Abstract
We found 10 individuals from 7 unrelated families among 170 severe combined immunodeficiency (SCID) patients who exhibited 9 different Janus kinase 3 (JAK3) mutations. These included 3 missense and 2 nonsense mutations, 1 insertion, and 3 deletions. With the exception of 1 individual with persistence of transplacentally transferred maternal lymphocytes, all infants presented with a T–B+NK– phenotype. The patient mutations all resulted in abnormal B-cell Janus kinase 3 (JAK3)–dependent interleukin-2 (IL-2)–induced signal transducer and activator of transcription-5 (STAT5) phosphorylation. Additional analyses of mutations permitting protein expression revealed the N-terminal JH7 (del58A) and JH6 (D169E) domain mutations each inhibited receptor binding and catalytic activity, whereas the G589S JH2 mutation abrogated kinase activity but did not affect γc association. Nine of the 10 patients are currently alive from between 4 years and 18 years following stem cell transplantation, with all exhibiting normal T-cell function. Reconstitution of antibody function was noted in only 3 patients. Natural killer (NK) function was severely depressed at presentation in the 4 patients studied, whereas after transplantation the only individuals with normal NK lytic activity were patients 1 and 5. Hence, bone marrow transplantation is an effective means for reconstitution of T-cell immunity in this defect but is less successful for restoration of B-cell and NK cell functions.
Introduction
The syndrome of severe combined immunodeficiency (SCID) is characterized by absent T- and B-lymphocyte function and is uniformly fatal in infancy without successful immune reconstitution.1-4 In the 30 years since the discovery that adenosine deaminase (ADA) deficiency causes SCID,5 mutations in 7 additional genes encoding proteins important in lymphocyte development or function have been found to cause this syndrome.6-15 Of these 7, 2 are due to mutations in genes encoding components of cytokine receptors, the common gamma chain (γc)6,7 or the interleukin-7 receptor α chain (IL-7Rα).13-15 γc is a shared element of receptors for interleukins 2, 4, 7, 9, 15, and 21 16-22 and is mutated in X-linked SCID (SCID-X1).6,7 Janus kinase 3 (JAK3), the only member of the Janus family of intracellular protein tyrosine kinases primarily expressed in hematopoetic cells,23-25 associates with γc26 and is required for signal transduction by γc-containing receptors.27 It follows that mutations in human JAK3 have also been reported to result in autosomal recessive SCID, with a clinical phenotype nearly identical to that of SCID-XI 6,7 characterized by absence of circulating T and natural killer (NK) cells with normal numbers of poorly functioning B cells (T–B+NK–).8,9 IL-7Rα mutations impact only the γc- and JAK3-containing receptor for IL-7 and lead to SCID in humans manifested by isolated absence of T cells (T–B+NK+).13-15 The characteristic lymphocyte phenotypes noted in γc-, JAK3-, or IL-7Rα–deficiency in humans are consistent with other data examining function of γc-containing receptors that demonstrate IL-7 receptor signaling is required for T-cell development,28,29 while IL-1530 signaling is important for normal NK cell development. The clinical phenotype of JAK3 deficiency demonstrates the importance of JAK3 in lymphocyte development and function. However, many details of the structure and function of JAK3 remain uncertain. JAK3 protein consists of 7 Janus homology (JH) domains based on sequence homology with other JAKs.31 Adjacent to the C-terminal JH1 catalytic domain of JAK3 is the JH2 pseudokinase domain, a unique structural feature of JAKs. While lacking kinase activity, the JH2 domain is essential for normal JAK3 function by virtue of its complex regulation of catalytic activity and autophosphorylation.32-35 The N-terminal region of JAK3, consisting of the JH7, JH6, and JH5 domains, forms a band 4.1, ezrin, radixin, moesin (FERM) homology protein-protein interaction domain that is required for γc binding and maintenance of normal catalytic activity.36-39 Little is known regarding the functions of the JH4 and JH3 domains, although the JH4 domain contains an SH2 motif with uncertain activity.40
Naturally occurring JAK3 mutations that result in human SCID can serve as valuable tools for delineation of the phenotype and clinical course of JAK3-deficient SCID following bone marrow transplantation and for JAK3 structure-function analysis. To date, only one such evaluation of JAK3 mutations has been reported for an extensive series of patients, the 23 known individuals with JAK3-deficient SCID in Europe.41 The present report extends these observations by analyzing immunologic and clinical data from novel JAK3 mutations found in the first reported series of 10 JAK3-deficient SCID patients from the United States.
Patients, materials, and methods
Patients and immunologic phenotype analysis
Patients described in this study all fulfilled criteria of the World Health Organization for the diagnosis of SCID.2 IL2RG sequence analysis was normal in the 5 male patients. Donor marrow was depleted of T cells by agglutination with soybean lectin, followed by 2 cycles of rosetting with aminoethylisothiuronium bromide–treated sheep erythrocytes, as previously described.42-45 None of the recipients of HLA-identical marrow or T cell–depleted HLA-haploidentical marrow received pretransplantation chemotherapeutic conditioning or posttransplantation graft-versus-host disease (GVHD) prophylaxis. Peripheral blood lymphocyte subsets were analyzed by flow cytometry using commercial antibodies and standard staining techniques. Maternal lymphocytes were detected by restriction fragment length polymorphism (RFLP) analysis, fluorescence in situ hybridization (FISH), karyotyping, or HLA typing using microcytotoxicity, flow cytometry, or polymerase chain reaction (PCR). Serum immunoglobulin levels were measured as previously reported.46,47 Lymphocyte proliferation was assessed by measuring [3H]thymidine incorporation in mononuclear cells following in vitro culture with optimal concentrations of the indicated mitogens, antigens, or irradiated allogeneic cells, as previously described.43 Natural killer cell function was measured as percent specific lysis of 51Cr-labeled K562 target cells by peripheral blood lymphocyte effector cells at 6 effector-target ratios between 100:1 and 3:1. B-cell chimerism was detected on the basis of RFLP or FISH analysis of Epstein-Barr virus (EBV)–transformed B-cell lines. Primary and memory antibody responses to bacteriophage ϕX174 immunization were quantified as described.48 Control subjects were healthy adult volunteers. All studies were done with the approval of the Duke University Health System's Institutional Review Board (IRB) for Clinical Investigations and with the written, informed consent of the patients' parents.
