Abstract
Elevated plasma levels of fibrinogen are associated with the presence of cardiovascular disease, but it is controversial whether elevated fibrinogen causally imparts an increased risk, and as such is a true modifier of cardiovascular disease, or is merely associated with disease. By investigating a transgenic mouse model of hyperfibrinogenemia, we show that elevated plasma fibrinogen concentration (1) elicits augmented fibrin deposition in specific organs, (2) interacts with an independent modifier of hemostatic activity to regulate fibrin turnover/deposition, (3) exacerbates neointimal hyperplasia in an experimental model of stasis-induced vascular remodeling, yet (4) may suppress thrombin generation in response to a procoagulant challenge. These findings provide direct experimental evidence that hyperfibrinogenemia is more than a by-product of cardiovascular disease and may function independently or interactively to modulate the severity and/or progression of vascular disease.
Introduction
Numerous epidemiological studies have documented the association of elevated plasma fibrinogen levels with cardiovascular disease.1-7 Cross-sectional prospective studies imply that elevated plasma fibrinogen levels are an independent and predictive parameter for increased risk of coronary artery disease, stroke, and peripheral vascular disease. The magnitude of the increase in fibrinogen levels correlates with the presence and severity of peripheral arterial disease, the extent of myocardial necrosis in infarcted patients, and cerebrovascular accidents. The disease risk associated with hyperfibrinogenemia is disproportionally augmented in those individuals with the highest fibrinogen levels, indicating a threshold effect. Notwithstanding its recognized value as a marker for the presence of cardiovascular disease, it remains controversial whether an elevated fibrinogen level itself imparts an increased risk, and therefore represents a true modifier of vascular disease, or is merely associated with disease. Fibrinogen production in the liver is regulated by cytokines and is greatly enhanced in the acute phase response to infection and other inflammatory processes.8-11 It is therefore possible that moderately elevated fibrinogen levels simply report a state of inflammation associated with vascular disease. On the other hand, augmented fibrinogen levels, per se, could alter the hemodynamic properties of blood or enhance concentration driven enzyme-substrate interactions between thrombin, fibrinogen, and platelets, and thus lead to increased intravascular fibrin deposition and thrombosis. These views are not mutually exclusive, as augmentation of fibrinogen levels secondary to an inflammatory challenge could nevertheless amplify or accelerate the disease process. Moreover, fibrinogen and fibrin degradation products might in turn enhance the inflammatory aspect of vascular lesions by regulating cytokine production and leukocyte-endothelial interactions.12-17
Recently described genetically altered mice with chronically increased plasma fibrinogen levels in the absence of underlying inflammation provide a novel experimental tool to investigate the cause-effect relationship between hyperfibrinogenemia and vascular disease.18 The mutant mouse strain (hereafter referred to as Hifib mice) was derived by pronuclear oocyte microinjection of the entire mouse fibrinogen locus, including all 3 fibrinogen chains as well as several kilobases of flanking sequence. The added transgenic fibrinogen allele is inherited in a Mendelian fashion, and the mice were reported to exhibit approximately 2-fold augmented plasma fibrinogen levels yet were free from overt signs of disease and displayed normal reproductive performance.18 Transgene-dependent hyperfibrinogenemia had no effect on diet-induced atherogenesis in C57Bl6 mice19 or in ApoE*3-Leiden transgenic mice.20
The objective of the current study was to investigate whether chronically elevated fibrinogen in Hifib mice (1) causes increased intravascular fibrin deposition or altered fibrin(ogen) turnover; (2) modifies platelet-thrombus formation in response to acute endothelial injury; (3) affects the incidence or extent of stasis-induced thrombosis and vascular remodeling; or (4) augments thrombosis in mice with an underlying hypercoagulable state. The last goal was approached by crossing Hifib mice with a genetically engineered mouse strain carrying a mutation (TMPro) in the thrombomodulin (TM) gene,21,22 to produce Hifib/TMPro mice. The TMPro mutation drastically reduces the cofactor activity of TM in the thrombin-dependent activation of protein C and thereby elicits a hypercoagulable state. Analysis of Hifib/TMPro mice therefore allowed us to investigate the consequences of hyperfibrinogenemia in animals that displayed increased susceptibility to thrombosis.
Materials and methods
Transgenic animals
The TMPro mouse strain has been described in detail earlier.21,22 The mutation in TMPro mice causes 2- to 3-fold reduced TM expression and disrupts protein C activation. Homozygous TMPro mice enrolled in the current study were derived by crossing nonlittermate, heterozygous N9 C57Bl/6 TMPro/wt mice and intercrossing the resultant homozygous animals to establish a colony of inbred homozygous TMPro mice on a C57Bl/6 background. The C57Bl/6-derived Y-chromosome was introduced by breeding N8 C57Bl/6 TMPro/wt females with wild-type C57Bl/6 males. Normal C57Bl/6 mice were obtained from Charles River Laboratories (Wilmington, MA). Hyperfibrinogenemic mice carrying an extra copy of the entire mouse fibrinogen locus (Hifib mice) have been described previously.18 Hifib mice used in the current study were maintained on a C57Bl/6 background by repeated backcrosses to wild-type C57Bl/6 mice. TMPro/Hifib animals (homozygous for TMPro-mutation; hemizygous for the fibrinogen transgene) were obtained by intercrossing C57Bl/6 TMPro mice with C57Bl/6 Hifib mice. Fibrinogen-deficient mice lacking the fibrinogen Aα-gene23 were kindly provided by Dr J. Degen (Children's Hospital Research Foundation, Cincinnati, OH). Experiments involving animals were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin.
