Abstract
Fludarabine in addition to cytosine-arabinoside (ARA-C) increases the accumulation of ARA-C–5′-triphosphate (ARA-CTP), which is responsible for the cytotoxic effect in leukemic blasts. In a randomized phase 3 trial, patients with high-risk myelodysplastic syndrome (MDS) (n = 91) or elderly patients with acute myeloid leukemia (AML) (n = 43) were randomized to receive 2 induction courses consisting of ARA-C (2 g/m2 days 1 through 5) and granulocyte colony-stimulating factor (G-CSF) (filgrastim, 5μg/kg) during and after chemotherapy with or without fludarabine (25 mg/m2, days 1 through 5) (FLAG versus AG). Consolidation consisted of daunorubicin (45 mg/m2, days 1 through 3) and ARA-C (200 mg/m2, days 1 through 7). Complete remission (CR) rate following AG was 65% versus 71% with FLAG (P = .49). Overall survival (OS) at 24 months was 24% for AG treatment and 39% for FLAG (P = .32). Event-free survival (EFS) at 2 years was 10% and 19% (P = .31) for the AG and FLAG treatments, respectively. Platelet and granulocyte recovery times after the second cycle were prolonged in the FLAG treatment group. Grades 3 to 4 neurotoxicities were more often reported in the FLAG arm (14% versus 3%, P = .03), whereas no significant differences in other toxicities were observed. In a cohort of patients, the in vivo accumulation of ARA-CTP in leukemic cells was determined. Although ARA-CTP accumulation in leukemic cells after FLAG was enhanced, clinical outcome in terms of CR rate, OS, EFS, and disease-free survival (DFS) was not significantly improved by combining fludarabine with ARA-C. (Blood. 2004;103:2908-2913)
Introduction
Cytosine-arabinoside (ARA-C) is one of the most effective drugs in the treatment of myeloid malignancies. High doses of ARA-C (up to 3 g/m2 for 6 doses) as postremission therapy in acute myeloid leukemia (AML) have been shown to reduce the probability of relapse and may result in better disease-free survival.1 However, especially for patients older than 60 years, the benefits appear to be counterbalanced by increased toxicity. The clinical effects of ARA-C are highly dependent on the intracellular conversion of ARA-C to an active metabolite: ARA-C–5′-triphosphate (ARA-CTP).2 ARA-CTP is subsequently incorporated into DNA, resulting in leukemic cell death. The MD Anderson group has nicely shown, in vitro as well as in vivo, that the accumulation of ARA-CTP can be increased by the addition of fludarabine to the ARA-C therapy.3,4 On the basis of these observations, a schedule of combination chemotherapy consisting of fludarabine, ARA-C, and granulocyte colony-stimulating factor (G-CSF), designated FLAG, has been introduced. Results from a variety of single-arm studies suggested that FLAG would provide effective antileukemic therapy, and thus FLAG is now commonly employed for treating patients with high-risk myelodysplastic syndrome (MDS) and AML.5,6 It has been suggested that this synergistic combination is not associated with the toxic effects that are seen after treatment with high-dose ARA-C. Depending on diagnosis and prognostic factors, complete remission rates varied between 45% and 60%.
The value of FLAG has never been critically assessed in appropriate comparative studies.7 The Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) therefore set out to evaluate in a prospective randomized study the potential value of FLAG in AML in elderly patients and in high-risk MDS in direct comparison with ARA-C plus G-CSF. In a subset of patients, the in vivo accumulation of ARA-CTP in the leukemic cells was studied.
Patients, materials, and methods
Patients
Patients with a primary or secondary hematologic diagnosis of myelodysplasia classified according to the French-American-British (FAB) classification as refractory anemia with excess of blasts (RAEB), RAEB-transformed (RAEB-t), chronic myelomonocytic leukemia (CMML), or other forms of MDS with multiple cytogenetic anomalies and/or profound cytopenia (neutrophils fewer than 0.5 × 109/L or platelets fewer than 20 × 109/L) were eligible. After amendment of the study in 1999, AML patients older than 60 years and for whom high-dose chemotherapy followed by stem cell transplantation was not feasible could also be included.
Risk classification for MDS was estimated according to the International Prognostic Scoring System (IPSS).8 Patients previously treated with chemotherapy for other diseases within 6 months before entry to the study, or presenting with active uncontrolled infection or a second active primary malignancy, were not eligible.