EBV-transformed B-cell lines
B-cell lines were established from peripheral blood mononuclear cells of healthy donors, patients, and their parents by transformation with Epstein-Barr virus (EBV)–containing supernatant and maintained as previously described.49 Patient cell lines used for JAK3 sequence analysis were established prior to bone marrow transplantation, whereas those used for analysis of patient B-cell chimerism were established after transplantation.
Immunoblotting
EBV-transformed B cells were solubilized in 0.5% Triton X-100 lysis buffer. Clarified whole cell lysates were resolved by 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose. Blocked filters were blotted with rabbit polyclonal antibody directed against the C-terminus of JAK3.23 Membranes were then washed and incubated with horseradish peroxidase (HRP)–conjugated goat antirabbit immunoglobulin (Ig) (Organon Teknika, West Chester, PA). Antibody binding was detected by enhanced chemiluminescence (ECL) (Amersham Pharmacia Biotech, Piscataway, NJ). To assess lysate integrity and gel loading, JAK3 blots were stripped and reprobed with rabbit α-STAT5A antiserum (Santa Cruz Biotechnology, Santa Cruz, CA).
Analysis of JAK3 mRNA levels
For Northern blots, total RNA isolated from EBV-transformed B-cell lines by lysis in Trizol reagent (Invitrogen, Carlsbad, CA) was used to prepare polyA-positive RNA (PolyATtract mRNA Isolation System; Promega, Madison, WI). Three micrograms of polyA-positive RNA were electrophoresed in a 1.5% agarose-formaldehyde gel, transferred to a nylon membrane, and UV cross-linked. Transcripts were detected with a 32P-labeled full-length JAK3 cDNA probe, and RNA loading was assessed with a 32P-labeled β-actin cDNA probe. For reverse transcriptase (RT)–PCR analysis, serial dilutions of EBV-transformed B-cell cDNA were PCR amplified using JAK3 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers. Products were resolved on agarose gels, ethidiumstained, and photographed.
JAK3 sequence analysis
PolyA-positive RNA isolated from EBV-transformed B cells as described for Northern blotting was used for random hexamer-primed first-strand cDNA synthesis (SuperScript II Preamplification System; Invitrogen). PCR amplification of cDNA templates with primer pairs –84F/400R (–84F: GGACTGAGGGGCTTTTTCTC; 400R: GGACTGGCAGGTCAAGGATA), 5F/2711R (5F: CACCTCCAAGTGAAGAGACG; 2711R: TACTCCATGACCAGCCGCAGCTCT), and 2322F/3594R (2322F: TCGTGACCTCAATAGCCTCATCTC; 3594R: CCACTGACACATATGCCCATCTGT) generated overlapping products spanning the JAK3 cDNA coding sequence23 that were purified (Qiaex II Gel Extraction Kit; Qiagen, Santa Clarita, CA) and used as templates in sequencing reactions (Big Dye Terminator Cycle Sequencing System; PerkinElmer Life Sciences, Boston, MA). Sequencing reactions representing both strands were analyzed using an ABI 377 Prism DNA (PerkinElmer) instrument and software package. Mutations were confirmed by sequencing patient and parental genomic DNA PCR products amplified from suspect regions using procedures identical to those employed for RT-PCR.
IL-2–induced STAT5 phosphorylation
EBV-transformed B cells were acid washed and resuspended in serum-free RPMI medium at 5 × 106 cells per milliliter; 100 μL aliquots were stimulated with 1000 U/mL IL-2 for 20 minutes at 37°C and then fixed by incubation with 100 μL fixation reagent (Reagent A, Fix & Perm; Caltag Laboratories, Burlingame, CA) for 3 minutes at 25°C. Cells were then vortexed gently while 3 mL ice-cold methanol was added to each tube for 10 minutes at 4°C. Washed cells were incubated with 100 μL permeabilization reagent (Reagent B, Fix & Perm; Caltag) and an optimized amount of unlabeled primary antibody for 30 minutes at 25°C. Cells were washed twice in phosphate-buffered saline (PBS) with 2% fetal calf serum (FCS) and incubated with 1 μg labeled secondary antibody for 30 minutes at 25°C. Primary antibodies utilized included mouse IgG1,κ antiphosphoSTAT5 monoclonal antibody (mAb) (Zymed Laboratories, San Francisco, CA), mouse IgG1,κ (MOPC 31C) clarified ascites isotype control Ab (Sigma, St Louis, MO), rabbit polyclonal IgG α-STAT5a (Santa Cruz Biotechnology), and normal rabbit IgG isotype control Ab (Caltag). Labeled secondary antibodies used were fluorescein isothiocyanate (FITC)–conjugated goat antimouse IgG (Caltag) and goat antirabbit IgG FITC (Caltag). Washed cells were then resuspended in PBS for analysis on a FACScan instrument (Becton Dickinson, San Jose, CA) equipped with CellQuest software. Fluorescence data collected on forward scatter– and side scatter–gated live cells were reported as (1) fluorescence index (FI) = (geometric mean channel fluorescence of stimulated cells)/(geometric mean channel fluorescence of unstimulated cells) and (2) percentage of cells staining positive (% positive) = (percentage of cells staining positive following incubation with the indicated α-STAT Ab and FITC-conjugated secondary Ab) – (percentage of cells staining positive with isotype control Ab plus FITC-conjugated secondary Ab).
Plasmids, mutagenesis, and transfection
Generation of cDNA expression constructs containing wild-type JAK3 (pME18sJak3) or the catalytically inactive K855A mutant (pME18sK855A) has been previously reported.50 JAK3 cDNA expression vectors containing either the del58A, D169E, or G589S mutation were generated by site-directed mutagenesis of pME18sJak3 using the Transformer Site-Directed Mutagenesis Kit (BD Biosciences Clontech, Palo Alto, CA). Chimeric IL-2Rαγγ (Tac-γc) cDNA (Warren Leonard, National Heart, Lung, and Blood Institute, Bethesda, MD) was cloned into the pME18s vector. cDNA constructs (5 μg) were transiently transfected into JAK3-negative COS-7 cells using diethylaminoethyl (DEAE)–dextran (Promega) according to the manufacturer's instructions.