Acute carotid injury model
FeCl3-induced arterial injury was induced according to published procedures.24,25 The left common carotid artery was exposed by blunt dissection, and blood flow was recorded with Doppler flow probes (model 0.5VB; Transonic Systems, Ithaca, NY) positioned around the distal end of the artery. Ten minutes after probe placement, a 1.0 × 0.6 mm2 strip of filter paper soaked in 20% FeCl3 was applied to the adventitial surface of the artery for 1 minute. The field was flushed with saline and flow was continuously monitored. In some experiments, the procedure was then repeated on the right common carotid. Occlusion times were expressed as t75, the time at which pulse amplitude flow dropped to 25% of the initial flow, defined as the average flow occurring between minute 2 and minute 4 after injury. Data were evaluated for statistical significance by Wilcoxon rank sum analysis and Student t test.
Carotid artery ligation
The left common carotid artery was dissected, and blood flow was permanently interrupted by applying a tight ligature near the carotid bifurcation as described.26 After 4 weeks, all animals were perfused at physiological pressure with 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.3. The left and right common carotid arteries were dissected, embedded in paraffin, and serial sections (5-μm-thick) were cut at 150-μm intervals spanning the entire length of the vessel. Digitized images of these vessels were analyzed using image analysis software (NIH Image 1.60; National Institutes of Health, Bethesda, MD) as described.26
Determination of plasma fibrinogen levels, thrombin-antithrombin complexes, D-dimer, tissue fibrin deposition, and in vitro thrombin generation
Ten minutes prior to collection of samples for analysis of fibrin deposition in tissues, plasma levels of fibrinogen, D-dimer, and thrombin-antithrombin (TAT)–complex, mice were injected intraperitoneally with 1000 U heparin (unfractionated porcine heparin, sodium-salt, MW 6000-30 000, Life Technologies, Grand Island, NY). Blood samples for measurement of D-dimer and TAT-complex were then collected into 1:10 volume 3.2% sodium citrate and analyzed using enzyme-linked immunosorbent assay (ELISA) systems (Asserachrome D-Di; American Bioproducts, Parsippany, NJ; and Enzygnost TAT micro; Behring Diagnostics, Westwood, MA). Fibrinogen levels were measured by sandwich ELISA using rabbit anti–human fibrinogen antibody (DAKO, Carpinteria, CA) and peroxidase-conjugated goat anti–mouse fibrinogen antibody (Accurate International, Westbury, NY). The assay was calibrated with purified mouse fibrinogen. Whole clottable protein in plasma was measured by a modification of a previously described assay.27 Briefly, 25 μL of plasma was mixed with 750 μL of a solution containing 0.15 M NaCl; 0.01 M sodium phosphate pH 6.4; 30 units/mL aprotinin, and a small amount of glass powder. Clotting was initiated by addition of 2 U α-thrombin, followed by incubation at room temperature for 1 hour. Clotted protein was recovered by centrifugation (10 000g; 2 minutes); the glass powder was washed 3 times in the above buffer and once in water, suspended in 100 μL 0.75 N NaOH, boiled for 15 minutes, then cooled and mixed with 1 mL of 2% (wt/vol) Na2CO3. Protein concentration was then determined with diluted Folin reagent. The amount of cross-linked fibrin present in mouse tissues was analyzed by semiquantitative Western blot analysis essentially as described in a previous publication.22 In vitro thrombin generation as a function of plasma fibrinogen concentration was determined by a modification of a previously described method.28 The thrombin-generating reaction mixture contained 50 μL citrated plasma prepared from afibrinogenemic fibrinogen Aα-chain knockout mice, 50 μL actin FS (Dade Behring, Deerfield, IL), plus 100 μL 25 mM CaCl2 solution or 25 mM CaCl2 solution containing variable amounts of mouse fibrinogen. At various time intervals, 20-μL aliquots were transferred to 980 μL of a Tris[tris(hydroxymethyl)aminomethane]-buffered solution (pH 7.8) containing 100 μM S-2238 (Diapharm), 5 mM EDTA (ethylenediaminetetraacetic acid), 20 mM Tris, 0.14 M NaCl. After 2 minutes incubation, Phe-Pro-Arg-chloromethylketone (PPACK) (10 μM, final concentration) was added to inhibit further thrombin generation, and S-2238 amidolytic activity was measured spectrophotometrically at 405 nm. Thrombin activity was expressed in U/mL using murine α-thrombin (Enzyme Research Laboratories, South Bend, IN) for calibration. The presence of fibrinogen in the second stage of this assay did not affect thrombin activity as determined by S-2238 hydrolysis. Thrombin-stimulated TAT-complex formation in vivo was initiated by injecting 0.05 U human α-thrombin (Enzyme Research Laboratories) into the femoral vein. Blood samples were collected from the inferior vena cava after 10 minutes into 0.38% sodium citrate (final concentrations) and analyzed for thrombin-antithrombin (TAT) complex as above.
