Abstract
Fanconi anemia (FA) is an autosomal recessive syndrome featuring diverse symptoms including progressive bone marrow failure and early occurrence of acute myeloid leukemia. Nine genetic subtypes have been described for FA (A, B, C, D1, D2, E, F, G, and L), all of which have been connected to distinct disease genes, except B. Here we report on 8 unrelated FA patients who were excluded from the known subtypes on the basis of phenotypic correction or genetic data. Four of these cell lines failed to complement each other in somatic cell hybrids and therefore represent a new group, termed FA-I. The remaining cell lines complemented group FA-I but did not complement each other, thus representing a second new group, FA-J. Both FA-I and -J cell lines were capable of forming an FA multiprotein core complex. This complex is required for activation of the FANCD2 protein by mono-ubiquitination, a key downstream event in the FA pathway. In FA-I cells FANCD2 was not mono-ubiquitinated, indicating a defect upstream in the FA pathway, whereas in FA-J cells FANCD2 was mono-ubiquitinated, indicating a downstream defect. Our results suggest that the FA pathway of genome stabilization may be controlled by at least 11 different genes, including FANCI and FANCJ.
Introduction
Fanconi anemia (FA) is an autosomal recessive disorder characterized by diverse clinical symptoms including congenital abnormalities and a predisposition to bone marrow failure and malignancies.1-3 Cells from FA patients exhibit spontaneous chromosomal instability and are hypersensitive to chromosomal breakage by cross-linking agents such as diepoxybutane (DEB) and mitomycin C (MMC). This trait has been exploited to assess genetic heterogeneity in FA patients through complementation analysis. Eight complementation groups have been distinguished: A, B, C, D1, D2, E, F, and G.4-6 For 6 of these groups distinct disease genes have been identified: FANCA,7,8 FANCC,9 FANCD2,6 FANCE,10 FANCF,11 and FANCG/XRCC9.12 Four FA-D1 patients were shown to possess biallelic mutations in the breast cancer susceptibility gene BRCA2,13 suggesting that BRCA2 is a disease gene for FA and that the BRCA2 polypeptide is functionally connected to the FA pathway. Recently, a new FA gene (FANCL) was discovered based on protein association studies.14 A key event in the FA pathway is the activation of FANCD2 by mono-ubiquitination, which critically depends on the association of FANCA, -C, -E, -F, -G, and -L in a nuclear multiprotein “core complex,” in which FANCL is likely to function as the E3 ubiquitin ligase.14 The activated isoform of FANCD2 (FANCD2-L) has been shown to interact and colocalize with the breast cancer susceptibility gene product BRCA1 in DNA-damage—inducible subnuclear foci.15 Current data thus suggest the existence of an integrated FA/BRCA pathway of genomic maintenance. However, the precise molecular mechanisms, function, and the number of participating components are unknown. Here we provide evidence for the existence of at least 2 additional FA genes whose encoded proteins are likely to play an essential role in the FA/BRCA pathway.