In vitro kinase assay
COS-7 cells were solubilized 48 hours after transfection in 0.5% Brij lysis buffer containing 50 mM Tris (tris(hydroxymethyl)aminomethane) (pH 7.5), 300 mM NaCl, 2 mM EDTA (ethylenediaminetetraacetic acid), 200 μM Na3VO4, and protease inhibitors. Clarified whole cell lysates were immunoprecipitated with rabbit polyclonal antibody directed against the C-terminus of JAK3.23 JAK3 immunoprecipitates washed 3 times in lysis buffer and once in 100 mM NaCl and 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.5) were resuspended in 50 μL kinase reaction buffer containing 1 μCi (0.037 MBq) [γ-32P]adenosine triphosphate ([γ-32P]ATP) (Amersham Pharmacia) for 1 or 5 minutes at room temperature. Eluted samples were resolved by 8% SDS-PAGE, transferred to nitrocellulose, and subjected to autoradiography. The membrane was then immunoblotted with anti-JAK3 Ab to assess gel loading.
JAK3/γc association assay
COS-7 cells coexpressing one wild-type or mutant JAK3 allele and Tac-γc were lysed 48 hours after transfection and immunoprecipitated with anti-Tac Ab (David Nelson, National Cancer Institute, Bethesda, MD). Washed immunoprecipitates were eluted, resolved by 8% SDS-PAGE, and immunoblotted with anti-JAK3 Ab.23 The membrane was then stripped and reprobed with anti-γc Ab (Amersham Pharmacia). Aliquots of cell lysates that had not been subjected to immunoprecipitation were also immunoblotted with anti-JAK3 Ab to quantify expression levels of the various JAK3 proteins.
Results
Analysis of patient JAK3 expression levels and immunologic phenotypes
Investigation of our population of infants with SCID of undetermined etiology revealed 10 individuals from 7 unrelated families who were JAK3 deficient. All patients were from the United States. Five individuals were male, and 5 were female. There were 7 white and 3 African American infants among this group of JAK3-deficient patients. Patients 4, 8, 9, and 10 are full siblings affected with JAK3-deficient SCID, and pretransplantation samples were available from patient 4 for the studies reported here. All patients presented with recurrent infections except the 3 infants (patients 4, 9, and 10) undergoing transplantation in the newborn period. Analyses of JAK3 protein expression in immunoblots of EBV-transformed B-cell lines established at presentation from the 7 unrelated patients undergoing transplantation at our institution are depicted in Figure 1A. In this and similar experiments, diminished levels of JAK3 protein with normal-appearing electrophoretic mobility were observed in lysates from patients 2 and 3 (Figure 1A upper panel, lanes 3 and 4) compared with that noted in the healthy control. JAK3 was not detectable in lysates from patients 1, 4, 5, 6, or 7 (Figure 1A upper panel, lanes 2 and 5-8). Reprobing with antibody directed against signal transducer and activator of transcription-5a (STAT5a) (Figure 1A lower panel) affirmed that the abnormalities in JAK3 protein expression noted in this study were not due to gel loading disparities. As seen in Figure 1B, Northern blot analysis revealed levels of JAK3 mRNA expression in EBV-transformed B-cell lines from 6 of the 7 patients that correlated with levels of detectable JAK3 protein. Specifically, patients 2 and 3 expressed significant levels of both JAK3 protein (Figure 1A upper panel, lanes 3 and 4) and apparently normal-sized JAK3 mRNA (Figure 1B upper panel, lanes 3 and 5), whereas patients 1, 4, 5, and 6 lacked detectable JAK3 protein (Figure 1A upper panel, lanes 2 and 5-7) or mRNA (Figure 1B upper panel, lanes 2, 4, 6, and 7) expression. Sequential hybridization with a labeled actin probe verified comparable loading of all RNA samples in this experiment (Figure 1B lower panel). Semiquantitative RT-PCR also revealed nondetectable levels of JAK3 mRNA expression in EBV-transformed B cells from recently diagnosed patient 7 (Figure 1C).
Analysis of patient JAK3 protein and mRNA expression levels. (A) Lysates of EBV-transformed B cells from a healthy control ([lane 1]) and 7 SCID patients [lanes 2-8] were electrophoresed and immunoblotted with anti-JAK3 Ab (top blot). The membrane was stripped and reprobed with anti-STAT5A antiserum (bottom blot) to assess sample loading. (B) EBV-transformed B-cell RNA samples from a healthy control (lane 1) and SCID patients 1 to 6 (lanes 2-7) were analyzed on a Northern blot sequentially hybridized with 32P-labeled JAK3 (top blot) and actin (bottom blot) probes. (C) EBV-transformed B-cell cDNA from a healthy control (lane 1) and SCID patient 7 (lane 2) were PCR amplified using JAK3- or GAPDH-specific primer pairs and template concentrations previously determined to yield similar quantities of control and patient GAPDH product. Ethidium-stained reaction products are shown.
Analysis of patient JAK3 protein and mRNA expression levels. (A) Lysates of EBV-transformed B cells from a healthy control ([lane 1]) and 7 SCID patients [lanes 2-8] were electrophoresed and immunoblotted with anti-JAK3 Ab (top blot). The membrane was stripped and reprobed with anti-STAT5A antiserum (bottom blot) to assess sample loading. (B) EBV-transformed B-cell RNA samples from a healthy control (lane 1) and SCID patients 1 to 6 (lanes 2-7) were analyzed on a Northern blot sequentially hybridized with 32P-labeled JAK3 (top blot) and actin (bottom blot) probes. (C) EBV-transformed B-cell cDNA from a healthy control (lane 1) and SCID patient 7 (lane 2) were PCR amplified using JAK3- or GAPDH-specific primer pairs and template concentrations previously determined to yield similar quantities of control and patient GAPDH product. Ethidium-stained reaction products are shown.
Analyses of the immunologic phenotype of the patients at the time of diagnosis (Table 1) revealed low numbers of circulating CD3-bearing T cells and CD16+ NK cells but significant numbers of circulating CD20+ B cells (T–B+NK–)8,9 in JAK3-deficient patients 1, 2, 4, 5, 6, and 7. The unexpectedly high number of T and NK cells present in patient 3 was due to documented persistence of transplacentally transferred maternal lymphocytes.51 Fundamental to the diagnosis of SCID, T-cell proliferative responses were also profoundly depressed in each patient at the time of presentation (Table 1). Serum IgA was undetectable in 5 patients, and IgG was undetectable in 2 (Table 1). The number of circulating NK cells present at diagnosis was quite low in all infants except patient 3 who had documented persistence of transplacentally transferred maternal lymphocytes (Table 1; data not shown). NK function was uniformly absent or severely depressed in all patients studied before transplantation (Table 2).