Study design
This study was approved by the VU University Medical Center (Amsterdam, The Netherlands). Informed consent was provided according to the Declaration of Helsinki. After informed consent, patients were randomly assigned to induction chemotherapy consisting of (1) 2000 mg/m2 cytosine-arabinoside (ARA-C) intravenously in a 4-hour infusion on days 1 through 5, plus 5 μg/kg/d G-CSF (filgrastim; Amgen, Breda, The Netherlands) subcutaneously starting 24 hours before ARA-C and continuing until neutrophil recovery (absolute neutrophil count [ANC] exceeding 0.5 × 109/L) (AG regimen); or (2) the same regimen with the addition of 25 mg/m2 fludarabine intravenously in 30 minutes on days 1 through 5 starting 4 hours before ARA-C infusion (FLAG regimen). G-CSF was postponed or interrupted in case of white blood cell (WBC) count greater than 30 × 109/L and resumed as soon as WBC count had dropped to below 20 × 109/L.
Patients in complete remission (CR) or partial remission (PR) after cycle 1 proceeded to an identical chemotherapy, cycle 2. It was planned that patients in CR or PR following cycle 2 would receive cycle 3, consisting of the combination 200 mg/m2/d ARA-C (continuous infusion) on days 1 through 7 and 45 mg/m2/d daunorubicin (intravenous [IV] bolus infusion) on days 1 through 3 plus 5 μg/kg/d G-CSF. The objectives of the study were to assess whether the response rate, event-free survival, disease-free survival, and overall survival time improved when fludarabine was added to ARA-C as compared with ARA-C alone and to assess whether the ARA-CTP accumulation in leukemic blasts in a subset of patients increased as the consequence of fludarabine added to ARA-C.
Intracellular ARA-CTP accumulation
Bone marrow from 12 patients was obtained immediately after the infusion of ARA-C.
Mononuclear cell fraction was obtained with the use of Lymphoprep (Axis-Shield, Oslo, Norway) gradient centrifugation. Determination of the nucleotide pools, ARA-CTP, and fludara-ARA–adenosine triphosphate (F-ARA-ATP) was performed on leukemic blast cells from AML patients entered in this study. Cells were washed, transferred to an Eppendorf vial, centrifuged, and rapidly frozen in liquid nitrogen and stored at -80°C until extraction. Extraction of the nucleotides including ARA-CTP and F-ARA-ATP was performed as described previously.9 Briefly, cell pellets were resuspended in 150 μL cold phosphate-buffered saline (pH, 7.4), and subsequently 50 μL 40% ice-cold trichloroaceticacid (TCA) (wt/vol) was added. The suspension was kept on ice for 20 minutes. Proteins were spun down (5 minutes, 32 168g [12 000 rpm], 4°C), and the supernatant was neutralized with 400 μL tri-octylamine/1,1,2-tri-chloro-tri-fluor-ethane (1:4, vol/vol). Nucleotides were separated with the use of anion-exchange HPLC (26). The HPLC system consisted of 2 M300 pumps (Gynkotek, Munich, Germany); a Promis 2 autosampler (Spark Holland, Emmen, The Netherlands); a Partisphere Sax column (Whatman, Maidstone, Kent, United Kingdom) (internal diameter [ID], 4.6 mm; length, 12.5 cm; particle size, 5 μm); a 1000S diode-array detector set at 280 and 254 nm (Applied Biosystems, Foster City, CA); and a Chromeleon data aquisition system (Dionex, Sunnyvale, CA) with pump control. Elution was performed isocratically with 0.25 M KH2PO4 containing 0.5 M KCl (pH, 4.5) at a flow of 1.5 mL per minute. The retention times of the nucleotides were (depending on the various age of the column) as follows: adenosine diphosphate (ADP), 2.5 minutes; uridine 5′-triphosphate (UTP), 3.9 minutes; CTP, 4.5 minutes; ARA-CTP, 5.4 minutes; ATP, 6.8 minutes; and guanosine triphosphate (GTP), 9.5 minutes.
Supportive care
Prophylactic antibiotics and antifungal agents were administered according to local recommendations of each of the participating centers. Empiric broad spectrum antibiotics were started in case of fever higher than 38.5°C. Prophylactic platelet transfusions were given in case of thrombocytopenia below 20 × 109/L. Therapeutic infusions were administered in cases of active bleeding. Filtrated red blood cells were transfused to maintain the hematocrit above 0.3 (30%).
