Abstract
It has been reported that interferon α (IFN-α) enhances humoral immunity and that dendritic cells of the myeloid lineage promote B-cell differentiation. Here we studied whether the plasmacytoid dendritic cell (PDC), a subset of dendritic cells specialized for the production of IFN-α, is involved in regulating B-cell differentiation and immunoglobulin production. The recently identified class of CpG oligonucleotides (CpG-C) was used to activate both B cells and PDCs via Toll-like receptor 9 (TLR9). The presence of PDCs synergistically enhanced CD86 expression, cytokine production (interleukin 6 [IL-6], tumor necrosis factor α, and IL-10) and plasma cell differentiation of isolated human peripheral blood B cells stimulated through CpG-C and B-cell antigen receptor (BCR) ligation. This stimulation protocol was sufficient to drive purified naive B cells into IgM-producing plasma cells and to trigger IgG synthesis in memory B cells. PDCs contributed to B-cell activation via IFN-α secretion. Up-regulation of TLR9 on B cells was not involved. These results demonstrate that CpG-stimulated PDCs induce plasma cell differentiation in naive and memory B cells in the absence of T-cell help, providing an explanation for the excellent activity of CpG oligonucleotides as a humoral vaccine adjuvant. (Blood. 2004;103:3058-3064)
Introduction
T cells and B cells are 2 principal players in the immune response, with T cells controlling much of the activity of B cells. B-cell activation by protein antigens requires binding of the antigen to the B-cell surface immunoglobulin (B-cell receptor [BCR]) and costimulation by antigen-specific T cells through CD40-CD40 ligand interaction and T-cell–derived cytokines. Appropriately activated B cells proliferate and differentiate to plasma cells or to long-lived memory cells. Recent developments in B-cell biology indicate that not only the specific subset of B1 cells in peritoneal and pleural cavities1 but also the activities of conventional B2 cells can be regulated in a T-cell–independent manner.
Similar to other antigen-presenting cells, B cells express Toll-like receptors (TLRs). The family of TLRs establishes a combinatorial repertoire to discriminate among a wide spectrum of pathogen-associated microbial molecules.2 Human B cells express a limited set of TLRs including TLR7 and TLR9.3,4 The natural ligands for TLR9 are CpG motifs within microbial DNA.5-7 Activation of murine and human B cells by CpG motif containing DNA (CpG DNA) is well established.8,9 In fact, CpG motifs were discovered based on B-cell stimulation,10 and B cells were the first immune cell subset in humans identified to respond to CpG DNA in a CpG-specific fashion.11,12
In humans, the only other cell type identified so far that expresses TLR9 is the plasmacytoid dendritic cell (PDC). The PDC is characterized by the production of high amounts of type I interferon α and β (IFN-α and IFN-β) on viral infection. CpG DNA represents a unique microbial molecule recognized by PDCs, leading to activation and maturation of PDCs.13-16 Different classes of CpG oligonucleotides (ODNs) have been identified. CpG-A (prototype ODN 221617 ) induces the production of large amounts of type I IFN in PDCs. Despite its potent IFN-α induction in PDCs, CpG-A is not recognized by human B cells.18 In earlier studies, other CpG ODNs were developed based on the activation of human B cells (CpG-B, prototype ODN 200611 ). This class of CpG ODN is poor at inducing IFN-α in PDCs. Recently a new group of CpG ODN sequences (CpG-C) was identified that induces high levels of IFN-α in PDCs while retaining the ability of directly activating B cells.18,19
CpG-C provides an excellent tool to study the functional consequence of TLR-mediated stimulation of B cells and PDCs. Two independent aspects of B-cell biology prompted us to study the interaction of B cells with PDCs. First, IFN-α was found to potently enhance humoral immunity,20 and second, a number of studies highlighted an important role for dendritic cells of the myeloid lineage in regulating B-cell differentiation by soluble factors21 and via cell-to-cell contact.22 In the present study we identified the functional activity for PDCs to license B cells to undergo plasma cell differentiation and immunoglobulin production without a requirement for T-cell help.
Materials and methods
Media and reagents
The medium used throughout was RPMI 1640 (PAA Laboratories, Linz, Austria) supplemented with 10% (vol/vol) heat-inactivated (56°C, 30 minutes) fetal calf serum (FCS; HyClone, Logan, UT), 1.5 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma, Munich, Germany). F(ab′)2 fragments of rabbit antihuman IgG plus IgA plus IgM antibody from Jackson ImmunResearch Laboratories (Dianova, West Grove, PA) were added at a final concentration of 10 μg/mL unless indicated otherwise. Recombinant IFN-α was from Strathmann Biotech (Dengelsberg, Germany). Completely phosphorothioate-modified ODNs were provided by Coley Pharmaceutical Group (Wellesley, MA) or were purchased from Metabion (Martinsried, Germany). The CpG ODN M352, a representative of CpG-C class of CpG ODN18 with the sequence 5′ TCG TCG AAC GTT CGA GAT GAT 3′ and a non-CG control sequence was used. In some experiments, the CpG-B ODN 2006 5′ TCG TCG TTT TGT CGT TTT GTC GTT 3′ was used for selective microbial stimulation of B cells while minimizing IFN-α secretion by small amounts of contaminating PDCs (< 0.01% contaminating PDCs in isolated B cells are sufficient to produce relevant amounts of IFN-α in response to CpG-C ODN). ODNs were dissolved in Tris-EDTA (tris(hydroxymethyl)aminomethane-ethylenediaminetetraacetic acid [TE]) buffer. ODNs were used at a final concentration of 3.0 μg/mL unless indicated otherwise.