Patient JAK3 gene mutation analysis
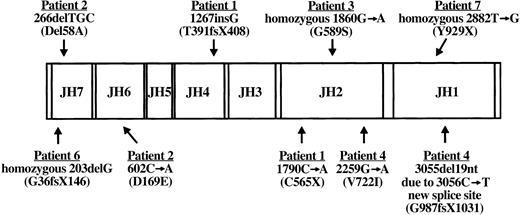
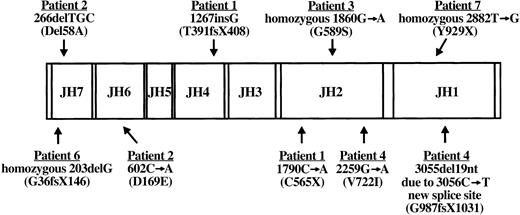
RT-PCR amplification of EBV-transformed B-cell RNA permitted sequence analysis of the entire JAK3 coding region in all 7 JAK3-deficient patients. Patient JAK3 cDNA abnormalities detected by this approach were further evaluated by sequencing patient and parental genomic DNA PCR products amplified from suspect regions. Results of this analysis are shown in Figure 2. Patient 1 mutations have been previously reported.9 She was a compound heterozygote with 2 mutations, the first being a single G insertion following nucleotide 1267 (amino acid 391) in the JH4 domain inherited from her father, whereas the second was a nonsense mutation at nucleotide 1790 (amino acid 565) in the JH2 domain (1790C>A) inherited from her mother.9 The G insertion and resultant frameshift led to premature termination after 17 missense amino acids. Patient 2 was a compound heterozygote with a different N-terminal mutation on each JAK3 allele. The paternal allele of patient 2 exhibited deletion of adjacent nucleotides 266, 267, and 268 (266delTGC) that led to an in-frame deletion of alanine 58 (del58A) in the JH7 domain. Patient 2 inherited a point mutation at nucleotide 602 (602C>A) from his mother that gave rise to the substitution of glutamic acid for aspartic acid at amino acid 169 (D169E) in the JH6 domain. An analysis of the functional consequences of each patient 2 mutation has been reported previously.39 Patient 3 was found to be homozygous for a G to A transition at nucleotide 1860 predicted to change amino acid 589 in the JH2 domain from glycine to serine (G589S). Both parents of patient 3 were heterozygous for the (1860G>A) mutation in exon 11. Patient 4 was a compound heterozygote with 2 different mutations. From her father, she inherited a G>A mutation at nucleotide 2259 that changed amino acid 722 in the JH2 domain from valine to isoleucine (V722I). The second abnormality in patient 4 was a splice mutation inherited from her mother that manifested as a cDNA deletion of nucleotides 3055 to 3073, the 3′ terminal 19 bases encoded by exon 20. Analysis of genomic DNA PCR products amplified from this region revealed a C>T point mutation at nucleotide 3056 in exon 20 that created a new 5′ splice site with 3055G, 3056T. Utilization of this new donor splice site would lead to the observed aberrant removal of 19 nucleotides at the 3′ boundary of exon 20 from the JAK3 transcript. The resultant frameshift, commencing at amino acid 987 in the JH1 domain, would lead to premature termination after 43 missense codons. Despite lacking detectable JAK3 protein or message, no mutations have been identified yet for patient 5. Sequence analysis revealed patient 6 to be homozygous for deletion of a single G nucleotide at position 203 in the JH7 domain that resulted in a frameshift at amino acid 36 and subsequent premature stop codon at amino acid position 146. The mother and father of patient 6 were both carriers of the 203delG mutation. Patient 7 was found to be homozygous for a nonsense T>G mutation at nucleotide 2882 in the JH1 kinase domain (Y929X). The mother of patient 7 was found to be a carrier for the 2882T>G mutation, but his consanguineous father was not available for analysis.
Diagram of patient JAK3 mutations. The coding region of wild-type JAK3 with its 7 JH domains is shown along with JAK3 mutations identified in the 6 SCID patients described in this report. For each patient, the cDNA mutation and resultant protein abnormality (in parentheses) are indicated. Note that patients 1, 2, and 4 are compound heterozygotes bearing 2 different mutations, whereas patients 3, 6, and 7 are homozygous for a single mutation. No mutations have been detected yet in patient 5. Nucleotide numbers correspond to the published cDNA sequence (U09607).23 X indicates stop codon; and fs, frameshift.
Diagram of patient JAK3 mutations. The coding region of wild-type JAK3 with its 7 JH domains is shown along with JAK3 mutations identified in the 6 SCID patients described in this report. For each patient, the cDNA mutation and resultant protein abnormality (in parentheses) are indicated. Note that patients 1, 2, and 4 are compound heterozygotes bearing 2 different mutations, whereas patients 3, 6, and 7 are homozygous for a single mutation. No mutations have been detected yet in patient 5. Nucleotide numbers correspond to the published cDNA sequence (U09607).23 X indicates stop codon; and fs, frameshift.
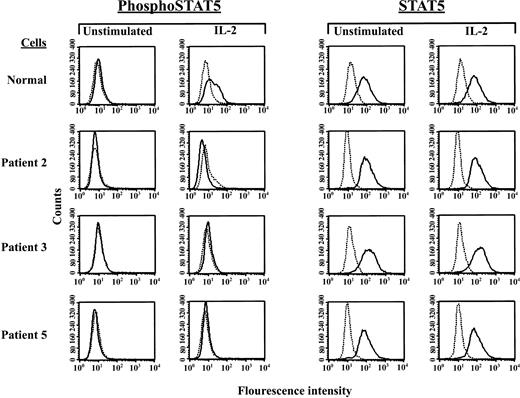
Defective IL-2–induced phosphorylation of STAT5 in JAK3-deficient SCID patient B cells
We next wished to examine whether the patient JAK3 gene sequence alterations and diminished JAK3 protein expression levels we detected had demonstrable functional consequences by analyzing JAK3-dependent, IL-2–induced STAT5 phosphorylation52 in patient EBV-transformed B cells using a sensitive flow cytometric assay.53 In these experiments, resting and IL-2–stimulated cells were fixed and permeabilized to allow intracellular staining with antibodies specific for either total STAT5 (STAT5) or the IL-2–activated, tyrosine-phosphorylated form of STAT5 (phosphoSTAT5). Antibody binding was then detected by flow cytometry and used to quantify IL-2–induced STAT5 phosphorylation by comparing (1) geometric mean channel phosphoSTAT5 fluorescence of stimulated and unstimulated cells and (2) the percentage of stimulated versus unstimulated cells staining positive for phosphoSTAT5.