Materials and methods
Patient cell lines
Epstein Barr virus—immortalized lymphoblastoid cell lines were established from blood samples derived from FA patients who had been referred to the European Fanconi Anemia Research (EUFAR) cell repository for genetic subtyping, as described.16 Cell lines BD952 and AG656 were obtained from the European Collection of Cell Cultures (www.ecacc.org.uk). The FA diagnosis was based on clinical symptoms suggestive of FA in combination with a positive result from a chromosomal breakage test using MMC or DEB as a cross-linking agent. Recorded clinical symptoms were as follows: patient EUFA592 died at 6.5 years of age from aplastic anemia; patient BD952 was diagnosed at 8 years because of aplastic anemia; patient EUFA816 died with aplastic anemia at 9 years of age; patient EUFA961 had hematological symptoms but managed quite well with androgens (at 18 years of age). Patient EUFA543 was diagnosed at 3 years of age with café-au-lait spots, thumb abnormalities, abnormal kidney, microphthalmia, microcephaly, and growth delay; aplastic anemia started at age 6. Patient AG656 had neonatal hydrocephalus, retarded growth, and skeletal abnormalities. Patient EUFA696 had growth retardation and skeletal abnormalities and died from aplastic anemia at 7 years of age. Patient EUFA1333 had thumb abnormalities, café-au-lait spots, low birth weight, genital abnormalities, microcephaly, and kidney/urinary tract abnormalities, but normal bone marrow (at age 2 years). All FA cell lines used in this study were hypersensitive to growth inhibition by MMC relative to those from healthy controls. Reference cell lines representing the known complementation groups were those described previously.4 Cell line EUFA1289, derived from an FA-D2 patient, was used as a negative control in FANCD2 Western blot analysis. Approval was obtained from the VU University Medical Center's institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Cell culture
Cell lines were grown in RPMI 1640 culture medium supplemented with 1 mM glutamine (Gibco, Invitrogen, Carlsbad, CA), 1 mM sodium pyruvate, and 10% fetal calf serum (FCS; Gibco). Selection media contained in addition (combinations of) the antibiotic G418 sulfate/neomycin (400 μg/mL Geneticin; Gibco), hygromycin B (100 μg/mL; Roche, Basel, Switzerland), or hypoxanthine (13.6 μg/mL)/aminopterin (0.176 μg/mL)/thymidine (3.88 μg/mL) known as HAT (Gibco). For the induction of ubiquitinated FANCD2, cells were treated with either 100 nM MMC or 1 mM hydroxyurea (HU, Sigma-Aldrich, St Louis, MO) for 24 hours.
Selection markers
The patient cell lines EUFA592, EUFA816, EUFA961, EUFA696, and AG656 were transfected with pSV2neo to generate sublines with stable resistance to G418/neomycin. Cell lines EUFA592, BD952, EUFA696, and EUFA543 were transfected with pSV2hph conferring hygromycin resistance, as described.17 Hybrids of neomycin- and hygromycinresistant fusion partners were selected in medium containing both compounds (400 μg/mL and 100 μg/mL, respectively). Most reference cell lines for the known complementation groups were doubly marked (NT), having next to G418/neomycin resistance (N) a mutation in HPRT, conferring resistance to 6-thioguanine (T).16 The HPRT mutation also confers sensitivity to hypoxanthine, aminopterine, and thymidine (HAT), which blocks de novo synthesis of purines and kills HPRT- cells. Hybrids between NT-marked and unmarked cell lines were selected in medium containing both HAT and G418/neomycin. Hybrids with the reference group A cell line HSC72OT were selected in medium supplemented with HAT and ouabain (738 ng/mL).16
Cell fusion
Polyethylene glycol (PEG)—induced fusions were carried out as described,16 using as fusion partners the doubly marked cell lines HSC72OT, HSC230NT, HSC536NT, HSC62NT, EUFA130NT, EUFA121NT, EUFA143NT (representing the complementation groups A, B, C, D1, E, F, and G, respectively4 ), or cell lines marked with either hygromycin (H) or G418/neomycin (N) resistance. Fusion experiments involving group FA-D2 had exceedingly low success rates; therefore, a retroviral vector expressing FANCD2 was used instead. The cell fusion procedure entailed mixing 2 × 107 lymphoblasts from each fusion partner. After centrifugation (250 × g for 5 minutes) and resuspension in serum-free medium, the cells were divided into 4 equal portions for 4 parallel fusion experiments. After centrifugation, cell fusion was induced by gentle resuspension of the cells in 1 mL of a solution containing 50% PEG (type 1000; Merck, Darmstadt, Germany) in serum-free medium. After 1 minute, the samples were carefully mixed with 4 mL of serum-free medium, followed 2 minutes later by another 4 mL of serum-free medium, after which the cells were left at room temperature for 20 minutes. The cells were then washed once by centrifugation at 111 × g and resuspended in 10 mL of complete medium. After recovery at 37°C for 48 hours, selection medium was added. In successful experiments outgrowth of the hybrids typically occurred after 2-5 weeks. Success rates were usually 80%-90% but strongly depended on the (combination of) cell lines used. In cases of multiple failures a modified protocol was attempted, in which 2.5 × 107 lymphoblasts from each fusion partner were mixed, centrifuged, and gently resuspended in 2.5 mL 50% PEG solution. After 2 minutes at room temperature, 8 mL serum-free medium was added. After another 2 minutes the cells were washed by addition of 10 mL of serum-free medium and centrifuged. After 3 additional cycles of resuspension and centrifugation at 63 × g, pellets were resuspended in 28 mL complete medium and divided into 4 equal portions. Following 72 hours at 37°C, selective medium was added.