Isolation of cells
Human peripheral blood mononuclear cells (PBMCs) were obtained from buffy coats provided by the blood bank of the University of Greifswald (Greifswald, Germany). Cells were resuspended in complete medium at a final concentration of 2 × 105 cells/mL. For B-cell isolation, B cells were labeled with anti-CD19 antibody coupled to colloidal paramagnetic microbeads (B-cell isolation kit; Miltenyi Biotec, Bergisch-Gladbach, Germany) and passed through a magnetic separation column (LS; Miltenyi Biotec). The purity of isolated B cells was more than 95% as assessed by flow cytometric analysis (CD20). Viability (> 96%) was determined by trypan blue exclusion. Isolated B cells contained less than 1% CD3+, less than 1% CD14+, and less than 0.01% PDCs (BDCA-2+, lin-, major histocompatibility class II positive [MHCII+], CD123+). PDCs were positively isolated using the PDC-specific BDCA-4 antibody according to the manufacturer's protocol (BDCA-4 cell isolation kit; Miltenyi Biotec). The purity of isolated PDCs was more than 95% as assessed by flow cytometric analysis. Naive and memory B cells were purified from PBMCs by magnetic cell separation. First, negatively selected B cells were obtained by using the B-cell isolation kit (Miltenyi Biotec). CD27 memory B cells were isolated from negatively selected untouched B cells using CD27 microbeads (Miltenyi Biotec). The negative fraction was used as the naive B-cell population. The purity of naive B cells and memory B cells was more than 95% as analyzed by flow cytometry (naive B cells, IgD+CD27-; memory B cells, IgD-CD27+).
Cell culture
B cells (1 × 105 cells in 200 μL complete medium/well) were cultured with or without PDCs (1 × 104 cells in 200 μL complete medium/well) in 96-well round-bottom plates. Cells were stimulated by CpG-C ODN M352 (3 μg/mL) and anti-Ig (10 μg/mL). After 3 days supernatants were collected for analysis of cytokine secretion by enzyme-linked immunosorbent assay (ELISA), and cells were harvested for flow cytometric analysis. For plasma cell development, cells were incubated for 8 days. Immunoglobulin production was measured after 13 days of incubation. In some experiments, B cells and PDCs were separated by using a transwell system (Costar, Wilmington, MA). B cells (5 × 105 cells) were seeded in the lower compartment and PDCs (5 × 104 cells) in the upper compartment of 24-well plates (total volume of 1.0 mL/well). For blockade of IFN type I-mediated effects, a combination of polyclonal rabbit anti–IFN-α antibody (5000 neutralizing U/mL), rabbit anti–IFN-β antibody (2000 neutralizing U/mL), and 20 μg/mL of a monoclonal mouse antihuman IFN-α/-β receptor chain 2 antibody was used (all from PBL, New Brunswick, NJ).
Flow cytometry
At the time points indicated, surface antigen staining was performed as described previously.4 Fluorescence-labeled monoclonal antibodies (mAbs) against human CD20 and CD38, CD40, CD86, HLA-DR, CD123, CD11c, BDCA-2, and appropriate isotype control antibodies were purchased from BD Pharmingen (Heidelberg, Germany). Flow cytometric data were acquired on a Becton Dickinson FACSCalibur equipped with 2 lasers (excitation at 488- and 635-nm wavelength). Data were analyzed using Cellquest software (Becton Dickinson, Heidelberg, Germany).
Detection of cytokines by ELISA
The following ELISAs were used: human interleukin 6 (IL-6) OptEIA ELISA (range, 4.7-300 pg/mL); human IL-10 OptEIA ELISA (range, 7.8-500 pg/mL); human tumor necrosis factor α (TNF-α) OptEIA ELISA (range, 7.8-500 pg/mL) (all from BD Pharmingen); and the IFN-α module set (Bender MedSystems, Vienna, Austria), (detection range, 8-500 pg/mL). Detection of immunoglobulins in tissue culture supernatant was measured after 13 days by IgM and IgG ELISA (Bethyl Laboratories, Montgomery, TX).
Reverse transcription–PCR
Purified peripheral blood B cells were stimulated as indicated. After 12 hours cells were lysed and RNA was extracted using the total RNA isolation kit (High pure; Roche, Mannheim, Germany). Real-time polymerase chain reaction (PCR) was performed as described.4 The copy number of the different TLRs was normalized by the housekeeping genes β-actin and cyclophilin-B and is presented as number of transcripts per 103 copies of cyclophilin B.
Statistical analysis
Data are expressed as mean ± SEM. Statistical significance of differences was determined by the paired 2-tailed Student t test. Differences were considered statistically significant for P values less than .05. Statistical analyses were performed using StatView 4.51 software (Abacus Concepts, Calabasas, CA).