As seen in Figure 3, IL-2–induced STAT5 phosphorylation was readily detectable in normal EBV-transformed B cells by this technique. In this experiment, the fluorescence index for phosphoSTAT5 of IL-2–treated normal control cells was 1.6, and the percentage of cells staining positive for phosphoSTAT5 increased from 0% to 18.1% following stimulation (Figure 3), while control total STAT5 staining remained essentially unchanged (Figure 3). Phosphorylation of STAT6, which is not induced by IL-2 binding, was not detected in these experiments (data not shown). In contrast to the results noted with normal control EBV-transformed B cells, STAT5 phosphorylation was not observed following IL-2 stimulation of EBV-transformed B cells established prior to bone marrow transplantation from any of the SCID patients studied (Figure 3). Shown in Figure 3 are single-color histograms of phosphoSTAT5 and total STAT5 staining of IL-2–stimulated cells from the 2 patients with JAK3 mutations and detectable levels of JAK3 protein (patients 2 and 3) and from patient 5, the infant who lacked detectable JAK3 mRNA and protein expression but had no identifiable JAK3 gene mutations. Similar results were obtained from an identical experiment performed with cells from the 4 patients with JAK3 gene mutations and no detectable JAK3 protein expression (patients 1, 4, 6, and 7) (data not shown).
Lack of IL-2–induced STAT5 phosphorylation by SCID patient EBV-transformed B-cell lines. Depicted are 1-color fluorescence histograms of healthy volunteer or SCID patient cells that had been incubated for 20 minutes with or without 1000 U/mL IL-2, fixed and permeabilized, subjected to indirect staining with Ab specific for tyrosine-phosphorylated STAT5 (phosphoSTAT5) or total STAT5 (STAT5), and analyzed by flow cytometry. Dashed lines represent staining with isotype control Ab and FITC-labeled secondary Ab, whereas solid lines signify staining with the indicated anti-STAT Ab and FITC-conjugated secondary reagent.
Lack of IL-2–induced STAT5 phosphorylation by SCID patient EBV-transformed B-cell lines. Depicted are 1-color fluorescence histograms of healthy volunteer or SCID patient cells that had been incubated for 20 minutes with or without 1000 U/mL IL-2, fixed and permeabilized, subjected to indirect staining with Ab specific for tyrosine-phosphorylated STAT5 (phosphoSTAT5) or total STAT5 (STAT5), and analyzed by flow cytometry. Dashed lines represent staining with isotype control Ab and FITC-labeled secondary Ab, whereas solid lines signify staining with the indicated anti-STAT Ab and FITC-conjugated secondary reagent.
Abnormal in vitro kinase activity of mutants derived from patients expressing detectable levels of JAK3 protein
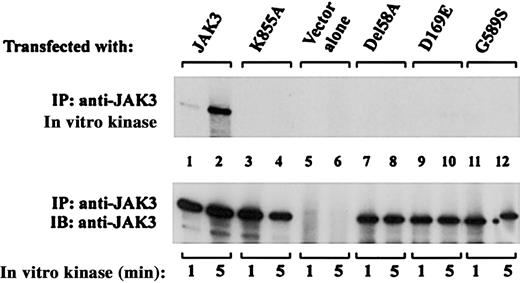
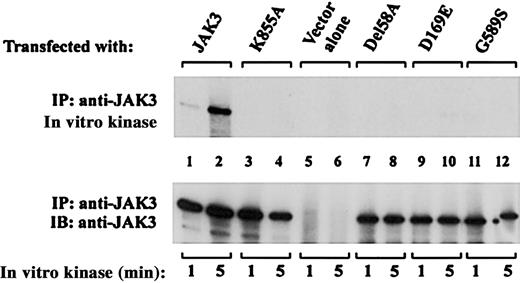
The experiment presented in Figure 3 demonstrated that JAK3-dependent STAT5 phosphorylation was abnormal in B cells from the 7 patients studied. However, for patients 2 and 3 who expressed detectable levels of JAK3 protein it is unclear whether this result represented (1) qualitatively normal JAK3 signal transduction that was attenuated due to the presence of limiting amounts of JAK3 protein or (2) defective signaling by an inherently abnormal JAK3 protein. Interpretation of these data is further complicated by the coexistence of 2 different mutant JAK3 alleles in EBV-transformed B cells from compound heterozygote patient 2 and uncertainty regarding the relative contributions of each mutant to the observed phenotype (Figure 3). To address these concerns, we next analyzed the catalytic activity of each patient 2 and patient 3 mutant JAK3 protein expressed individually in COS cells. JAK3 cDNA expression constructs containing either the del58A or D169E mutation from patient 2, or the patient 3 G589S mutation, were generated by site-directed mutagenesis and transiently transfected into JAK3-negative COS-7 cells. Autophosphorylation of immunoprecipitated JAK3 from transfected cells was then assessed by in vitro kinase assay. As seen in Figure 4, control wild-type JAK3 displayed brisk catalytic activity (Figure 4 upper panel, lanes 1 and 2), while the previously described catalytically inactive control construct bearing a K855A mutation within the ATP binding site50 had no demonstrable kinase activity (Figure 4 upper panel, lanes 3 and 4). Lack of [γ-32P]ATP incorporation was likewise noted in JAK3 immunoprecipitates from COS-7 cells transfected with pME18s vector alone (Figure 4 upper panel, lanes 5 and 6), confirming the absence of detectable background kinase activity under these experimental conditions. In contrast to wild-type JAK3, none of the 3 patient-derived mutants exhibited detectable kinase activity in this experiment (Figure 4 upper panel, lanes 7-12). Reprobing of the membrane with anti-JAK3 antibody affirmed comparable loading of the different JAK3 constructs (Figure 4 lower panel, lanes 1-4 and 7-12) and lack of endogenous JAK3 expression by COS-7 cells (Figure 4 lower panel, lanes 5 and 6) in this experiment. Taken together, these data support the hypothesis that the abnormal JAK3 signaling displayed by del58A, D169E, and G589S mutant proteins was not simply due to limited expression of an otherwise normal JAK3 kinase. Rather, each of the 3 mutant JAK3 proteins exhibits inherently abnormal catalytic function that is not corrected by overexpression in COS-7 cells.