Authentication of fusion hybrids
Tetraploidy of the hybrids was verified by flow cytometry (Partec PAS, Partec GmbH, Münster, Germany) using Hoechst 33258 (bisbenzimide; Sigma-Aldrich) fluorescence or by counting the number of chromosomes in 20 randomly selected metaphases. Results from noncomplemented hybrids that showed significant loss of chromosomes were excluded from the analysis. Fusion hybrids were further authenticated by verifying the presence of polymorphic variable number of tandem repeats microsatellite marker alleles from both parental cell lines.
Transfection, transduction, and segregation of polymorphic markers
The cell lines EUFA592, EUFA816, BD952, EUFA696, EUFA543, and AG656 were electroporated with expression plasmids containing FANCA, FANCG, FANCF as described before.18 Cells surviving hygromycin selection (100 μg/mL) were tested for complementation in an MMC-induced growth inhibition test as described in the next paragraph. Cell lines EUFA592, EUFA961, EUFA696, EUFA543, and AG656 were transduced with the retroviral vector pMMP containing FANCD2 cDNA.15 In brief, 5 × 106 cells were spun down and resuspended in 1.5 mL of FANCD2-containing virus supernatant plus freshly thawed polybrene (8 μg/mL). After 3-4 hours at 37°C the cells were resuspended in 5 mL complete medium. After 2 days, selection medium containing 15 nM MMC was added. Negative (FA-A) and positive (FA-D2) control cell lines were included in all experiments. It should be pointed out that the puromycin resistance marker located at the retroviral vector6,15 was not used because in our hands the puromycin concentration required to select against nontransduced cells showed considerable interstrain variation. Cells surviving the 15 nM MMC selection (usually appearing after 1-2 weeks) were tested for complementation in an MMC-induced growth inhibition test, as described in the next paragraph. Families with multiple affected siblings and/or consanguinity were also genotyped with fluorescently labeled microsatellite markers tightly linked to and flanking the known FA genes and electrophoresed on an ABI 377 DNA analyzer (Applied Biosystems, Melbourne, Australia) to test for cosegregation with the phenotype.8 Markers used were D3S1597, D3S1304, D3S2403, D6S1645, D6S439, D6S1281, D6S1019, D8S88, D8S1119, GAAT1A4, D9S287, D9S1874, D9S741, D9S301, D9S910, D9S938, D11S1359, D11S1981, D11S1392, D13S220, D13S787, D13S1493, D16S3026, and D16S539.
Growth inhibition assay
Following successful selection of cell lines and fusion hybrids, their cross-linker sensitivity was determined by a growth inhibition test, according to a previously published protocol.16,19 Briefly, cultures were initiated with 5 × 104 cells/mL in multiple 25-cm2 tissue-culture flasks (NUNC, Roskilde, Denmark) in the presence of different concentrations of MMC (0-100 nM). The cultures were allowed to grow until the unexposed cultures had undergone 3 population doublings, which varied between 3 and 14 days. All parallel cultures were then counted using a Z1 cell and particle counter (Beckman Coulter, Fullerton, CA). IC50 values were estimated from growth inhibition curves as the concentration of MMC that reduced growth by 50%. IC50 values for FA cell lines and noncomplemented hybrids were typically below 10 nM MMC, except for HSC62 (FA-D1) cells, which showed values in the range of 10 to 15 nM. Values for wild-type cell lines were in the range of 15 to 100 nM; transfected/transduced cell lines and hybrids with values in this range were considered complemented.