Results
PDCs synergistically increase B-cell activation in response to CpG and B-cell receptor ligation
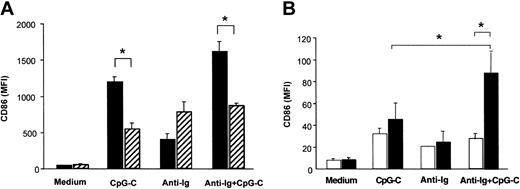
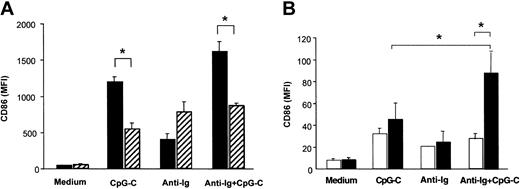
PBMCs isolated from healthy donors were incubated with CpG ODN M352 (CpG-C), with B-cell receptor ligation (anti-Ig), or a combination of both for 72 hours. Both with and without anti-Ig, CpG-C strongly up-regulated CD86 expression in CD19+ B cells (Figure 1A). The depletion of PDCs from PBMCs resulted in a marked decrease of CpG-induced CD86 expression in B cells both in the presence or absence of anti-Ig (Figure 1A solid versus hatched bars). PDC-dependent CD86 expression in B cells was not observed for stimulation with anti-Ig alone. Next B cells and PDCs were isolated from PBMCs and coincubated in the presence of CpG-C, anti-Ig, or a combination of both for 72 hours. CD86 expression in B cells stimulated with CpG-C and anti-Ig was synergistically increased in the presence of PDCs; CD86 expression on B cells stimulated with anti-Ig alone was not further enhanced when PDCs were present (Figure 1B).
PDCs contribute to B-cell activation within PBMCs. Cells were incubated with CpG-C (3 μg/mL), anti-Ig (10 μg/mL), or a combination of CpG-C and anti-Ig. After 72 hours CD86 was analyzed on CD19+ B cells by flow cytometry. Data are presented as mean fluorescence intensity (MFI ±SEM). (A) Total PBMCs (▪) or PBMCs depleted of PDCs (▨) were used (n = 4 independent experiments). (B) Isolated B cells were incubated alone (1 × 105 B cells/200 μL/well) or cocultured with PDCs (1 × 104 PDC/200 μL/well; n = 5 independent experiments; □, B cells alone; ▪, B cells and PDCs).
PDCs contribute to B-cell activation within PBMCs. Cells were incubated with CpG-C (3 μg/mL), anti-Ig (10 μg/mL), or a combination of CpG-C and anti-Ig. After 72 hours CD86 was analyzed on CD19+ B cells by flow cytometry. Data are presented as mean fluorescence intensity (MFI ±SEM). (A) Total PBMCs (▪) or PBMCs depleted of PDCs (▨) were used (n = 4 independent experiments). (B) Isolated B cells were incubated alone (1 × 105 B cells/200 μL/well) or cocultured with PDCs (1 × 104 PDC/200 μL/well; n = 5 independent experiments; □, B cells alone; ▪, B cells and PDCs).
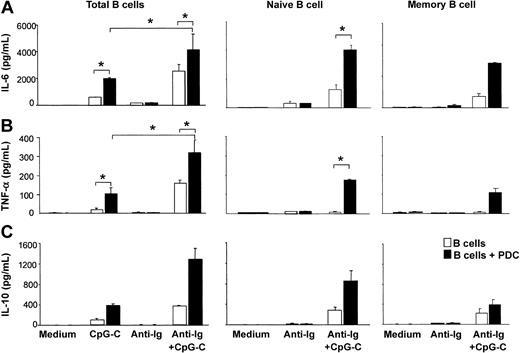
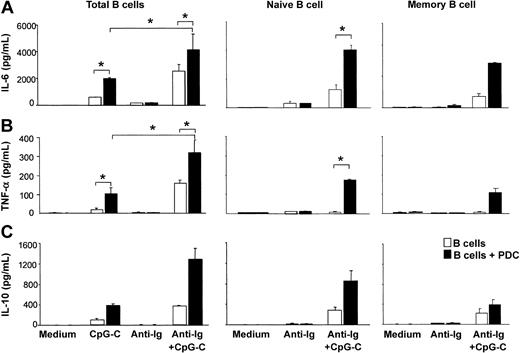
CpG-C alone induced the production of small amounts of IL-6, TNF-α, and IL-10 from B cells. The production of these cytokines was further enhanced in the presence of PDCs. The highest levels of IL-6, TNF-α, and IL-10 were found in cocultures of B cells and PDCs stimulated with CpG-C and anti-Ig (Figure 2). Next naive B cells (CD27-IgD+) and memory B cells (CD27+IgD-) were isolated from PBMCs. Although lower in naive B cells, considerable levels of TLR9 mRNA were found in both naive and memory B cells (naive B cells, 106 ± 8 copies TLR9 mRNA/105 copies of the housekeeping gene β-actin, n = 2; memory B cells, 237 ± 25 copies TLR9 mRNA, n = 2; data not shown). PDCs increased the production of IL-6, TNF-α, and IL-10 in both naive and memory B cells stimulated with CpG-C and anti-Ig (Figure 2). Despite the lower level of TLR9 mRNA in naive B cells, cytokine levels were consistently higher in naive B cells as compared to memory B cells.
The presence of PDCs enhances CpG-stimulated cytokine production in naive and memory B cells. Total B cells, naive B cells, or memory B cells were incubated alone (□ 1 × 105 B cells/200 μL/well) or in the presence of PDCs (▪ 1 × 104 PDCs/200 μL/well) and were stimulated with CpG-C (3 μg/mL), anti-Ig (10 μg/mL), or a combination of CpG-C and anti-Ig. After 72 hours, IL-6 (A), TNF-α (B), or IL-10 (C) was measured in the supernatants by ELISA. Data are shown as means ± SEM of 4 (IL-6 and TNF-α) or of 2 (IL-10) independent experiments.