Mutants derived from patients 2 and 3 lack JAK3 catalytic activity. COS-7 cells transfected with the indicated cDNAs were lysed and immunoprecipitated with anti-JAK3 antibody. In vitro kinase assays of JAK3 autophosphorylation were performed at room temperature on the immunoprecipitates for 1 or 5 minutes as indicated. Samples were then resolved by SDS-PAGE, transferred to nitrocellulose, and subjected to autoradiography to visualize32P incorporation (top blot). The membrane was probed with anti-JAK3 Ab to assess expression level and gel loading of the various JAK3 proteins (bottom blot).
Mutants derived from patients 2 and 3 lack JAK3 catalytic activity. COS-7 cells transfected with the indicated cDNAs were lysed and immunoprecipitated with anti-JAK3 antibody. In vitro kinase assays of JAK3 autophosphorylation were performed at room temperature on the immunoprecipitates for 1 or 5 minutes as indicated. Samples were then resolved by SDS-PAGE, transferred to nitrocellulose, and subjected to autoradiography to visualize32P incorporation (top blot). The membrane was probed with anti-JAK3 Ab to assess expression level and gel loading of the various JAK3 proteins (bottom blot).
Effect of JAK3 mutations on receptor association
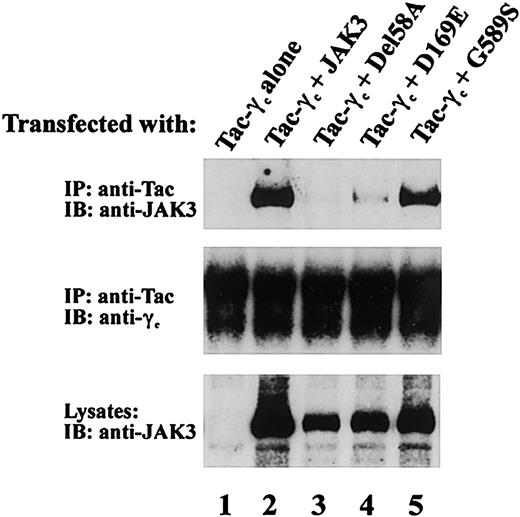
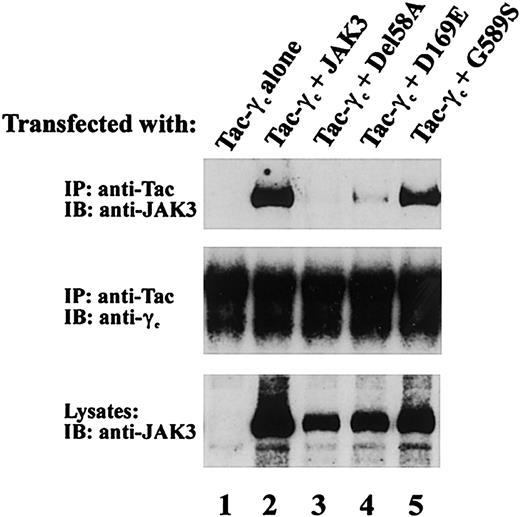
To further characterize the functional phenotype of JAK3 mutations from patient 2 and patient 3 that permitted protein expression, we examined the ability of del58A, D169E, and G589S mutants to associate with γc. JAK3-γc binding is required for IL-2 signaling and was assessed in COS-7 cells coexpressing one mutant JAK3 allele and Tac-γc, a chimeric construct containing the cytoplasmic and transmembrane domains of γc fused to the IL-2Rα chain extracellular domain. Use of Tac-γc in these studies allowed ready detection of receptor-JAK3 complexes54 as seen in Figure 5 where anti-Tac mAb coprecipitated JAK3 from cells expressing Tac-γc and wild-type JAK3 (Figure 5 upper panel, lane 2) but not Tac-γc alone (Figure 5 upper panel, lane 1). Analysis of receptor association by patient JAK3 proteins revealed the G589S mutant found in patient 3 also displayed substantial γc binding (Figure 5 upper panel, lane 5). In contrast, γc binding was severely affected by both patient 2 JAK3 mutations (Figure 5 upper panel), because no receptor interaction was noted with del58A (Figure 5 upper panel, lane 3) and minimal detectable JAK3 coprecipitated with Tac-γc in cells expressing D169E (Figure 5 upper panel, lane 4) despite similar levels of Tac-γc (Figure 5 middle panel) and JAK3 (Figure 5 lower panel) expression in these cells. These findings demonstrate that the JH2 domain mutation G589S did not interfere with receptor association whereas the 2 N-terminal mutations, del58A in JH7 and the JH6 substitution D169E, each had a significant adverse effect on γc binding in this assay system.
Effects of patient JAK3 mutations on JAK3/γc association. COS-7 cells were transfected with 5 μg of the indicated cDNAs. Cell lysates were immunoprecipitated with anti-Tac antibody and blotted with an anti-Jak3 antibody (top blot) or anti-γc (middle blot). Lysates were also blotted with anti-JAK3 Ab (bottom blot) to assess expression of the various JAK3 proteins.
Effects of patient JAK3 mutations on JAK3/γc association. COS-7 cells were transfected with 5 μg of the indicated cDNAs. Cell lysates were immunoprecipitated with anti-Tac antibody and blotted with an anti-Jak3 antibody (top blot) or anti-γc (middle blot). Lysates were also blotted with anti-JAK3 Ab (bottom blot) to assess expression of the various JAK3 proteins.