Immunoprecipitation and Western blotting
Immunoprecipitation (IP) was performed to determine FA protein complex formation, as described.20,21 Briefly, total cell extracts were made by lysis of 107 exponentially growing cells in 0.5 mL lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane] HCl, pH 7.4, 150 mM NaCl, 1% Nonidet NP40, 0.5 mg/mL PefaBloc (Roche, Basel, Switzerland), 1 μg/mL aprotinine, 1 μg/mL pepstatine, 1 μg/mL leupeptine (Sigma-Aldrich). FANCC protein was immunoprecipitated with 5 μL guinea pig antiserum against the N-terminus of FANCC (amino acid 5-106) and 30 μL protein A-agarose beads (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). After resuspension in sodium dodecyl sulfate (SDS) sample buffer, the proteins were separated on an 8% SDS-polyacrylamide gel and transferred to an Immobilon-P membrane (Millipore, Bedford, MA). The membrane was blocked with 5% dry milk in TBST (10 mM Tris HCl, pH 7.5,150 mM NaCl, 0.1% Tween20) and incubated with a rabbit polyclonal antibody against the C-terminus of FANCA (antiserum 89) or against FANCF(1-374) (1:500 diluted in 2% dry milk in TBST).21 The membrane was incubated with anti-rabbit horseradish-peroxidase (HRP)—labeled antibody (1:5000 diluted in TBST) (DAKO, Glostrup, Denmark). FANCD2 was visualized by direct Western blot, using aliquots of whole cell extract loaded on a 3%-8% Tris-Acetate NuPAGE gradient gel (Invitrogen) according to the manufacturer's protocol. Western blots were incubated with a FANCD2(1-292) affinity purified rabbit polyclonal antibody or a FANCD2(1-272) mouse monoclonal antibody (Santa Cruz Biotechnologies, Santa Cruz, CA; diluted 1:1000) and visualized by incubating the membrane with anti-rabbit or anti-mouse HRP-labeled antibody.
Results
Exclusion of FA cell lines from known complementation groups
Six lymphoblastoid cell lines (EUFA592, EUFA816, EUFA961, EUFA696, EUFA543, and AG656) were fused with the reference cell lines for the groups FA-A to FA-G (except group D2), and the resulting hybrids were tested for complementation. As shown in Table 1, all successfully established hybrid cell lines had IC50 values well above 15 nM MMC and were therefore considered complemented, indicating that the cell lines were functionally different from their fusion partners. In addition to the absence of data with a FA-D2 fusion partner, several of the combinations were not informative (EUFA816 × F; EUFA961 × C, F, and G; EUFA696 × F; and EUFA543 × C and F). However, as summarized in Table 2, most cell lines, including the cell line BD952, could be excluded from known groups by the lack of complementation by cDNA transfection, by transduction with FANCD2-containing retroviral particles, or by the lack of segregation of polymorphic markers with some of the known FA genes (detailed results not shown). Exclusion from group FA-D1 was done not only by cell fusion experiments but also by lack of segregation of polymorphic markers with BRCA2 (Table 2). In spite of all these efforts, 3 patient cell lines were still not excluded from several of the complementation groups, for instance, EUFA816 (not excluded from D2), BD952 (not excluded from B), and EUFA961 (not excluded from C, F, and G). However, in these remaining cell lines the FA core complex was formed, making their assignment to groups B, C, F, and G unlikely, since in these groups the core complex is not formed.21 Assignment of EUFA816 to group FA-D2 also was considered unlikely since the FANCD2 protein was readily detectable in cell lysates (Figure 3A). Additionally, the recently identified FANCL gene was excluded from being the disease gene in I and J group cell lines on the basis of Western blot data14 and linkage analysis for group I. Based on the various types of evidence, these 7 cell lines were therefore considered candidates for representing new complementation group(s).