The presence of PDCs enhances CpG-stimulated cytokine production in naive and memory B cells. Total B cells, naive B cells, or memory B cells were incubated alone (□ 1 × 105 B cells/200 μL/well) or in the presence of PDCs (▪ 1 × 104 PDCs/200 μL/well) and were stimulated with CpG-C (3 μg/mL), anti-Ig (10 μg/mL), or a combination of CpG-C and anti-Ig. After 72 hours, IL-6 (A), TNF-α (B), or IL-10 (C) was measured in the supernatants by ELISA. Data are shown as means ± SEM of 4 (IL-6 and TNF-α) or of 2 (IL-10) independent experiments.
Enhanced B-cell activation and differentiation in the presence of PDCs is reduced in transwell assays and is partially mediated via IFN-α
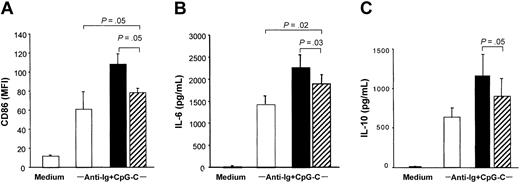
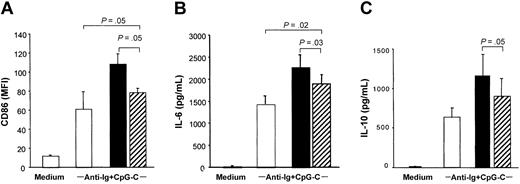
Separation of PDCs and B cells in transwells significantly reduced the costimulatory capacity of PDCs to increase CD86 expression and IL-6 and IL-10 production in B cells stimulated with CpG-C and anti-Ig (Figure 3; the transwell is shown in hatched bars). Despite reduced B-cell activation and differentiation, B cells separated from PDCs by the transwell system (Figure 3 hatched bars) still expressed higher levels of CD86, IL-6, and IL-10 as compared to B cells incubated in the absence of PDC (Figure 3 open bars). This suggested a contribution of soluble mediators.
PDCs contribute to B-cell activation by soluble factors. A transwell system was used to separate PDCs and B cells. B cells were cultured in the lower compartment of transwells (5 × 105 B cells/well) and PDCs in the upper compartment (5 × 104 PDC; ▨). B cells and PDCs were also cocultured without transwell (▪), and B cells were cultured without PDC (□). Cells were stimulated with CpG-C (3 μg/mL) and anti-Ig (10 μg/mL). After 3 days the MFI of CD86 was analyzed (A), and IL-6 (B) and IL-10 (C) were measured in the supernatants by ELISA. The means of 3 independent experiments ± SEM are shown.
PDCs contribute to B-cell activation by soluble factors. A transwell system was used to separate PDCs and B cells. B cells were cultured in the lower compartment of transwells (5 × 105 B cells/well) and PDCs in the upper compartment (5 × 104 PDC; ▨). B cells and PDCs were also cocultured without transwell (▪), and B cells were cultured without PDC (□). Cells were stimulated with CpG-C (3 μg/mL) and anti-Ig (10 μg/mL). After 3 days the MFI of CD86 was analyzed (A), and IL-6 (B) and IL-10 (C) were measured in the supernatants by ELISA. The means of 3 independent experiments ± SEM are shown.
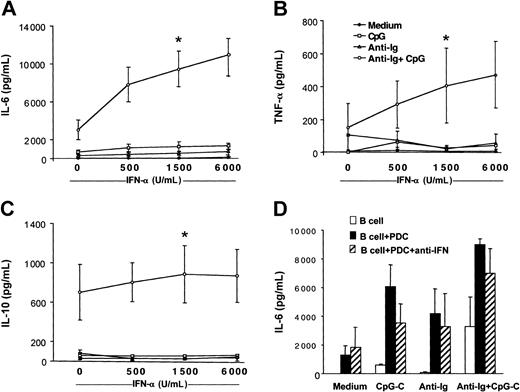
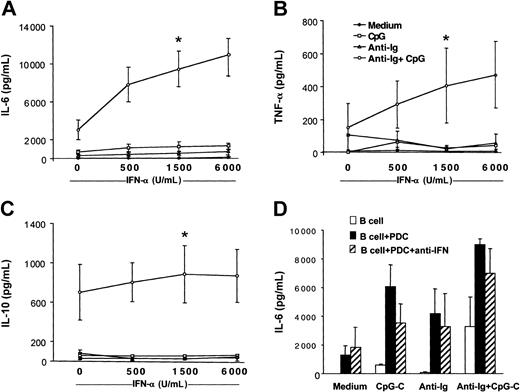
IL-10 has been reported to inhibit PDC function. Because B cells stimulated with CpG-C and anti-Ig released IL-10 (Figure 2), we examined whether PDCs coincubated with B cells are still capable of producing IFN-α. High levels of IFN-α were detected in cocultures of B cells and PDCs stimulated with CpG-C with or without anti-Ig for 72 hours (CpG-C, 18.838 ± 5.129 pg/mL; CpG-C, 13.305 ± 7.456 pg/mL; n = 2). In wells without PDCs, IFN-α was below 50 pg/mL (data not shown). To study the involvement of PDC-released IFN-α in B-cell activation, recombinant IFN-α was added to B cells stimulated with CpG, anti-Ig, or both. Increasing doses of recombinant IFN-α enhanced the production of IL-6, TNF-α, and IL-10 in B cells costimulated with CpG and anti-Ig (Figure 4A-C open circles). Not only cell-to-cell contact but also high local concentrations of IFN-α at the interface of B cells and PDCs may be eliminated in the transwell assays shown in Figure 3. A combination of blocking antibodies against type I IFN confirmed that B-cell activation is not exclusively mediated by IFN-α/-β (Figure 4D).