Posttransplantation course of JAK3-deficient SCID patients
Table 3 lists the number and type of stem cell transplantations used in treatment of the 10 patients in our series, 9 of whom are alive from between 4 years and 18 years after transplantation and are not prone to recurrent or opportunistic infections.45 Patient 1 was the only individual to receive chemotherapeutic conditioning that was given prior to a successful cord blood transplantation performed after 2 T cell–depleted haploidentical transplantations had resulted in T-cell chimerism but no improvement in T-cell function. Patient 3, who had persistence of transplacentally acquired maternal lymphocytes with grade I GVHD before transplantation, received an unfractionated HLA-identical transplant from his sister without preconditioning. After transplantation he developed grade III acute graft-versus-graft disease—successfully treated with steroids and cyclosporine—and a severe idiopathic seizure disorder that has persisted.51 Patient 7, who is deceased, failed to show evidence of T-cell function following 2 maternal T cell–depleted haploidentical transplantations. However, his course was complicated by a persistent vaccine-derived varicella infection and a drug-resistant Candida albicans infection, both present at the time of SCID diagnosis at age 14 months. With the exception of patient 7, all other JAK3-deficient SCID patients in our series have displayed development of 100% donor-origin T cells with an increase in numbers of circulating CD3+ cells and T-cell subsets (Table 4) and normalization of in vitro T-cell proliferative responses (Table 5).45 Restoration of B-cell function has been less effective, with most B cells in these individuals remaining of host origin, even following transplantation of HLA-identical marrow in patients 3 and 5 (Table 6).45 In addition, patient 6 underwent a second T cell–depleted maternal transplantation in an unsuccessful attempt to reconstitute humoral immunity in the face of long-standing normal T-cell function. The sole patient with 100% donor B cells in our series is patient 1, the only infant who underwent chemoablation (Table 6). Comparison of serum Ig levels before and after transplantation in patients 1 to 6 (Tables 1 and 6) revealed increased quantities of IgA or IgM after transplantation in all individuals. Available posttransplantation data were similar for patients 8 to 10 (Table 6). However, patients 3, 4, 6, 8, 9, and 10 remain on intravenous immune globulin (IVIG) because of persistently abnormal antibody responses to bacteriophage ϕX174 immunization.48 All patients mounted normal or near-normal IgM responses to ϕX174 but failed to demonstrate significant class switching to IgG (Table 7). After transplantation, the only individuals with significant improvement in numbers of circulating CD16+ cells and normal NK lytic activity were patients 1 and 5 (Table 2). Patient 1 underwent chemoablation, while patient 5 received HLA-identical marrow. Patient 4 demonstrated an increase in the number of CD16-bearing cells after transplantation without any demonstrable increase in NK function (Table 2).
Discussion
This report presents an analysis of the first reported series of JAK3-deficient SCID patients from the United States, consisting of 10 individuals from 7 unrelated families among the group of 170 SCID patients evaluated to date at Duke University Medical Center.4,45 This 5.9% prevalence of JAK3 deficiency makes it the fourth most common known form of SCID noted in our patient population behind SCID-X1 (78 of 170; 45.9%), ADA deficiency (28 of 170; 16.5%), and IL-7Rα chain deficiency (16 of 170; 9.4%) (J.L.R., S.M.B., R.H.B., unpublished data, June 2003). To our knowledge, only one of the mutations noted in our group of patients, 2259G>A, has been previously reported in other series, in one parent of a SCID patient who died before the infant's JAK3 gene sequencing could be performed.41
Functional examination of patient JAK3 mutations revealed that JAK3-dependent IL-2–induced STAT5 phosphorylation was absent in available EBV-transformed B-cell lines established from patients 1 to 7 prior to bone marrow transplantation (Figure 3). Further analysis of patient del58A, D169E, and G589S mutations that allowed protein expression revealed each transfected mutant protein exhibited severely diminished in vitro kinase activity (Figure 4), whereas del58A and D169E also hindered receptor binding (Figure 5). Taken together, these results demonstrate that the patient JAK3 sequence abnormalities we detected were diseasecausing mutations. The observed phenotype of del58A and D169E mutant proteins is explained by the fact that each resides within the 300–amino acid N-terminal FERM domain of JAK3 that mediates receptor binding and regulates catalytic activity of the distal C-terminal kinase domain.36-39 G589S resides within the JH2 domain, the site of the largest number of reported JAK3 sequence abnormalities with 3 of 9 mutations in the present series and 13 of 27 mutations reported from SCID patients in Europe41,55 involving this region. Previous reports demonstrated that, like the G589S mutant protein examined in this report, SCID patient JH2 domain mutations C759R and del586-592 each associated normally with γc but lacked in vitro kinase activity.33 These findings demonstrate that a nonconservative substitution of serine for glycine at residue 589 alone results in a similar phenotype, in terms of catalytic activity and γc binding, to that observed when G589 is the central residue deleted in the more extensive del586-592 mutation.33 While formal interpretation of this finding awaits delineation of the JAK3 crystal structure, it suggests residue 589 plays a significant role individually in the regulation of JAK3 kinase activity. However, regulation of catalytic activity by JH2 appears not to be restricted to residues 586-592 because patient C759R and a constructed E639K JH2 mutation also abrogated kinase activity.33
The current study also provides the second set of reported mutations in the JAK3 JH1 kinase domain. Patient 7 was found to be homozygous for the nonsense mutation Y929X that lies upstream of the putative activation loop, while 1 of the 2 mutations detected in compound heterozygote patient 4 (3056C>T) led to aberrant splicing and frameshift at G987 in the activation loop itself. Neither patient 7 nor patient 4 expressed detectable JAK3 protein. These JH1 domain mutations are distinct from the 3 previously reported L910S, Y1023X, and C1024fsX1037 kinase domain mutations.41,55 As previously mentioned, no JAK3 mutations have been found to date in patient 5 following sequence analysis of the cDNA open reading frame, all exons and surrounding intron splice sites in genomic DNA, and potential regulatory elements (J.O'S., unpublished data, June 2003). This individual may prove to have mutations in as yet to be identified cis-acting JAK3 regulatory elements or other genes encoding proteins that positively regulate JAK3 expression.
Nine of the 10 JAK3-deficient patients in this report are currently alive from between 4 years and 18 years after transplantation, with all 9 exhibiting normal T-cell function. These results confirm and extend our previous observation that bone marrow transplantation is an effective life-saving treatment modality for reconstitution of T-cell immunity in this defect.45 Restoration of B-cell function has been less successful in this group of patients, with 6 of 9 still requiring monthly IVIG therapy for persistently abnormal antibody function. While true that patient 1 in this report had 100% donor B cells and normal antibody function following chemoablation, the experience from a larger series of patients in Europe has demonstrated the use of preconditioning regimens does not enhance development of B-cell chimerism or function following bone marrow transplantation for T–B+ SCID.56 A potential basis for the abnormal B-cell function noted in JAK3-deficient patients after transplantation is the fact that most B cells in these individuals remain of host origin, even following transplantation of HLA-identical marrow in patients 3 and 5 (Table 6). The residual host-origin B cells in these patients are JAK3 deficient and likely exhibit suboptimal in vivo IL-4 and IL-21 signaling required for normal B-cell activation and class switching.57-61 The observed phenotype of JAK3-deficient patient ϕX174 antibody responses after transplantation is consistent with this notion, as is the finding that residual γc-deficient host B cells have remained poorly functional in 36 of 48 patients with related SCID-X1 undergoing transplantation at our institution.45
NK cell numbers at presentation in the group of JAK3-deficient infants reported here were quite low in all instances except patient 3, the single individual with documented persistence of maternal lymphocytes. Again, this finding is similar to that noted in our larger group of patients with SCID-X1 due to mutations in the γc protein that physically associates with JAK3 in cell surface receptors for several cytokines, including IL-1521,30 and IL-21,62,63 that are important in the regulation of NK cell development and function. Reconstitution of NK cell lytic activity was only noted in patient 5 after transplantation of HLA-identical marrow and in patient 1 following unrelated cord blood transplantation with preconditioning.