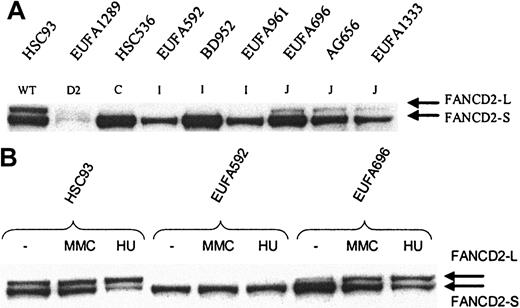
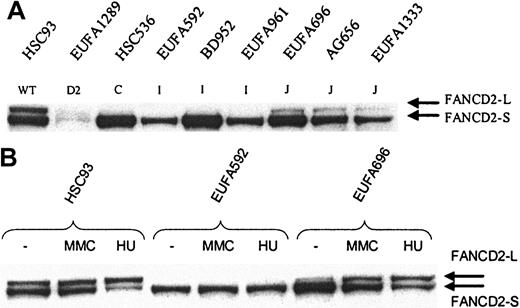
FANCD2 mono-ubiquitination in FA-I and FA-J cells. Whole cell extract was analyzed by direct Western blotting, using affinity-purified FANCD21-292 antibody (A) or mouse anti-FANCD21-272 monoclonal antibody (B). (A) FANCD2-L is clearly detectable in FA-J cells, but apparently absent in FA-I cells; EUFA816 (FA-I) and EUFA543 (FA-J) cells (results not shown) were in agreement with the FA-I and -J cell lines shown in the figure. EUFA1289 (FA-D2) cell line was used as a negative control for FANCD2. (B) No induction of FANCD2-L in FA-I cells and normal induction in FA-J cells. Cells were either untreated or treated with MMC or hydroxyurea (HU) for 24 hours, before analysis by direct Western blotting.
FANCD2 mono-ubiquitination in FA-I and FA-J cells. Whole cell extract was analyzed by direct Western blotting, using affinity-purified FANCD21-292 antibody (A) or mouse anti-FANCD21-272 monoclonal antibody (B). (A) FANCD2-L is clearly detectable in FA-J cells, but apparently absent in FA-I cells; EUFA816 (FA-I) and EUFA543 (FA-J) cells (results not shown) were in agreement with the FA-I and -J cell lines shown in the figure. EUFA1289 (FA-D2) cell line was used as a negative control for FANCD2. (B) No induction of FANCD2-L in FA-I cells and normal induction in FA-J cells. Cells were either untreated or treated with MMC or hydroxyurea (HU) for 24 hours, before analysis by direct Western blotting.
To assess possible heterogeneity within this set of 7 cell lines, an additional series of cell fusion experiments was carried out, which included an eighth patient cell line, EUFA1333. This cell line was considered either D1 or a candidate-new group on the basis of its ability to mono-ubiquitinate FANCD2 (Figure 3A).
Assignment of 8 putative non—(A-G) FA cell lines to 2 new complementation groups
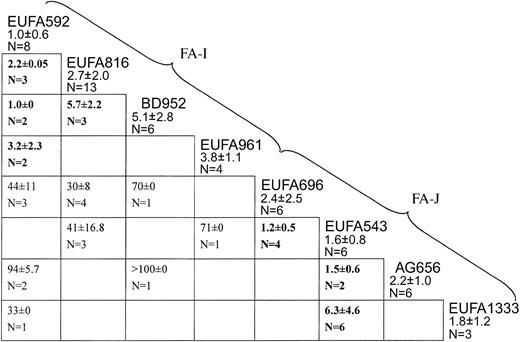
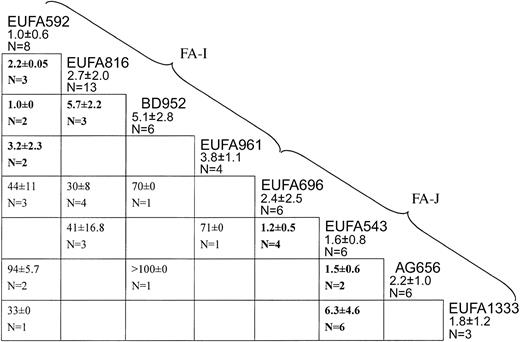
Figure 1 summarizes results from successful cell fusion experiments carried out among the panel of 8 candidate-new complementation group cell lines. EUFA592 failed to complement the cell lines EUFA816, BD952, and EUFA961 (IC50 values well below 10 nM), indicating that they belong to the same genetic subtype. The lack of complementation between EUFA816 and BD952 (IC50, 5.7 nM) confirmed this conclusion. Therefore, these 4 cell lines were considered to represent a new complementation group, FA-I.