Results
Fibrin(ogen) metabolism is altered in hyperfibrinogenemic mice
Plasma fibrinogen levels in 8- to 16-week-old Hifib mice, as determined by ELISA, were approximately 1.5-fold higher than in wild-type mice (3.5 ± 0.4 versus 2.4 ± 0.4 mg/mL ± SD; P ≤ .0005; n = 12/group). Values obtained by ELISA correlated with the amount of whole clottable protein in platelet-poor plasma. Fibrinogen levels in TMPro- and in Hifib/TMPro mice were the same as in wild-type and Hifib mice, respectively, demonstrating that the TMPro mutation does not alter ambient fibrinogen levels.
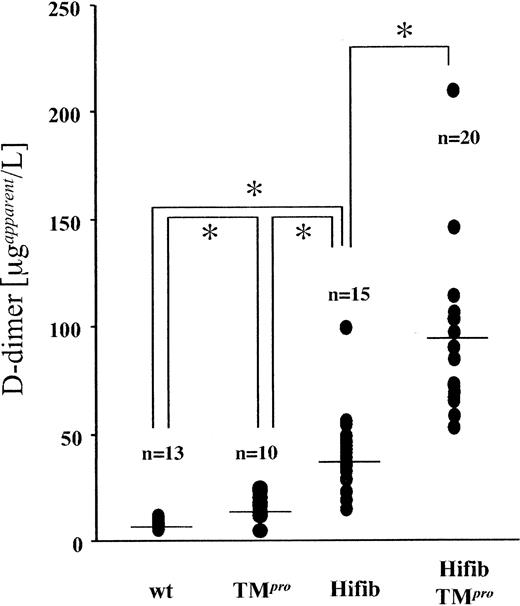
Plasma levels of the D-dimer fibrin split product were substantially elevated in Hifib mice (Figure 1) compared to wild-type mice (40 ± 17 versus 7.9 ± 2 μg/mL ± SD; P ≤ .0005) and were also higher than those measured in TMPro animals (16.6 ± 6 μg/mL ± SD, P ≤ .005). Reconstitution of plasma samples from fibrinogen-deficient mice with purified mouse fibrinogen at concentrations up to 5 mg/mL did not yield a detectable D-dimer signal. Likewise, spiking of normal mouse plasma with excess fibrinogen to achieve a plasma concentration of 5 mg/mL did not alter the measured D-dimer concentration. These control experiments eliminate the possibility that the approximately 5-fold elevation of D-dimer in Hifib mice is attributable to cross-reactivity of the assay antibodies with mouse fibrinogen. In Hifib/TMPro-mice, D-dimer levels were even further augmented (93 ± 40 μg/mL ± SD). This shows that elevation of plasma fibrinogen concentration itself caused augmented generation of fibrin degradation products, and—by inference—augmented fibrin formation. This effect is clearly observed in Hifib mice and is more pronounced in Hifib/TMPro animals, demonstrating that both mutations interact in a synergistic manner with respect to fibrin(ogen) turnover.
Plasma concentration of D-dimer fibrin degradation product in mutant mice. Steady-state D-dimer concentration in the plasma of hyperfibrinogenemic mice (Hifib and Hifib/TMPro), wild-type (wt), and thrombomodulin-deficient mice (TMPro) was determined by ELISA. Values are given as apparent D-dimer concentration determined from a standard constructed with purified human D-dimer. Augmented D-dimer levels in hyperfibrinogenemic mice enhanced formation and subsequent degradation of fibrin. Bars indicate the average; asterisks denote statistical significance below the 0.05 confidence level (Student t test).
Plasma concentration of D-dimer fibrin degradation product in mutant mice. Steady-state D-dimer concentration in the plasma of hyperfibrinogenemic mice (Hifib and Hifib/TMPro), wild-type (wt), and thrombomodulin-deficient mice (TMPro) was determined by ELISA. Values are given as apparent D-dimer concentration determined from a standard constructed with purified human D-dimer. Augmented D-dimer levels in hyperfibrinogenemic mice enhanced formation and subsequent degradation of fibrin. Bars indicate the average; asterisks denote statistical significance below the 0.05 confidence level (Student t test).