Recombinant IFN-α enhances cytokine production in B cells costimulated with B-cell antigen and CpG. B cells (2 × 105 B cells/200 μL/well) were incubated in the presence or absence of CpG-B (3 μg/mL), anti-Ig (10 μg/mL), and increasing concentrations of recombinant IFN-α (500, 1500, and 6000 IU/mL). After 72 hours, IL-6 (A), TNF-α (B), and IL-10 (C) concentrations were determined in the supernatants by ELISA (medium, closed symbols; CpG, open squares; anti-Ig, open triangles; and CpG + anti-Ig, open circles. The means ± SEM of 4 independent experiments are shown (*P < .05, evaluation for B cells stimulated with recombinant IFN-α as compared to B cells stimulated with anti-Ig and CpG alone). (D) B cells were incubated alone (□) or in the presence of PDCs (▪) and were stimulated with CpG-C, anti-Ig, or a combination of CpG-C and anti-Ig. The biologic activity of type I IFN (IFN-α and IFN-β) was blocked by a combination of anti–IFN-α, anti–IFN-β, and anti–IFN-α/-β receptor antibodies (▨). After 72 hours, IL-6 was measured by ELISA (means of 2 independent experiments).
Recombinant IFN-α enhances cytokine production in B cells costimulated with B-cell antigen and CpG. B cells (2 × 105 B cells/200 μL/well) were incubated in the presence or absence of CpG-B (3 μg/mL), anti-Ig (10 μg/mL), and increasing concentrations of recombinant IFN-α (500, 1500, and 6000 IU/mL). After 72 hours, IL-6 (A), TNF-α (B), and IL-10 (C) concentrations were determined in the supernatants by ELISA (medium, closed symbols; CpG, open squares; anti-Ig, open triangles; and CpG + anti-Ig, open circles. The means ± SEM of 4 independent experiments are shown (*P < .05, evaluation for B cells stimulated with recombinant IFN-α as compared to B cells stimulated with anti-Ig and CpG alone). (D) B cells were incubated alone (□) or in the presence of PDCs (▪) and were stimulated with CpG-C, anti-Ig, or a combination of CpG-C and anti-Ig. The biologic activity of type I IFN (IFN-α and IFN-β) was blocked by a combination of anti–IFN-α, anti–IFN-β, and anti–IFN-α/-β receptor antibodies (▨). After 72 hours, IL-6 was measured by ELISA (means of 2 independent experiments).
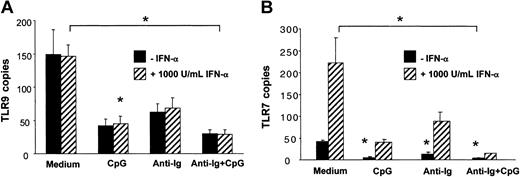
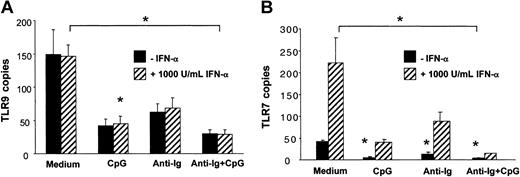
Unlike for TLR7, IFN-α did not up-regulate TLR9 mRNA expression in B cells (Figure 5A-B). CpG and anti-Ig independently down-regulated both TLR9 and TLR7. Expression of TLR9 and TLR7 was lowest when B cells were incubated in the presence of CpG and anti-Ig (Figure 5A-B). Similar results were obtained when data were normalized to the housekeeping gene β-actin instead of cyclophilin B. Together these data indicated that PDCs contribute to B-cell activation by the release of soluble IFN-α and possibly by cell-to-cell contact and that up-regulation of TLR9 is not involved.
The expression of TLR9 mRNA in human B cells is independently down-regulated by CpG and antigen. B cells (1 × 106/mL/well in 24-well plates) were stimulated with CpG-B (3 μg/mL), anti-Ig (10 μg/mL), and recombinant IFN-α (1000 IU/mL, ▨) for 12 hours. Expression of TLR9 and TLR7 mRNA was determined by quantitative real-time PCR and is depicted as the number of transcripts per 103 copies of the housekeeping gene cyclophilin-B (CPB). The means ± SEM of 3 independent experiments are shown. P < .05