Cell fusion results assigning 8 FA cell lines to 2 new complementation groups, I and J. Each box indicates a fusion experiment between 2 cell lines, for instance, the one on top of the vertical column and the one at the end of the horizontal row. Figures are IC50 values for MMC (means ± standard deviations). Values higher than 10 nM are complemented hybrids; values lower than 10 nM (bold face) are noncomplemented hybrids. Empty cells: no data available, either because fusions had failed multiple times or because the combination of properly marked parental cell lines was not available. EUFA592 failed to complement EUFA816, BD952, and EUFA961, defining complementation group I. EUFA543 failed to complement EUFA696, AG656, EUFA1333, defining group J.
Cell fusion results assigning 8 FA cell lines to 2 new complementation groups, I and J. Each box indicates a fusion experiment between 2 cell lines, for instance, the one on top of the vertical column and the one at the end of the horizontal row. Figures are IC50 values for MMC (means ± standard deviations). Values higher than 10 nM are complemented hybrids; values lower than 10 nM (bold face) are noncomplemented hybrids. Empty cells: no data available, either because fusions had failed multiple times or because the combination of properly marked parental cell lines was not available. EUFA592 failed to complement EUFA816, BD952, and EUFA961, defining complementation group I. EUFA543 failed to complement EUFA696, AG656, EUFA1333, defining group J.
Hybrids between one or more of the FA-I cell lines with the remaining 4 cell lines (EUFA696, EUFA543, AG656, and EUFA1333) were all complemented, with IC50 values well above 20 nM, excluding these cell lines from the new group FA-I. EUFA696 failed to complement EUFA543, which in turn did not complement AG656 and EUFA1333, indicating that these 4 cell lines belong to another new genetic subtype, termed FA-J.
Molecular phenotype of FA-I and FA-J cells
FA cells can be distinguished at the molecular level in terms of their ability to form a nuclear core complex and their capacity to mono-ubiquitinate FANCD2. Cell lines belonging to groups A, B, C, E, F, G, or L (the non-D groups) are unable to form the core complex and fail to mono-ubiquitinate FANCD2. Both D1 and D2 cells do form the core complex, but only D1 cells can ubiquitinate FANCD2, while D2 cells lack functional FANCD2 protein. FANCD1/BRCA2 is thought to act downstream of the FANCD2 ubiquitination step, whereas all other proteins function upstream. To find out whether FA-I and FA-J cells would fit in either of these phenotypes, we assessed their capacities to form a core complex and to ubiquitinate FANCD2. Figure 2 shows that in FA-I and FA-J cells FANCA, -C, and -F were coimmunoprecipitated from cellular extracts, indicating their capacity to form the FA protein core complex. However, as illustrated in Figure 3, FA-I cells were unable to convert FANCD2 into its ubiquitinated isoform even after being challenged with MMC or hydroxyurea (HU), the latter of which is a very potent inducer of this form (T. Taniguchi and A. D. D'Andrea, personal written communication, September 30, 2003). In contrast, FA-J cells were able to ubiquitinate FANCD2 and did respond to MMC and HU treatment. FA-J cells thus resembled FA-D1 and wild-type cells, whereas FA-I cells were unique in that they were able to form a complex but unable to generate the mono-ubiquitinated FANCD2 isoform. FANCI therefore likely functions downstream of FANCL but upstream of FANCD2 ubiquitination.
