proliferative capacity that MSCs retain after the cells have been expanded in culture.9,16 Therefore, Bl/6 mMSCs that were expanded to passage 4 under different conditions were assayed by both the t-CFU assay in which 100 cells were plated on a 58-cm2 plate or by an sc-CFU assay in which single cells are deposited into separate wells of a 96-well plate. As indicated in Figure 3, the t-CFU values of the expanded cells remained high. Of special note was that the number of colonies obtained in the t-CFU assay was greater than the number of cells plated (Figure 3D). Also, several wells of the 96-well plate in the sc-CFU assay contained multiple clones (Figure 1B). The results suggested, therefore, that some of the cells from the initially formed colonies detached and generated new colonies. Similar results were previously observed with experiments to assay CFUs with rat MSCs.15
Differentiation assays
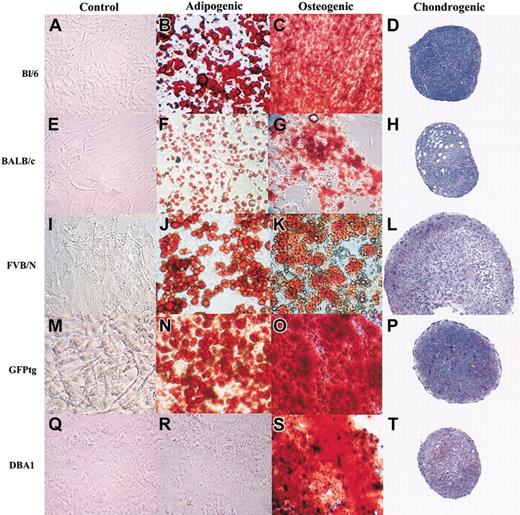
Passage 7 mMSCs readily differentiated into mineralizing cells or adipocytes when incubated in differentiation medium (Figure 5). However, differences were seen in the differentiation capabilities of MSCs from the different strains. mMSCs from Bl/6 mice (Figure 5A-C), GFPtg mice (Figure 5M-O), and DBA1 mice (Figure 5Q-S) more readily differentiated into mineralizing cells than into adipocytes. In contrast, mMSCs from BALB/c mice (Figure 5E-G) and FVB/N mice (Figure 5I-K) more readily differentiated into adipocytes. Differences were also seen in the efficiency of the mMSC strains to differentiate into chondrocytes under the conditions used for human MSCs.8,16 GFPtg, FVB/N, and DBA1 mMSCs readily differentiated into proteoglycan-producing pellets (Figure 5P, L, and T, respectively), as evidenced by the light purple staining in the pellets. BALB/c mMSCs produced little proteoglycans (Figure 5H), and the Bl/6 mMSCs showed little evidence of proteoglycan secretion, although they did produce stable pellets (Figure 5D). Control cells grown in CEM did not stain for either mineral or lipids (Figure 5A,E,I,M,Q). Interestingly, the DBA1 cells did not differentiate into mineralizing cells or adipocytes when the IMDM-containing media was used. It was only when the differentiation media was produced in DMEM that the cells were able to mineralize.
Differentiation of mMSCs in culture. mMSCs were incubated to confluency in CEM and then transferred to adipogenic or osteogenic medium for 21 days. Chondrogenesis was produced by incubating 200 000 mMSCs as a micromass pellet in chondrogenic media for 21 days. All mMSCs were passage 7, except GFPtg cells, which were passage 4. Original magnification, × 10.
Differentiation of mMSCs in culture. mMSCs were incubated to confluency in CEM and then transferred to adipogenic or osteogenic medium for 21 days. Chondrogenesis was produced by incubating 200 000 mMSCs as a micromass pellet in chondrogenic media for 21 days. All mMSCs were passage 7, except GFPtg cells, which were passage 4. Original magnification, × 10.
Epitope analyses
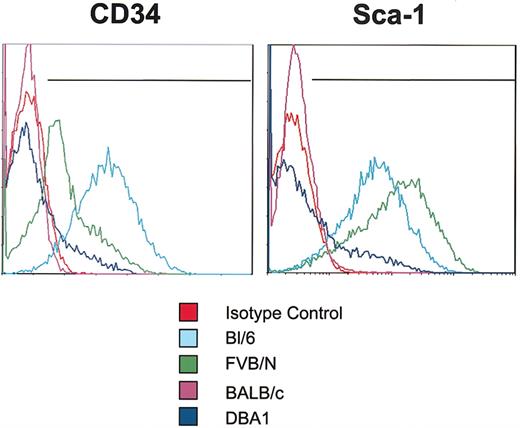
mMSCs from the 4 standard inbred strains were negative for the endothelial markers: FLK1 (VEGF-R2), CD31 (PECAM), CD90 (Thy1), and CD117 (c-kit) (Table 1). They were also negative in assays with the lineage panel of antibodies used to identify epitopes on many hematopoietic cells: CD11b, Ter-119, CD45R/B220, Ly6G and Ly-6C, and CD3e. In addition, all the mMSCs tested were negative for the hematopoietic cell marker CD45. However, they varied in their expression of CD34 as assayed by the mouse antibody (Figure 6) that is not specific for murine hematopoietic cells as it is in human cells.22 Bl/6 cells expressed high levels of CD34, FVB/N cells expressed moderate levels, and both BALB/c and DBA1 cells expressed low levels. The mMSCs also differed in their expression of Sca-1 (Figure 6). The Bl/6 and FVB/N cells expressed high levels of Sca-1, DBA1 cells expressed low levels, and the BALB/c cells were negative. Finally, the strains differed in their expression of CD106 (VCAM-1). The Bl/6 and FVB/N mMSCs expressed moderate levels of VCAM-1, whereas BALB/c and DBA1 mMSCs expressed very low levels.