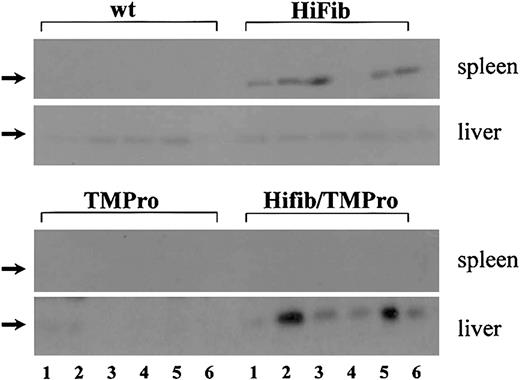
To determine whether augmented fibrin formation in Hifib mice leads to microvascular thrombosis, we measured the amount of insoluble fibrin present in the tissues of wild-type and mutant mice via Western blot analysis of tissue extracts with fibrin-specific antibodies (Figure 2). Fibrin deposition in the heart, liver, lung, brain, and kidney of Hifib mice was indistinguishable from that observed in wild-type mice, but the spleen of Hifib mice exhibited an augmented content of fibrin. A different pattern of organ-specific fibrin deposition was observed in Hifib/TMPro mice: fibrin levels in the heart, kidney, lung, and brain were again identical to those measured in wild-type animals, but in addition the spleen appeared spared from the excess fibrin deposition seen in Hifib mice. However, we detected significant amounts of fibrin in the liver of Hifib/TMPro mice, exceeding the extent of fibrin deposits detected in the liver of wild-type mice, TMPro mice with isolated TM deficiency, or Hifib mice. These results were reproduced in an independent population of mice that had been backcrossed twice more onto a C57Bl/6 background. As reported earlier, C57Bl/6 TMPro mice do not display fibrin deposits in excess of those seen in wild-type mice of the same genetic background.21 Thus, hyperfibrinogenemia causes an organ-specific, increased fibrin deposition in the spleen. Superimposing the Glu387 = > Pro thrombomodulin mutation onto hyperfibrinogenemia abrogates the splenic fibrin deposition seen in Hifib mice but elicits excess fibrin deposition in the liver. This provides additional evidence that TM deficiency and hyperfibrinogenemia have interacting effects on organ-specific fibrin(ogen) turnover.
Deposition of cross-linked fibrin in tissues of hyperfibrinogenemic mice. Equivalent amounts (normalized to initial wet tissue weight) of the urea-insoluble fraction of tissue extracts from wild-type (wt), thrombomodulin-deficient (TMPro), and hyperfibrinogenemic mice (Hifib and Hifib/TMPro) were subjected to SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis), and the fibrin β-chain (arrows) was detected with fibrin-specific antibodies recognizing the neoepitope generated by thrombin-mediated fibrinopeptide B release. Each lane represents analysis of one organ from one mouse; 6 lanes per mouse strain shown. Total protein content loaded in each lane was roughly equivalent, as ascertained by Poinceau S staining of blots (not shown). Excess fibrin is found only in the spleen of Hifib mice and not in other organs, including liver. Mice with combined hyperfibrinogenemia and thrombomodulin-deficiency (Hifib/TMPro) show excess fibrin in the liver but not in the spleen or other organs (heart, lung, kidney, brain not shown).
Deposition of cross-linked fibrin in tissues of hyperfibrinogenemic mice. Equivalent amounts (normalized to initial wet tissue weight) of the urea-insoluble fraction of tissue extracts from wild-type (wt), thrombomodulin-deficient (TMPro), and hyperfibrinogenemic mice (Hifib and Hifib/TMPro) were subjected to SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis), and the fibrin β-chain (arrows) was detected with fibrin-specific antibodies recognizing the neoepitope generated by thrombin-mediated fibrinopeptide B release. Each lane represents analysis of one organ from one mouse; 6 lanes per mouse strain shown. Total protein content loaded in each lane was roughly equivalent, as ascertained by Poinceau S staining of blots (not shown). Excess fibrin is found only in the spleen of Hifib mice and not in other organs, including liver. Mice with combined hyperfibrinogenemia and thrombomodulin-deficiency (Hifib/TMPro) show excess fibrin in the liver but not in the spleen or other organs (heart, lung, kidney, brain not shown).
Hyperfibrinogenemia suppresses thrombin generation
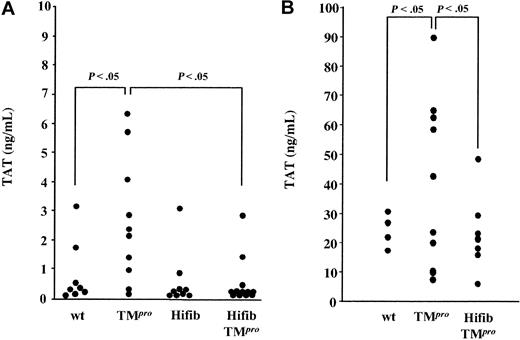
The ambient plasma concentration of the surrogate marker of thrombin generation, thrombin-antithrombin (TAT) complex, was somewhat lower in Hifib than in normal mice (Hifib: 0.5 ± 1.0 ng/mL; wild-type: 0.8 ± 1.0 ng/mL; Figure 3A), but this difference was statistically not significant. Confirming earlier findings,21 TMPro mice exhibited higher TAT levels than wild-type mice (2.6 ± 2.1 ng/mL; P ≤ .05). TAT levels in Hifib/TMPro mice (0.4 ± 0.9 ng/mL) resembled those seen in Hifib mice and were significantly lower than those seen in TMPro mice (P ≤ .05). Injection of 0.05 U of mouse α-thrombin into wild-type and TMPro mice increased plasma TAT levels to 30 ± 7 ng/mL and 56 ± 18 ng/mL, respectively, consistent with increased thrombin-dependent amplification of coagulation in TMPro mice. The same manipulation did not elicit enhanced TAT-complex formation in Hifib/TMPro mice (27 ± 9 ng/mL), as compared to wild-type mice (Figure 3B).