Evaluation and statistical analysis
Complete remission (CR) was defined by bone marrow containing fewer than 5% blasts, no circulating blasts, and absence of extramedullary disease. Recovery of peripheral blood values to a platelet count of more than 100 × 109/L and a neutrophil count of at least 1.5 × 109/L was required after induction cycle 2 and consolidation cycle 3. A partial response (PR) was defined by 1 of 3 endpoints of improvement: a 50% decrease in blasts in patients who initially had a blast percentage of more than 5%, an increment of polymorphic neutrophils of at least 100% and more than 1 × 109/L, or an increment in platelet count of at least 100% and a level greater than 50 × 109/L.
Failure of induction therapy was defined as absolute drug resistance, early death (before the completion of chemotherapy of the first induction cycle), or hypoplastic death (death after the completion of induction cycle 1 or 2 before hematologic recovery). Chromosomal abnormalities were described according to the International System for Human Cytogenetics Nomenclature. Standard cytogenetic techniques were used at diagnosis to karyotype the MDS and AML. Patients were classified into 3 distinct prognostic categories: favorable risk, standard risk, and poor risk. Favorable risk was defined by the presence of t(8;21), inv(16) or t(16;16), and t(15;17). Poor risk was defined as monosomies or deletions of chromosome 5 or 7, abnormalities of the long arm of chromosome 3(q21;q26), and the presence of complex cytogenetic abnormalities (more than 4 unrelated clones). Patients who did not meet the criteria for poor or favorable were classified as standard risk.10
Toxicity was evaluated according to Common Toxicity Criteria (CTC) criteria. Duration of neutropenia and thrombocytopenia was defined as the time (days) from the start of chemotherapy to the day of ANC recovery to more than 1 × 109/L and to platelet recovery to more than 20 × 109/L, respectively. Patients who died, progressed, or were lost to follow-up were censored at the dates when the last neutrophil or platelet count had been assessed. Overall survival (OS) was measured from the date of registration until death or last contact. Event-free survival (EFS) was measured from the date of registration until relapse after CR, death in CR, or last contact, whichever occurred first. If a patient did not reach CR on protocol treatment, EFS was set at 0. Disease-free survival (DFS) was restricted to patients who achieved CR and was measured from attainment of CR until the date of relapse, death, or last contact, whichever occurred first. Randomization was stratified by hospital and diagnosis.
The proportions of patients in the 2 treatment arms showing a CR on protocol treatment were compared by means of Pearson chi-square test, and the 95% confidence interval for the difference in proportions was calculated. Differences between patient characteristics at the time of randomization were compared by means of Pearson chi-square test, Fisher exact test, or the Kruskal-Wallis test, as appropriate. Univariate and multivariate logistic regression were used to reveal prognostic factors for response. Curves for OS, EFS, DFS, and hematologic recoveries were calculated by means of the actuarial method of Kaplan and Meier, and the treatment arms were compared by log-rank test. Univariate and multivariate Cox regression was used to determine prognostic factors for OS, EFS, and DFS. Variables included in the univariate analysis were treatment arm, sex, age, World Health Organization (WHO) performance, diagnosis (AML/MDS), FAB classification of MDS, bilirubin level, creatinin level, BM cellularity, hemoglobin level, platelet count, WBC count, ANC, number of cytopenias, IPSS for MDS, and cytogenetic classification. Furthermore, the Kruskal-Wallis test was used to compare the number of days in the hospital between the 2 treatment groups. The sample size was determined with CR rate as endpoint. With 100 patients, an increase of CR of 40% (based on results of monotherapy with ARA-C in AML)11 to 60% would have been detected with a power of 50%. An interim analysis was planned after 20 patients in each treatment arm had received 2 cycles of chemotherapy. Premature closure would have been considered for the study if the CR rate after 2 courses between the 2 treatment arms differed significantly at the 1% level.
Results
Between October 1996 and January 2001, 137 patients were randomized for study treatment. Two patients were not eligible owing to wrong diagnosis. One patient was lost to follow-up from the day of registration and was therefore excluded from analysis. Of 134 remaining patients, 65 were randomized to treatment with FLAG and 69 for treatment with AG. The clinical and hematologic characteristics are summarized in Table 1. A total of 91 MDS patients and 43 with AML were entered. There were no significant clinical differences between the 2 treatment groups. The majority of the patients had a good performance status; only 8% were classified higher than performance score (PS) 1. Cytogenetic studies were performed in 131 patients and were evaluable in 115 patients. Abnormal metaphases were found in 49 patients; these included del(7) (n = 20), del(5) (n = 10), trisomy 8 (n = 10), and complex karyotypic abnormalities (n = 16) alone or in combination. Thirty patients were classified as having poor prognosis, while 84 had standard risk. Only one patient presented with core binding leukemia t(8;21) and was therefore regarded as good risk. Of the MDS patients, 59 belonged to the intermediate-2 or high-risk group according to the IPSS. Twelve patients had antecedent MDS; 9 of them were allocated to the AG treatment arm.