Assays of CD34 and Sca-1 epitopes on mMSCs. Passage 7 mMSCs were incubated with antibodies for CD34 or Sca-1 and assayed by FACS. Each antibody was tested individually, and isotype controls were gated to less than 1%. Representative plots from 3 samples from each strain are shown.
Assays of CD34 and Sca-1 epitopes on mMSCs. Passage 7 mMSCs were incubated with antibodies for CD34 or Sca-1 and assayed by FACS. Each antibody was tested individually, and isotype controls were gated to less than 1%. Representative plots from 3 samples from each strain are shown.
Discussion
The technical difficulties of isolating and expanding MSCs from mice have limited the number of experiments that can be conducted with the many informative transgenic mice now available. The results presented here should enable such experiments in most lines of transgenic mice. The protocol to isolate the mMSCs from bone marrow differed from the protocols we previously used to isolate either human1-15,17 or rat16 MSCs in several aspects. One was that the marrow was isolated by centrifuging cut long bones18 instead of either the needle aspiration used for human cells or flushing the marrow from long bones with medium that was used with rat cells. This technique allowed rapid and efficient removal of the mMSCs with increased sterility. Another aspect was that the medium for culture of the murine cells contained equal proportions of FCS and HS instead of only FCS. Even more significant was that the cells were initially isolated by incubation as high-density cultures for 4 weeks followed by replating and another 1 to 2 weeks in high-density cultures in medium previously shown to inhibit growth of hematopoietic cells.19-21 Only the most easily detached cells were harvested. The cells were then expanded in a variety of basal media as low-density cultures. In contrast to the protocol for preparing mMSCs, rat MSCs were prepared by plating all the marrow cells at high density and incubating them until the cultures reached about 90% confluency before expanding the cells at low density. Human MSCs were prepared with essentially the same protocol as the rat MSCs except mononuclear cells were isolated from the marrow aspirate with a density gradient to prepare the high-density plates. These relatively subtle differences in the protocol for isolating the mMSCs greatly improved the yields and freed the cultures of hematopoietic and endothelial precursors. The isolated MSCs expanded rapidly, apparently because the cells did not synthesize the large amounts of extracellular matrix that previously made mMSCs difficult to lift from early passage cultures.10
The isolated mMSCs were similar to rat15 and human MSCs14,16 in that they expanded more rapidly if plated at very low density, formed single-cell–derived colonies, and readily differentiated into either adipocytes or mineralizing cells. The mMSCs were similar to human MSCs15 in that they differentiated into chondrocytes under the conditions in which human MSCs readily form cartilage.8 They were also similar to rat MSCs in that when plated at very low densities, single cells gave rise to more than one colony, because a few of the cells from the initial colonies detached and formed new colonies. In addition, the mMSCs were similar to the rat MSCs in that after incubation under optimal conditions, the murine cells expanded more than 2500-fold in about 12 days, whereas the human cells rarely expanded more than 1000-fold.17
Comparisons of MSCs isolated from 4 inbred strains and 1 transgenic strain expressing EGFP demonstrated that some features of the cells were strain specific. Two strains (Bl/6 and BALB/c) expanded more rapidly than the other 3, and the cells from the different strains had different media requirements for optimal growth. Also, cells from Bl/6, DBA1, and GFPtg mice differentiated more readily into mineralizing cells than adipocytes, but BALB/c and FVB/N mMSCs differentiated more readily into adipocytes. Chondrocyte differentiation also varied among the strains, with all the strains producing stable pellets, but only the GFPtg, FVB/N, and DBA1 mMSCs depositing significant proteoglycans. The mMSCs from different strains also differed in their expression of the cell-surface epitopes CD35, Sca-1, and VCAM-1.
The differences among mMSCs from different strains may well explain conflicting data recently published on the engraftment of mMSCs or other bone marrow cells into nonhematopoietic tissues. One of the most striking observations made here was that MSCs from the GFPtg mice expanded much more slowly under all the conditions tested than MSCs from Bl/6 mice from which the GFP-expressing mice were derived. Such differences in propagation rates and other features may in part explain why some laboratories have detected limited engraftment of GFP-labeled bone marrow cells into nonhematopoietic tissues, whereas other laboratories have seen relatively high levels of engraftment using either different strains of mice or different cellular markers23,24 (reviewed recently in Prockop et al17 ).
Prepublished online as Blood First Edition Paper, October 30, 2; DOI 10.1182/blood-2003-09-3070.
Supported in part by grants from the National Institutes of Health (grants HL 073755, RR 017447, and AR 48323), the Oberkotter Foundation, HCA the Healthcare Company, and the Louisiana Gene Therapy Research Consortium.
A.P. and J.A.M. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.