Plasma concentration of thrombin-antithrombin complex in mutant mice. Plasma levels of thrombin-antithrombin (TAT) complexes were measured by ELISA. (A) Steady-state TAT levels in hyperfibrinogenemic mice (Hifib) and in hyperfibrinogenemic mice with superimposed thrombomodulin deficiency (HiFib/TMPro) are comparable to that measured in wild-type mice (wt). (B) Mice were injected intravenously with a bolus of 0.05 U of mouse α-thrombin to induce TAT-complex formation, and TAT levels were measured 10 minutes after thrombin infusion. Hyperfibrinogenemia suppresses the augmentation of TAT formation observed in mice with isolated thrombomodulin deficiency (TMPro). Values are given as apparent TAT concentration determined from a standard constructed with purified human TAT.
Plasma concentration of thrombin-antithrombin complex in mutant mice. Plasma levels of thrombin-antithrombin (TAT) complexes were measured by ELISA. (A) Steady-state TAT levels in hyperfibrinogenemic mice (Hifib) and in hyperfibrinogenemic mice with superimposed thrombomodulin deficiency (HiFib/TMPro) are comparable to that measured in wild-type mice (wt). (B) Mice were injected intravenously with a bolus of 0.05 U of mouse α-thrombin to induce TAT-complex formation, and TAT levels were measured 10 minutes after thrombin infusion. Hyperfibrinogenemia suppresses the augmentation of TAT formation observed in mice with isolated thrombomodulin deficiency (TMPro). Values are given as apparent TAT concentration determined from a standard constructed with purified human TAT.
To further evaluate the effect of fibrinogen levels on thrombin generation, we measured in vitro generation of thrombin in platelet-poor afibrinogenemic mouse plasma (prepared from fibrinogen-deficient mice) that had been repleted with varying amounts of purified mouse fibrinogen (Figure 4). The peak level of thrombin activity, as well as the summed area of the thrombin generation curve, was inversely correlated with the plasma concentration of fibrinogen. This observation suggests that elevated fibrinogen levels diminish thrombin formation in mice, reproducing earlier findings obtained with fibrinogen-repleted plasma from afibrinogenemic human patients.28
Effect of fibrinogen on in vitro thrombin formation. In vitro thrombin generation as a function of plasma fibrinogen concentration was determined by measuring thrombin amidolytic activity generated over time in plasma of fibrinogen-deficient mice reconstituted with varying amounts of mouse fibrinogen. Thrombin generation correlates inversely with plasma fibrinogen concentration.
Effect of fibrinogen on in vitro thrombin formation. In vitro thrombin generation as a function of plasma fibrinogen concentration was determined by measuring thrombin amidolytic activity generated over time in plasma of fibrinogen-deficient mice reconstituted with varying amounts of mouse fibrinogen. Thrombin generation correlates inversely with plasma fibrinogen concentration.
Hyperfibrinogenemia does not accelerate arterial platelet-thrombus formation
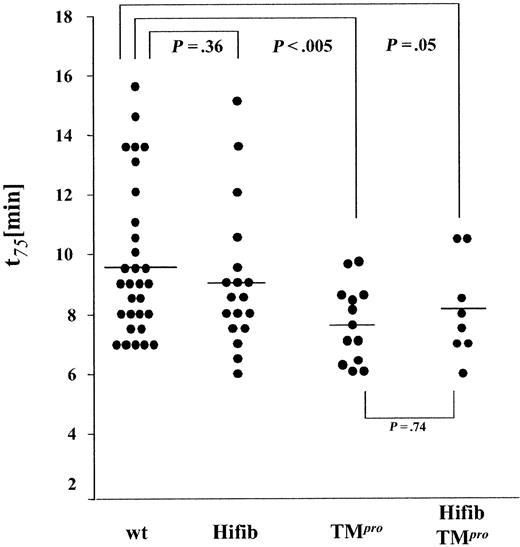
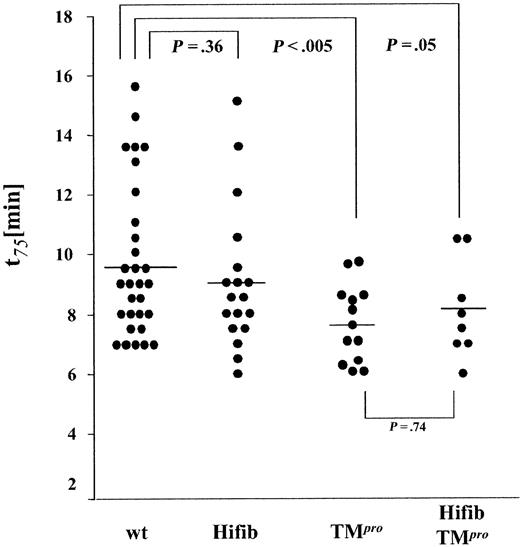
We then examined whether increased levels of fibrinogen in blood led to accelerated formation of an occlusive platelet thrombus. The formation of thrombi in the carotid artery of experimental animals was induced by application of a FeCl3 solution to the external surface of the dissected vessel. The time from application of the solution to a 75% reduction of initial flow (t75) was recorded as a measurement of thrombus growth (Figure 5). The incidence of occlusive thrombus formation was identical in all experimental groups. The occlusion time in Hifib mice was only marginally shorter than that determined in wild-type animals (9.0 ± 2.3 versus 9.7 ± 2.5 minutes ± SD, P = .36). Reproducing earlier findings,21 the average occlusion time in hypercoagulable TMPro mice was significantly shorter than in wild-type controls (7.6 ± 1.3 minutes ± SD; P < .005). Thrombus growth in Hifib/TMPro mice was nearly the same as that observed in TMPro mice (8.1 ± 1.6 minutes ± SD; P = .74 versus TMPro). These data imply that hyperfibrinogenemia does not alter the incidence and extent of platelet thrombus formation, even in sensitized TMPro animals with underlying thrombophilia.