Response to chemotherapy
The overall complete response rate was 68%. CR after completion of chemotherapy was achieved in 71% of patients in the FLAG arm as compared with 65% in the AG arm (P = .49) (Table 2). The CR rate was better in the AML patients (84%) than in the MDS patients (60%). FLAG induced a better CR rate in patients with AML as compared with AG-treated patients (95% versus 71%; P = .046). Reasons for not obtaining a complete remission were related to resistance to chemotherapy, early death, and hypoplastic death. Of 134 patients, 100 (75%) received at least the first 2 cycles of planned therapy, whereas 76 (57%) completed the 3 cycles of protocol treatment. Reasons for going off protocol treatment were progression to AML (n = 5); no response to cycle 1 (n = 6); extramedullary toxicity (n = 11); persistent hypoplasia (n = 8); death (n = 16); or miscellaneous (n = 12). No significant difference with regard to the received treatment cycles between the 2 treatment arms was observed.
Overall survival and disease-free survival
The median follow-up of 32 patients still alive is 24 months; 16 of these patients are in continuous CR. The probability of OS at 2 years after randomization was 39% in the FLAG group and 24% in the control group (P = .32) (Figure 1). Although the salvage treatment offered after failure or relapse was quite heterogeneous, no significant difference in overall survival after failure or relapse (median, 5 months) between the 2 treatment arms was observed. DFS probabilities at 2 years were 23% and 16%, respectively (P = .43) (Figure 1). No significant differences (19% versus 10%, P = .31) between FLAG and AG groups were apparent for EFS.
Overall survival, disease-free survival, and event-free survival by treatment arm. (A) Overall survival. (B) Disease-free survival. (C) Event-free survival.
Overall survival, disease-free survival, and event-free survival by treatment arm. (A) Overall survival. (B) Disease-free survival. (C) Event-free survival.
Hematopoietic recovery and hospitalization
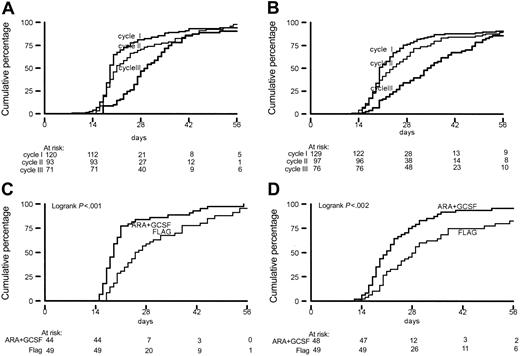
Median time to recovery toward an ANC greater than 1.0 × 109/L and a platelet count of more than 20 × 109/L progressively increased with each cycle of chemotherapy. Duration of cytopenia was more pronounced for the FLAG-treated patients (Figure 2). The median times to neutrophil recovery to 1.0 × 109/L after cycles 1, 2, and 3 were 19, 19, and 29 days, respectively, in the AG-treated patients and 19, 25, and 31, respectively, for the FLAG treatment group. Median duration of thrombocytopenia was 20, 21, and 34 days after cycles 1, 2, and 3 in the AG therapy group, which compared with median values of 21, 28, and 35 days in the FLAG cycles. Also, for recovery time of platelet count to greater than 100 × 109/L and ANC to greater than 0.5 × 109/L, identical increments were found with each subsequent cycle. The mean number of days of hospitalization for patients allocated to FLAG treatment exceeded those for AG patients: 26.2 days versus 23.6 days (P = .005).
Recovery time (in days) to ANC greater than 1 × 109/L and platelet count exceeding 20 × 109/L. (A-B) From start of induction chemotherapy by cycle for ANC (A) and platelet count (B). (C-D) From start of chemotherapy cycle 2 by treatment arm for ANC (C) and platelets (D).
Recovery time (in days) to ANC greater than 1 × 109/L and platelet count exceeding 20 × 109/L. (A-B) From start of induction chemotherapy by cycle for ANC (A) and platelet count (B). (C-D) From start of chemotherapy cycle 2 by treatment arm for ANC (C) and platelets (D).