The expression of TLR9 mRNA in human B cells is independently down-regulated by CpG and antigen. B cells (1 × 106/mL/well in 24-well plates) were stimulated with CpG-B (3 μg/mL), anti-Ig (10 μg/mL), and recombinant IFN-α (1000 IU/mL, ▨) for 12 hours. Expression of TLR9 and TLR7 mRNA was determined by quantitative real-time PCR and is depicted as the number of transcripts per 103 copies of the housekeeping gene cyclophilin-B (CPB). The means ± SEM of 3 independent experiments are shown. P < .05
PDCs support plasma cell differentiation and immunoglobulin production in peripheral blood B cells
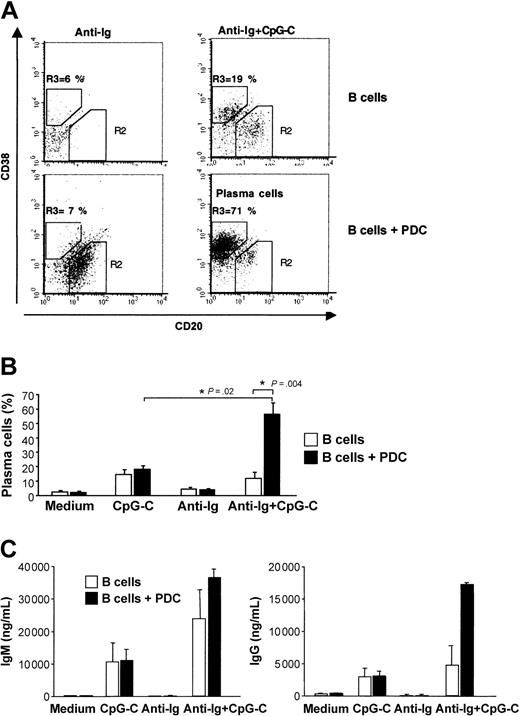
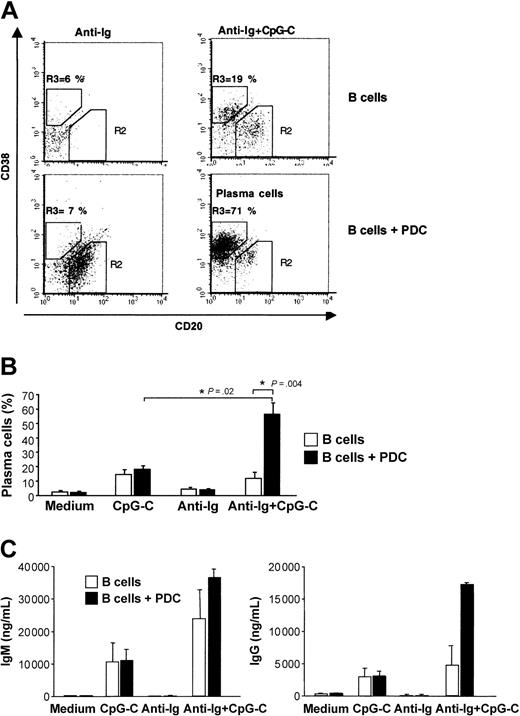
Plasma cell development is characterized by down-regulation of CD20 and up-regulation of CD38. The generation of plasma cells was strongly increased when CpG-C– and anti-Ig–stimulated B cells were cocultured with PDCs (Figure 6A-B). Consistent with the frequency of developing plasma cells, the highest levels of IgM and IgG were found in cocultures of B cells and PDCs stimulated with CpG-C and anti-Ig (Figure 6C).
PDCs enhance plasma cell development and immunoglobulin production of antigen- and CpG-stimulated B cells. B cells (1 × 105 B cells/200 μL/well) were incubated alone or in the presence of PDCs (1 × 104 PDCs/200 μL/well) and were stimulated with CpG-C (3 μg/mL), anti-Ig (10 μg/mL), or a combination of CpG-C and anti-Ig. (A) After 8 days the frequency of plasma cells (CD20-CD38+) was determined by flow cytometry. The frequency of CD20-CD38+ plasma cells is indicated in the upper left quadrant (top panel, B cells alone; bottom panel, B cells cocultured with PDCs). One representative dot plot is shown. (B) The mean plasma cell frequencies (± SEM) of 5 independent experiments are depicted. (C) After 13 days IgM (left panel) and IgG (right panel) were measured in the supernatants by ELISA. Data are shown as means ± SEM of 3 independent experiments (□, B cells alone; ▪, B cells plus PDCs).
PDCs enhance plasma cell development and immunoglobulin production of antigen- and CpG-stimulated B cells. B cells (1 × 105 B cells/200 μL/well) were incubated alone or in the presence of PDCs (1 × 104 PDCs/200 μL/well) and were stimulated with CpG-C (3 μg/mL), anti-Ig (10 μg/mL), or a combination of CpG-C and anti-Ig. (A) After 8 days the frequency of plasma cells (CD20-CD38+) was determined by flow cytometry. The frequency of CD20-CD38+ plasma cells is indicated in the upper left quadrant (top panel, B cells alone; bottom panel, B cells cocultured with PDCs). One representative dot plot is shown. (B) The mean plasma cell frequencies (± SEM) of 5 independent experiments are depicted. (C) After 13 days IgM (left panel) and IgG (right panel) were measured in the supernatants by ELISA. Data are shown as means ± SEM of 3 independent experiments (□, B cells alone; ▪, B cells plus PDCs).