Ferric chloride–induced thrombus formation in hyperfibrinogenemic mice. Thrombus growth was determined by measuring the time required to achieve a 75% reduction of preinjury flow rate. End-point time was not significantly shortened in hyperfibrinogenemic mice (Hifib and Hifib/TMPro) compared to wild-type (wt) or thrombomodulin-deficient mice (TMPro). Average time to 75% flow reduction indicated by horizontal bars.
Ferric chloride–induced thrombus formation in hyperfibrinogenemic mice. Thrombus growth was determined by measuring the time required to achieve a 75% reduction of preinjury flow rate. End-point time was not significantly shortened in hyperfibrinogenemic mice (Hifib and Hifib/TMPro) compared to wild-type (wt) or thrombomodulin-deficient mice (TMPro). Average time to 75% flow reduction indicated by horizontal bars.
Hyperfibrinogenemia does not cause thrombus formation in response to vascular stasis
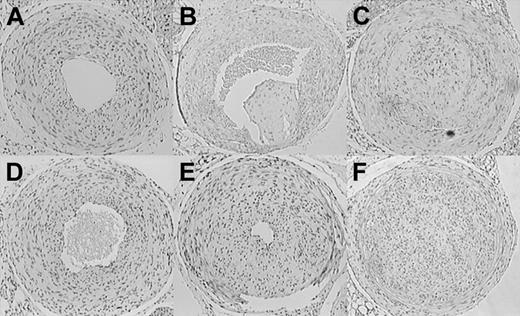
The effect of hyperfibrinogenemia on stasis-induced thrombus formation was investigated by employing an established experimental protocol that reduces blood flow in the common carotid artery to a residual, pulsatile mode and induces reproducible alterations of arterial wall function by a combination of neointimal hyperplasia and vascular remodeling.26,29 The model is distinguished by the continued presence of a functional endothelium and in animals with normal coagulation systems produces little thrombosis that remains limited to the site of the ligation.26,30 Of 12 wild-type mice analyzed, none showed evidence of organized thrombus formation. The incidence of neointima formation was 8/12 in this control group, and all of the ligated vessels had maintained lumen patency (Figure 6A). As shown previously, when applied to hypercoagulable TMPro mice, carotid artery ligation elicits extensive and persistent thrombotic occlusion with subsequent remodeling and thrombus organization.21 These findings were reproduced here in a group of 5 TMPro animals, all of which showed clear evidence of organized thrombi (Figure 6B-C). Two independent control experiments on fibrinogen-deficient animals (n = 6 and 8, respectively) showed that the absence of fibrinogen does not prevent neointimal hyperplasia/vascular remodeling associated with this experimental model (Figure 6D). Intimal hyperplasia was documented in 8 of 12 Hifib mice (Figure 6E). Morphometric analysis of serial sections derived from the ligated arteries showed that hyperfibrinogenemic mice, in comparison to wild-type mice, exhibited significantly reduced cross-sectional lumen area (387 ± 223 versus 499 ± 145 area units ± SD; P < .05). The extent of intimal hyperplasia was greater in Hifib mice (495 ± 394 versus 240 ± 134 area units ± SD; P < .05). All other parameters measured (length of internal and external elastic lamina, vessel circumference, medial area, intimal cell density) were not significantly different between Hifib mice and controls. Likewise, the morphology of the unligated carotid artery was identical in both groups. In 3 of 12 Hifib carotid arteries the lumen was completely replaced by cellular tissue along the entire length of the carotid from the site of the ligation to within a short distance from the aortic arch (Figure 6F). In general, the morphology of the lesions in Hifib mice differed from that seen in prothrombotic TMPro mice. In particular, we noted the absence of irregular tissue fragments in the lumen or in association with segments of the intima, which are characteristic features of the occlusions/organized thrombi in TMPro animals (compare Figure 6C and 6F). In contrast, the ligated carotid artery of Hifib mice resembled the appearance of the nonthrombotic vascular wall of ligated wild-type vessels, consistent with a minimal degree of thrombosis. Ligation of the carotid artery did not alter the plasma concentration of fibrinogen, TAT-complex, D-dimer, or interleukin-6 in this control group, as compared to sham-operated animals (measured at 7, 14, 21, and 28 days after injury; data not shown).