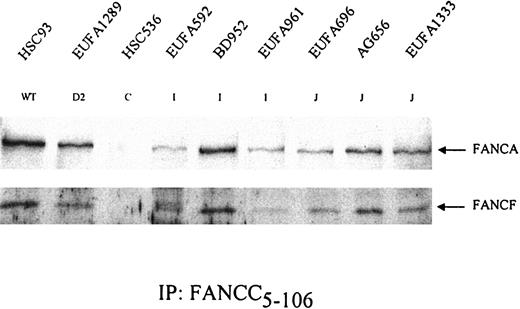
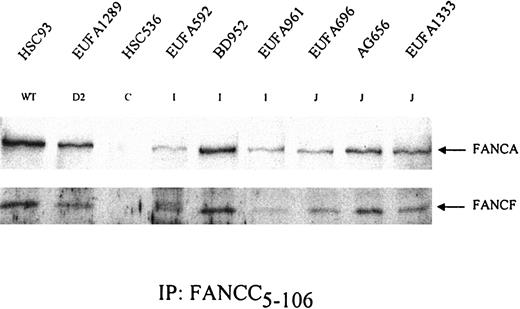
Formation of FA protein core complex in FA-I and FA-J cells. FANCC was precipitated from the indicated cell lysates. After gel electrophoresis, coimmunoprecipitation of the other core complex components FANCA and FANCF was examined. HSC536 (FA-C) was included as a negative control. Results show that both FA-I and FA-J cells are able to form the FA protein core complex. EUFA816 (FA-I) and EUFA543 (FA-J) (results not shown) were in agreement with the FA-I and -J cell lines shown.
Formation of FA protein core complex in FA-I and FA-J cells. FANCC was precipitated from the indicated cell lysates. After gel electrophoresis, coimmunoprecipitation of the other core complex components FANCA and FANCF was examined. HSC536 (FA-C) was included as a negative control. Results show that both FA-I and FA-J cells are able to form the FA protein core complex. EUFA816 (FA-I) and EUFA543 (FA-J) (results not shown) were in agreement with the FA-I and -J cell lines shown.
Discussion
Results reported in this paper indicate the existence of 2 new complementation groups in FA, termed FA-I and FA-J. The conclusions are based on different types of evidence, derived from cell fusion, cDNA-mediated complementation, immunoprecipitation, Western blot analysis, and segregation of polymorphic markers. Two caveats have been recognized in the assignment of FApatient cell lines to complementation groups. First, hybrid cell lines can lose chromosomes so that lack of complementation in a hybrid might be due to the loss of the chromosomes that contain the complementing gene. Although the chance of false assignments due to chromosome loss can be minimized by assessing the ploidy status of the hybrids using flow cytometry or chromosome counting, an example exists of 2 patient cell lines that were at first incorrectly assigned to group D, which after their mutated gene was identified (FANCD2) caused this complementation group to be split up into D2 (defective in FANCD2; reference 6) and D1 (defective in BRCA2; reference 13). Second, the MMC resistance of a “complemented” hybrid may have resulted from a genetic reversion of the disease gene rather than from genuine complementation. This has been shown to be the case with patient EUFA173, who was originally classified as FA-H but later reassigned to group A.5 Genetic reversion in “complemented” hybrids would normally go unnoticed, especially when this would occur simultaneously in multiple hybrids generated from the same set of parental cell lines. To minimize the chance that an erroneous nonassignment would define a new complementation group, it has been proposed that at least 2 cell lines should be identified to belong to this group on the basis of mutual noncomplementation in fusion hybrids.5 In the present study as many as 4 cell lines were identified within each of the new groups I and J, making the possibility of genetic reversion in a specific combination of cell lines extremely unlikely. Moreover, the segregation of polymorphic markers within a number of I and J families was incompatible with defects in known FA genes, which essentially proved exclusion from all known groups, except B. However, since the known FA-B cells (in contrast to FA-I and FA-J cells) are unable to form the nuclear FA multiprotein complex, I and J cells are unlikely to be defective in the putative FANCB gene.