PDCs trigger plasma cell development and IgM production in both naive and memory B cells and IgG production in memory B cells
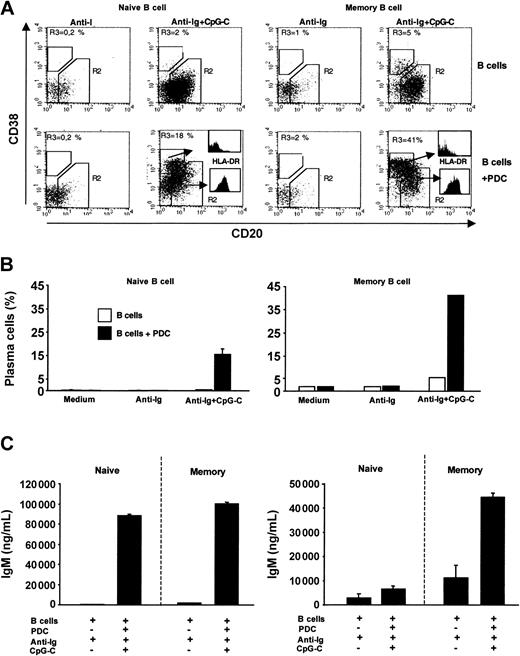
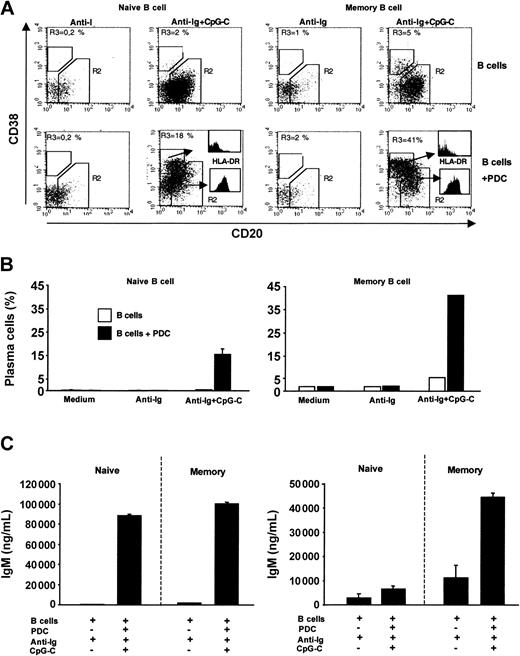
Naive and memory B cells were sorted from PBMCs. In the absence of PDCs, combined stimulation with anti-Ig and CpG-C was not sufficient to trigger plasma cell development in naive B cells (Figure 7A-B left panel). However, in the presence of PDCs a considerable proportion of naive B cells down-regulated CD20 and up-regulated CD38 (characteristic for plasma cell development; Figure 7A left panel). Developing plasma cells (CD20-CD38+) showed down-regulated levels of MHC II as compared to CD20+CD38- cells (Figure 7A histograms). Plasma cell development in cocultures of PDCs with naive or with memory B cells was associated with the production of high levels of IgM (Figure 7C). IgG synthesis was largely restricted to cocultures of PDCs and memory B cells (Figure 7C). Together these data demonstrate that CpG, anti-Ig, and the coculture with PDCs are sufficient to allow naive B cells to undergo plasma cell differentiation and IgM production and secretion.
PDCs trigger plasma cell differentiation of naive and memory B cells. Sorted naive or memory B cells (1 × 105 B cells/200 μL/well) were incubated alone or in the presence of PDCs (1 × 104 PDCs/200 μL/well) and were stimulated with anti-Ig (10 μg/mL) with or without CpG-C (3 μg/mL). (A) After 8 days the frequency of plasma cells (CD20-CD38+) and the MHC II expression was determined by flow cytometry. The frequency of plasma cells (gate R3) is indicated in the upper left quadrant (left panel, naive B cells; right panel, memory B cells; upper panel, B cells alone; lower panel, B cells plus PDCs). The expression of MHC II on plasma cells and nonplasma cells is shown by histograms. (B) The mean plasma cell frequency is depicted (naive B cells, mean ± SEM of 2 independent experiments; memory B cells, one experiment; □, B cells alone; ▪, B cells plus PDCs). (C) After 13 days IgM and IgG were measured in the supernatants by ELISA. Data are shown as means ± SEM of 3 independent experiments.
PDCs trigger plasma cell differentiation of naive and memory B cells. Sorted naive or memory B cells (1 × 105 B cells/200 μL/well) were incubated alone or in the presence of PDCs (1 × 104 PDCs/200 μL/well) and were stimulated with anti-Ig (10 μg/mL) with or without CpG-C (3 μg/mL). (A) After 8 days the frequency of plasma cells (CD20-CD38+) and the MHC II expression was determined by flow cytometry. The frequency of plasma cells (gate R3) is indicated in the upper left quadrant (left panel, naive B cells; right panel, memory B cells; upper panel, B cells alone; lower panel, B cells plus PDCs). The expression of MHC II on plasma cells and nonplasma cells is shown by histograms. (B) The mean plasma cell frequency is depicted (naive B cells, mean ± SEM of 2 independent experiments; memory B cells, one experiment; □, B cells alone; ▪, B cells plus PDCs). (C) After 13 days IgM and IgG were measured in the supernatants by ELISA. Data are shown as means ± SEM of 3 independent experiments.
Discussion
Here we report a T-cell–independent pathway of plasma cell development that involves costimulation of B cells with PDCs, a subset of dendritic cells specialized on antiviral immune responses. We found that PDCs synergistically enhance activation (CD86) and cytokine production (IL-6, TNF-α, IL-10) of human peripheral blood B cells that were stimulated by B-cell receptor ligation (anti-Ig) and a microbial molecule (CpG). PDC-derived soluble factors including IFN-α contributed to B-cell activation. PDCs strongly enhanced plasma cell differentiation and increased immunoglobulin production (IgM and IgG) of blood B cells. In purified naive B cells, PDCs triggered the development of plasma cells that produced mainly IgM and only little IgG.