Stasis-induced thrombosis and vascular remodeling in the carotid artery of mutant mice. Representative sections through the ligated carotid artery of mutant mice, obtained 4 weeks after ligation. H&E staining, cell nuclei appear dark. Neointima formation but no thrombosis in wild-type (A) and fibrinogen-deficient (D) mice. Neointima formation and severe thrombosis in thrombomodulin-deficient mice (B-C). Enhanced (E) and in some cases occlusive stenosis/intimal hyperplasia in hyperfibrinogenemic mice (F). Note the absence of organized thrombi in Hifib animals. Original magnification, × 40.
Stasis-induced thrombosis and vascular remodeling in the carotid artery of mutant mice. Representative sections through the ligated carotid artery of mutant mice, obtained 4 weeks after ligation. H&E staining, cell nuclei appear dark. Neointima formation but no thrombosis in wild-type (A) and fibrinogen-deficient (D) mice. Neointima formation and severe thrombosis in thrombomodulin-deficient mice (B-C). Enhanced (E) and in some cases occlusive stenosis/intimal hyperplasia in hyperfibrinogenemic mice (F). Note the absence of organized thrombi in Hifib animals. Original magnification, × 40.
These data show that preexisting hyperfibrinogenemia does not elicit thrombosis in excess of that seen in wild-type animals or alter the incidence of neointima formation in this model. On the other hand, hyperfibrinogenemia modified vascular remodeling elicited by cessation of blood flow, resulting in augmented intimal hyperplasia and narrowing of the vessel lumen.
Discussion
Our findings provide evidence that increased fibrinogen levels elicit augmented fibrin deposition in specific organs and exacerbate neointimal hyperplasia in an experimental model of stasis-induced vascular remodeling. On the other hand, hyperfibrinogenemia did not alter the extent or incidence of arterial thrombosis induced either by acute chemical injury or by stasis of blood flow. Indeed, augmented plasma fibrinogen levels in mice suppressed thrombin generation in plasma in vitro, as well as in the blood of animals subjected to a thrombotic challenge. These data establish a direct cause-effect relationship between a moderate elevation of fibrinogen concentration and alterations of hemostatic system function and of the response to vascular injury, respectively.
The abundance of fibrin degradation products in the plasma of hyperfibrinogenemic mice and the presence of excess fibrin deposition in some organs show that excess fibrinogen available for conversion to fibrin by thrombin leads to augmented fibrin formation. Importantly, this occurs in the absence of preexisting vascular disease or compounding risk factors that might cause a heightened activity of the coagulation system. Only in the spleen does systemically enhanced fibrin generation lead to a substantial accumulation of fibrin. While we have not investigated the basis of this selective effect on the spleen, we suspect that the high rate of platelet biosynthesis and clearance in the mouse spleen might generate a microenvironment that is more susceptible to fibrin deposition. The formation of fibrin degradation products is much more pronounced when hyperfibrinogenemia is superimposed onto a hypercoagulable state secondary to thrombomodulin deficiency. The previously reported augmentation of plasma D-dimer concentration in TMPro mice is caused by a combination of enhanced thrombin formation that produces more fibrin and possibly by enhanced fibrinolytic activity secondary to the suppression of thrombomodulin-dependent formation of the fibrinolysis inhibitor TAFI (thrombin activated fibrinolysis inhibitor).22 Since hyperfibrinogenemia and thrombomodulin dysfunction both determine the rate of fibrin formation, they interact to raise D-dimer levels to a value exceeding a simple additive effect of both mutations. The analysis of Hifib mice with superimposed TM deficiency shows that hyperfibrinogenemia interacts either negatively or positively to determine the net balance between fibrin deposition and removal in an organ-specific manner: in Hifib/TMpro mice, excess fibrin deposition is no longer found in the spleen, but instead occurs in the liver. A similar synergistic effect on liver fibrin deposition is obtained when TM deficiency is combined with a loss of plasminogen activator function.31 A high local concentration of fibrinogen at its site of synthesis might render the liver particularly sensitive to alterations that affect fibrin(ogen) turnover. On the other hand, the beneficial effect of the TMPro mutation on fibrin deposition in the spleen of Hifib mice might be explained by the enhancement of fibrinolysis secondary to diminished activation of TAFI by the TM-thrombin complex. Irrespective of the underlying mechanism, these observations reinforce the notion of organ-specific regulatory mechanisms of coagulation and fibrinolysis that have been documented previously in mice with other mutations affecting hemostatic function.32