Toxicity
The frequencies of maximal grades 3 to 4 side effects per patient related to hemorrhages, liver abnormalities, cardiac function, and rhythm disturbances and gastrointestinal toxicity were similar in the two groups. However, the patients in the FLAG group experienced significantly more neurologic abnormalities (P = .03), and the increased frequency of septicemia was of borderline significance (P = .06) (Table 3). After adjustment for poor cytogenetic risk, age, diagnosis, and creatinine level, the risk of septicemia was significantly greater in the FLAG treatment group (odds ratio, 2.12; P = .039).
In vitro ARA-CTP accumulation
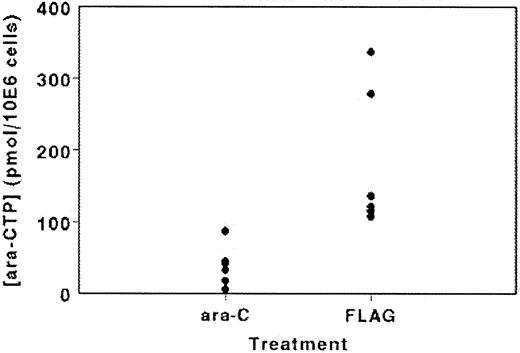
In a subgroup of patients, we studied the in vivo accumulation of ARA-CTP in the leukemic cells (Figure 3). ARA-CTP accumulation at the end of the ARA-C infusion on day 1 of AG therapy varied between 6.1 and 87.5 pmol/106 cells (mean ± standard deviation [SD], 38.7 ± 28.2; n = 6), whereas in the 6 FLAG-treated patients the ARA-CTP accumulation was significantly higher (P = .004), varying between 107.9 and 337.3 pmol/106 cells (mean ± SD, 183 ± 99.1). Concentrations of F-ARA-ATP were very low or undetectable, possibly because the samples were taken after the F-ARA-ATP peak could be expected.
ARA-CTP accumulation in leukemic cells isolated immediately after infusion of ARA-C (2 g/m2) with or without the addition of fludarabine in a selected cohort of 12 patients.
ARA-CTP accumulation in leukemic cells isolated immediately after infusion of ARA-C (2 g/m2) with or without the addition of fludarabine in a selected cohort of 12 patients.
Prognostic factors
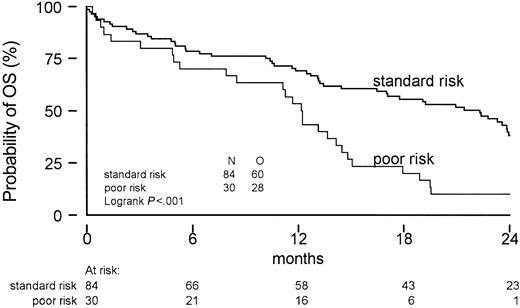
Logistic regression and Cox regression were used to evaluate prognostic factors for the probability of obtaining CR, OS, EFS, and DFS. The results of the univariate and multivariate analyses are listed in Tables 4, 5. Patients with lower platelet counts at diagnosis and a diagnosis of MDS, rather than AML, had a significantly reduced probability of attaining a complete remission. Notably, the treatment arm had no significant prognostic value for any of the endpoints (CR, OS, DFS, or EFS). In AML patients, FLAG resulted in a better CR rate (95% versus 71%, P = .046), but that was not translated into improved quality of remission evidenced by a better DFS, EFS, or OS. Poor cytogenetic risk, as defined for AML, also turned out to be a major prognosticator in this study including mainly MDS patients (Figure 4).
Overall survival according to cytogenetic risk profile. Only one good-risk patient was identified.
Overall survival according to cytogenetic risk profile. Only one good-risk patient was identified.
Discussion
Patients with myelodysplastic syndromes with high-risk features and elderly patients with AML who are candidates for intensive chemotherapy face low remission rates and a short disease-free survival. In 10 recently compiled studies performed between 1995 and 2000, the complete remission rates in high-risk MDS patients ranged from 38% to 79%. In addition, high relapse rates and short survival were noted in these studies. This has raised a question regarding the value of the use of intensive chemotherapy in patients with low, intermediate-1– and intermediate-2–risk MDS.12 In one of the few randomized studies in patients with poor-risk MDS, HOVON previously showed a complete response rate of 63% and a 2-year overall survival of 18%.13 Comparable results were reported in the HOVON/European Organization for the Research and Treatment of Cancer (HOVON/EORTC) AML-11 study in AML in the elderly showing a CR rate of 56% and overall survival of 22% at 2 years.14 These outcome results are in accordance with those of various other studies in AML in the elderly.15 The treatment outcome of patients with high-risk MDS and older patients with AML is bad and needs further improvement. Dose intensification should be considered and investigated for toxicity and feasibility.