Recent reports have demonstrated sustained reconstitution of T- and B-cell function in 5 newly diagnosed SCID-X1 patients up to 2.5 years following retroviral gene therapy without preconditioning.64,65 The clinical and biochemical similarities between SCID-X1 and JAK3 deficiency, along with reported successful T- and B-cell reconstitution in JAK3–/– mice by retroviral gene therapy,66-68 suggested the T- and B-cell defects in newly diagnosed JAK3-deficient human SCID infants might also be correctable by gene therapy. However, 2 of the gene therapy–corrected children with X-linked SCID unfortunately developed leukemia approximately 3 years after the gene transfer treatments. Reports of overexpression or constitutive activation of JAK kinases associated with malignant transformation also make the regulation of transduced JAK3 expression in gene therapy recipients an area of potential concern.69-73 In light of these serious considerations, allogeneic bone marrow transplantation will likely remain the treatment of choice for infants with SCID for the foreseeable future. While marrow transplantation resulted in life-saving T-cell reconstitution in 9 of the 10 JAK3-deficient patients in this report, it has not been a perfect therapy, because B-cell function has only developed in 3 patients and NK function has normalized in just 2 individuals after transplantation. Because there is no need for pretransplantation chemotherapy or GVHD prophylaxis for successful immune reconstitution by transplanted HLA-identical or haploidentical allogeneic marrow stem cells, these human SCID chimeras are a unique model for studying human T, B, and natural killer cell development. Longitudinal analyses of immune function in these individuals will provide an opportunity to gain insight into possible causes of the imperfections of bone marrow transplantation in JAK3-deficient and other forms of SCID and aid in the development of more effective therapies.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-06-2104.
Supported by National Institutes of Health grants HD35961, AI42951, and AI47605, and by grant M01-RR-30 from the National Center for Research Resources, General Clinical Research Centers Program, National Institutes of Health.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Jennifer Puck and Amy Hsu for providing patient IL2RG sequence information and help with the STAT phosphorylation assay. We are also grateful to Drs Warren Leonard and David Nelson for providing reagents.

![Figure 1. Analysis of patient JAK3 protein and mRNA expression levels. (A) Lysates of EBV-transformed B cells from a healthy control ([lane 1]) and 7 SCID patients [lanes 2-8] were electrophoresed and immunoblotted with anti-JAK3 Ab (top blot). The membrane was stripped and reprobed with anti-STAT5A antiserum (bottom blot) to assess sample loading. (B) EBV-transformed B-cell RNA samples from a healthy control (lane 1) and SCID patients 1 to 6 (lanes 2-7) were analyzed on a Northern blot sequentially hybridized with 32P-labeled JAK3 (top blot) and actin (bottom blot) probes. (C) EBV-transformed B-cell cDNA from a healthy control (lane 1) and SCID patient 7 (lane 2) were PCR amplified using JAK3- or GAPDH-specific primer pairs and template concentrations previously determined to yield similar quantities of control and patient GAPDH product. Ethidium-stained reaction products are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/6/10.1182_blood-2003-06-2104/6/m_zh80060458310001.jpeg?Expires=1767903809&Signature=B52XxdJOBe-SzJaE4MBa9aDG2G~-rqYZAbZ3htg8A6z78P4gZhfezZsBK-HKqBJp58ONB2DIScJHkcJZCxO~VQWWrCv28VhG0ha1OOO2Y8yDqehY76quJvP3as2B54ink-tCfdYTYmQ2je14frm2Y4OHysRDvK~o7uW0EhwBQCxNcO1F9JGRqYGQA7qpwp0BeiDajvpux5J0jKEh32YhAOnfzB5ChXh1PNGfIzl9D1bNlooeisphPPZqyIyTyoDDhvqB38b3zBhuCupQlyeoRx~NUOQPEnuxFEGREq8i96vD8PsbSFlyWmoCfyPE7dPEFeeT3lNPvz2uQ1EkoLqOLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Analysis of patient JAK3 protein and mRNA expression levels. (A) Lysates of EBV-transformed B cells from a healthy control ([lane 1]) and 7 SCID patients [lanes 2-8] were electrophoresed and immunoblotted with anti-JAK3 Ab (top blot). The membrane was stripped and reprobed with anti-STAT5A antiserum (bottom blot) to assess sample loading. (B) EBV-transformed B-cell RNA samples from a healthy control (lane 1) and SCID patients 1 to 6 (lanes 2-7) were analyzed on a Northern blot sequentially hybridized with 32P-labeled JAK3 (top blot) and actin (bottom blot) probes. (C) EBV-transformed B-cell cDNA from a healthy control (lane 1) and SCID patient 7 (lane 2) were PCR amplified using JAK3- or GAPDH-specific primer pairs and template concentrations previously determined to yield similar quantities of control and patient GAPDH product. Ethidium-stained reaction products are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/6/10.1182_blood-2003-06-2104/6/m_zh80060458310001.jpeg?Expires=1767989889&Signature=DtjEAKzXcLcvSWYLLBf4q4O2w39DDbPlAEBUn0E4hOh36KCLn3cp6nqAv9S-LvnaUc~3ounSEzbwKj-NXxU0R33ty-cD4Nd0JTatnW1T1Eq0qaB6mw8oRAOg8xFCKpjo~vg7oPgw-hIO5yWcS2p8o9pe96NH-vwAUXcx6AKMpBcWmNRKRVUCWvugfayt3KEmcP8ZCzHZoRtEPVEz1q189yRqdTFdeZUtztQ78IdavP5aV4kWAgbu8733WoofF2U56NTIueF4PkJiE90-gYema3RkJqQJT6CvwGP7-QYLzhLbTWCFtkFxlQ1H-~0iemm4cStm5dApXqmh6uUKGUMhVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)