FA is clinically heterogeneous and shows considerable overlap with a number of other syndromes.1 The hyperresponsiveness of FA cells to the clastogenic effect of cross-linking agents has been considered as the decisive criterium to distinguish patients with FA from non-FA patients. However, Nakanishi and coworkers22 reported an MMC-hypersensitive cell line that was presumed to be derived from an FA patient (EUFA1020) but appeared to carry pathogenic mutations in the Nijmegen breakage syndrome gene, NBS1. Since EUFA1020 cells were able to form the ubiquitinated isoform of FANCD2, this raised the possibility of NBS1 being a candidate for the gene defective in the new group FA-J. However, since the consanguineous FA-J patient EUFA543 was heterozygous for markers closely linked to the NBS1 locus and normal NBS1 protein expression was found in the cell lines EUFA543, EUFA696, and EUFA1333 (J.P.d.W., unpublished data, October 2002), this gene was excluded as a candidate for FANCJ.
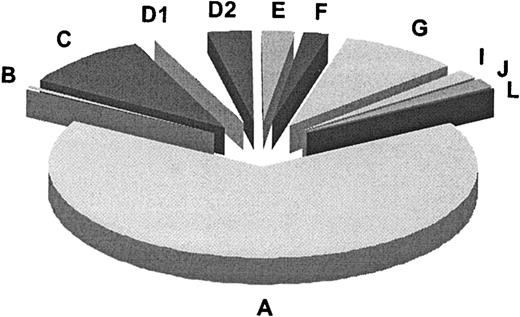
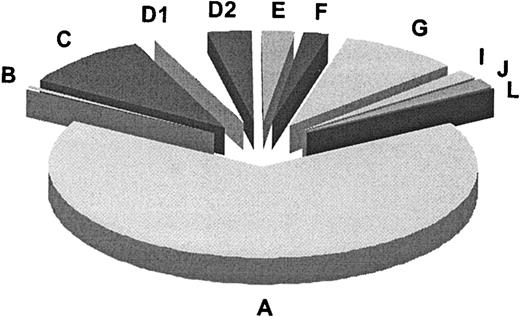
In summary (Table 3), our results along with published data add up to a total of 11 genetic subtypes or complementation groups in FA. Figure 4 summarizes the relative prevalences for these groups, as based on 241 FA families classified according to complementation group. For 3 groups (B, I, and J) the defective gene remains to be identified. Most of the FA genes known or implicated to date, including FANCI, control the FA pathway upstream of the FANCD2-ubiquitination step. FANCI may function, presumably indirectly,14 in the ubiquitination of FANCD2. FANCD1/BRCA2 and the predicted FANCJ protein are the only FA proteins so far that act downstream in the FA pathway, or independently. Experiments to test whether the FANCI and -J polypeptides engage in interactions with the other FA proteins await identification of the corresponding genes and proteins.
Relative prevalences of 11 complementation groups in FA. Results are based on the first 241 FA families classified by the European Fanconi Anemia Research Programme (1994-2003). This number includes the reference cell lines for groups A, B, C, D1, and D2 (Table 3). The absolute numbers per group were as follows: A, 159; B, 2; C, 23; D1, 8; D2, 8; E, 6; F, 5; G, 21; I, 4; J, 4; L, 1.
Relative prevalences of 11 complementation groups in FA. Results are based on the first 241 FA families classified by the European Fanconi Anemia Research Programme (1994-2003). This number includes the reference cell lines for groups A, B, C, D1, and D2 (Table 3). The absolute numbers per group were as follows: A, 159; B, 2; C, 23; D1, 8; D2, 8; E, 6; F, 5; G, 21; I, 4; J, 4; L, 1.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-08-2915.
Supported by the FA Research Fund, Eugene, OR; the Dutch Cancer Society, The Netherlands Organization for Health Research and Development; and the Deutsche FA Hilfe e.V., Unna-Siddinghausen, Germany.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the FA patients and their families for participating in this study; Drs S. Ball, R. Barr, J. Bodurtha, J. Burn, W. Ebell, S. Mohan, D. Schuler, and M. Schweiger for referring their patients; Mr R. Dietrich for invaluable help with the collection of blood samples; Marta Alonso Guervós, Carola van Berkel, and Neil Morgan for excellent technical assistance; Alan D'Andrea for anti-FANCD2 antibodies and the FANCD2-containing retroviral vector; Toshiyasu Taniguchi for advice; and Annette Medhurst for valuable comments on the manuscript.