ARA-C is one of the cornerstones of current chemotherapy in myeloid malignancies. For ARA-C, a clear dose-effect relationship has been established in clinical studies.2 High-dose ARA-C has been successfully administered in various combinations in patients who have experienced relapse and as postconsolidation therapy. It has been shown in a randomized study that induction therapy with high-dose ARA-C prolonged remission duration and disease-free survival.16 Unfortunately, the toxicity of high-dose ARA-C limits its applicability in older patients. The cytotoxic effects of ARA-C are highly dependent on the intracellular accumulation of ARA-CTP. Addition of fludarabine to ARA-C increases the accumulation of ARA-CTP in leukemic blasts.4 A schedule of combination chemotherapy consisting of fludarabine, ARA-C, and G-CSF, designated FLAG, has been suggested to provide effective antileukemic therapy without the toxic sequelae of high-dose ARA-C. FLAG has become a quite popular schedule and is commonly employed for treating patients with high-risk MDS and AML.17,18 In the absence of comparative studies addressing the value the FLAG regimen, we wished to investigate the value of the addition of FLAG by direct comparison with AG. G-CSF itself can affect ARA-CTP levels.19 Therefore, G-CSF was also administered in the control ARA-C treatment group in the interest of a clear comparison. In this randomized trial containing this defined group of AML and MDS patients, the CR rates in FLAG (71%) and the control arm (65%) were not significantly different. We also did not note a difference in OS, DFS, or EFS, although we realize that the power of this study is low. On the basis of the high response rates in this study as compared with published studies in comparable patient groups and the acceptable toxicity, it is feasible to deliver intensive chemotherapy to these elderly patients. In particular, the CR rate of 84% in the elderly AML patients was superior to other published CR rates in this patients. The toxicity profile was not favorable for FLAG-treated patients. Grades 3 to 4 septicemia and serious neurotoxicities were seen significantly more frequently after FLAG chemotherapy. Especially after the second and third cycle of chemotherapy, recovery times of platelets and granulocytes were considerably retarded in the FLAG arm. In the multivariate analysis, FLAG treatment was never a significant prognostic factor for OS, EFS, or DFS, while FLAG resulted in a significantly better CR rate only for AML patients. This subgroup result must, however, be viewed with caution, because a selection bias cannot be ruled out.
In a selection of patients, we examined whether ARA-CTP accumulation in leukemic blasts indeed was augmented following FLAG therapy as compared with AG treatment. As a matter of fact, the concentrations of ARA-CTP were found to be in the same range as initially reported by Gandhi et al.4 Despite the increased accumulation of ARA-CTP, which was hypothesized to result in a better outcome for these patients, survival was not significantly different between the 2 treatment groups. Therefore, these results do not provide support for the postulated added value of fludarabine as a component of the FLAG schedule. In fact, we observed additional toxicity (Table 3). For the time being, there is little direct evidence to support the incorporation of fludarabine in intensive treatment with ARA-C protocols for myeloid malignancies. Although the number of patients of this study limits the power of the analysis, it seems that the single-arm studies published until now provide a thin rationale for the general use of FLAG treatment. This study mimics the observation in all MDS treatment trials and in all treatment trials of AML in the elderly that CRs can be achieved but that the quality of these remissions is relatively poor, resulting in remissions of short duration. Future strategies should include, in addition to 1 or 2 courses of intensive induction therapy, some form of experimental maintenance therapy, such as monoclonal antibody-targeted therapy, signal transduction inhibitors, or reduced intensity stem cell transplantation to eradicate minimal residual disease to improve remission duration and overall survival.
Appendix
The following individuals are members of the HOVON study group: Dr B. Löwenberg (chair); Dr P.C. Huijgens; Dr E. Vellenga; Dr L.F. Verdonk; Dr W.E. Fibbe; Dr M.H.J. van Oers; Dr A. Schattenberg; Dr R. Schaafsma; Dr H.C. Schouten; Dr G.E.G. Verhoef; and Dr P.W. Wijermans.
Prepublished online as Blood First Edition Paper, December 4, 2003; DOI 10.1182/blood-2003-07-2195.
A complete list of the members of the HOVON Study Group appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.