Despite the clear evidence for augmented fibrin deposition in the spleen of Hifib mice and in the liver of Hifib/TMpro mice, we were unable to document a compounding effect of hyperfibrinogenemia on acute thrombosis. Elevated fibrinogen levels did not alter the incidence or initial growth of a platelet thrombus formed after chemical injury of the carotid aorta, even in sensitized TMPro animals with underlying thrombophilia. Likewise, increased fibrinogen concentration did not elicit stasis-induced thrombosis in excess of that seen in mice with normal fibrinogen levels. In fact, we noted a slight suppression of TAT complex formation in Hifib mice as compared to wild-type controls. This suppressive effect was substantially more pronounced in Hifib/TMPro mice: hyperfibrinogenemia abrogated elevated TAT complex formation—which is a reliable indicator of the prothrombotic state in TMPro mice—under baseline conditions, as well as after amplifying thrombin generation by infusion of a small amount of human thrombin. Likewise, fibrinogen suppressed in a dose-dependent manner the in vitro generation of thrombin in plasma, consistent with our in vivo observations. This phenomenon might be caused by a competition between fibrinogen and other thrombin substrates for interaction with thrombin and/or by increased sequestration of thrombin by fibrin. The latter is a well-characterized nonsubstrate binding phenomenon in the interaction between human thrombin and human fibrin that is attributed to (1) residual thrombin binding at or near the substrate interaction site in the fibrin E-domain, and (2) binding at a higher-affinity binding site in the γ′-variant of the fibrinogen γ-chain.33-37 The mouse γ-chain does not contain the thrombin-binding sequence motif,38 thus predicting the absence of a high-affinity thrombin-fibrin interaction. A preliminary analysis of binding of mouse thrombin to mouse fibrinogen failed to produce evidence for the existence of a high-affinity thrombin binding component in mouse fibrin (data not shown), consistent with the above prediction. These species-specific properties of fibrinogen must be considered when extrapolating findings in mice to human physiology. For example, one would predict that the high-affinity interaction of thrombin with human γ′-fibrin should significantly enhance the suppressive effect of fibrin on thrombin generation, and the γ′-chain might possess additional properties that affect the formation and structure of the fibrin network.39
Thus, elevated plasma fibrinogen appears to suppress thrombin formation in plasma but also to enhance fibrin deposition in some organs. One explanation for this potential paradox that is consistent with our experimental findings is that thrombin, once formed, may be sequestered on fibrin(ogen) and is then either rapidly cleared from the circulation40,41 or becomes associated with fibrin deposited at the vascular wall. Fibrin-associated thrombin is protected against inactivation by antithrombin and retains activity,42,43 and thereby may lead to the localized propagation and enhancement of fibrin deposition, while at the same time suppressing the formation of thrombin-antithrombin complex.
Hyperfibrinogenemia also significantly enhanced intimal thickening and stenosis after carotid artery ligation. On the other hand, complete absence of fibrinogen did not prevent neointima formation. The latter finding is consistent with observations in afibrinogenemic patients44 and in fibrinogen-knockout mice,45 but contrasts with the reported reduction of intimal hyperplasia upon ancrod-induced defibrinogenation46 in the same experimental model as employed here. However, in contrast to our analysis, the latter study examined lesions forming in close vicinity to the site of the ligation, where substantial mechanical injury occurs. Second, defibrinogenation with Ancrod not only eliminates fibrinogen but also elicits a burst of fibrinolytic activity47 that may exert a similar effect on neointima formation as the enhancement of fibrinolysis secondary to elimination of the fibrinolysis inhibitor, PAI-1.45,48-52 Plasmin-mediated proteolysis affects cell migration and tissue remodeling in the vascular wall through extracellular matrix degradation, activation of procollagenase and metalloproteases, and activation of latent growth factors such as TGFβ.52 Although not strictly required, fibrin(ogen) may augment these processes by providing a fibrin matrix that enhances net plasmin activity. Irrespective of the precise underlying mechanism, our results show that a moderate elevation of fibrinogen concentration may alter the outcome of vascular remodeling. This insight should facilitate the rationale design of future experiments to examine the consequences of hyperfibrinogenemia in models that are more relevant to human pathology. For example, we would predict that hyperfibrinogenemia aggravates advanced atherosclerosis in “humanized” apoE-knockout mice simultaneously expressing apo(a).53 Of note, in agreement with our findings, the latter study showed that the absence of fibrinogen ameliorates atherosclerotic lesion formation only in the presence of human apo(a), but not in normal mice.
In summary, our data provide direct experimental evidence that a moderate elevation of fibrinogen concentration results in augmented fibrin formation and subsequent fibrin degradation, altered coagulation system function, and an altered response to vascular injury. These findings establish that hyperfibrinogenemia is more than a by-product of cardiovascular disease, but may—in a positive or negative manner—determine the severity and/or progression of vascular disease. Of note, our findings predict that species-specific properties of fibrinogen, such as the high-affinity interaction of thrombin with the fibrinogen γ'-chain, may exert physiologically relevant effects in humans that may escape detection in mouse models employed to probe fibrinogen function.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-08-2886.
Supported by the American Heart Association (grant-in-aid 0150403N, H.W.) and the National Institutes of Health (HL60655, H.W.; HL68888, M.W.W.; and HL68836, S.L.).
B.K. and B.C.C. contributed equally to this work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful for the help of Dr David A. Meh and Cara Hartwig in establishing fibrinogen assays and in vitro thrombin generation.