In this study we take advantage of a recently identified group of CpG oligonucleotides (CpG-C) that represents the first well-defined microbial molecule that is able to simultaneously activate B cells and PDCs both expressing TLR9.18,19 Of note, lipopolysaccharide (LPS), another pathogen-derived microbial molecule, is not recognized by human B cells and PDCs due to the lack of TLR4 on both cell types.4
Down-regulation of TLR9 mRNA was observed in response to B-cell receptor ligation and to CpG-C. Therefore up-regulation of TLR9 expression is not likely involved in the synergistic effect of B-cell receptor ligation, CpG-C, and PDC-derived IFN-α. TLR9 down-regulation in this study confirms our earlier results of CpG-induced down-regulation of TLR9 mRNA expression in B cells after 3 hours and 15 hours.4 However, down-regulation of TLR9 in our study contradicts results obtained by others.3,23
In the literature, IL-12 secreted by CD40L-activated myeloid dendritic cells has been identified as a key soluble factor that supports differentiation of naive B cells into immunoglobulin-secreting plasma cells.21 Importantly, in that study not only myeloid dendritic cells but also naive B cells required additional stimulation with CD40L. Other studies suggested a role of dendritic cells of the myeloid lineage in plasma cell development.24,25 However, in all of the above studies on the interaction of myeloid dendritic cells and B cells, the addition of exogenous T-cell–derived stimuli such as CD40L or IL-2 or IL-10 was necessary for the induction of plasma cell differentiation. In our study, T-cell–derived stimuli were not required for the induction of plasma cell differentiation by PDCs.
It has been reported that type I IFN enhances the antibody response and induces isotype switching in vivo.20 Interestingly, in LeBon et al's paper isotype switching occurred even when dendritic cells were the only cell type responding to type I IFN. In our studies, despite the production of high amounts of IFN-α and the induction of plasma cell differentiation by PDCs, no evidence was found that PDCs support isotype switching in cocultures of PDCs and naive B cells. Therefore, PDCs alone seem unable to substitute for the important signaling between B cells and T cells that stimulates isotype switching. Evidence from the literature indicates that other dendritic cell subsets contribute to isotype switching of CD40L-stimulated naive B cells.25,26 One could speculate that myeloid dendritic cells mediate IFN-α–induced isotype switching in vivo in the study by Le Bon and colleagues.20
It is known that T-cell help (CD40L) is responsible for endogenous production of IL-10 and IL-6 in B cells and that both cytokines play a critical role in the differentiation of CD40-activated B cells.27 In our study, PDCs seem to substitute for this aspect of T-cell help by synergistically increasing the production of both IL-6 and IL-10 in CpG-stimulated B cells. We identified IFN-α as one of the PDC-derived factors that induced IL-6 in B cells stimulated with CpG and B-cell antigen; in the absence of CpG or B-cell antigen the effect of IFN-α on IL-6 production in B cells was low. In addition to PDC-derived IFN-α, transwell assays in our study suggested that direct cell-to-cell contact of B cells and PDCs may contribute to B-cell activation. The lack of complete reversal of B-cell activation by blocking antibodies against type I IFNs indicated that high local concentrations of IFN-α or IFN-β at the interface of B cells and PDCs are not responsible for this additional transwell-sensitive effect.
Plasma cell development from naive B cells in the absence of T-cell–derived costimulation is usually limited to certain thymus-independent (TI) antigens1 and to some viral infections in vivo.28 Our results demonstrate that appropriate microbial stimulation of B cells and PDCs along with a model antigen for B-cell receptor ligation (anti-Ig) is sufficient to drive plasma cell development of both naive and memory B cells without T-cell–derived costimulation. Thus, under certain circumstances, plasma cell differentiation can be a helper T-cell–independent phenomenon. Of note, in a recent publication, T-cell help was required for PDCs to induce plasma cell differentiation.29 In that publication, influenza virus was used to induce IFN-α production in PDCs, and CD40L was used to activate B cells. In our study, CpG-C seems to substitute for viral stimulation of PDCs and for CD40L-mediated stimulation of B cells.
Several studies are available on the stimulation of B cells with CpG with or without antigen.3,30-32 All of these studies are in agreement with the concept that stimulation with antigen and CpG is sufficient to drive memory B cells toward plasma cell development. However, stimulation with antigen and CpG is not sufficient to drive plasma cell differentiation from naive B cells unless additional stimulation is provided; this can be T-cell help (CD40L or cytokines) as demonstrated in the literature, or alternatively PDCs as demonstrated in our study.
It has been reported that mice that lack T-cell help (MHC II knockout mice) are still capable of mounting a neutralizing IgM response against the glycoprotein of vesicular stomatitis virus (VSV); in contrast to IgM, IgG was found to be strictly dependent on CD4 T-cell help.28 Similar to this, in our study PDCs substitute for T-cell help with regard to B-cell differentiation and IgM production. Therefore, PDCs may be responsible for supporting T-helper cell–independent humoral immune responses during viral infections in vivo. Interestingly, T cells and PDCs are colocalized in the T-cell zone of lymphoid tissue.33 From there PDCs, similar to T cells, could migrate to the outer T-cell zone in which T cells are known to interact with B cells that have taken up and processed antigen.34
Recent studies demonstrate that the gDNA of a virus can engage TLR9, resulting in the secretion of IFN-α by PDCs.35,36 However, there is limited information on how CpG motifs within microbial DNA are recognized by B cells under physiologic circumstances, that is, during bacterial or viral infections. DNA-containing chromatin-IgG complexes were found to activate B cells via a MyD88-dependent mechanism.37 The dual engagement of B-cell receptor and TLR in their study restricts the B-cell response to B cells recognizing their corresponding antigen, and thus polyclonal activation of B cells is avoided. However, it is known that the presence of CpG DNA alone at high concentrations leads to polyclonal B-cell stimulation. However, it is questionable whether microbial DNA in the extracellular space in B-cell areas of lymphatic tissue would reach high enough concentrations to stimulate B cells. Although this may happen during serious systemic infections such as sepsis, degradation of microbial DNA by nucleases will prevent this situation during the course of a normal limited infection. In DNA-containing immune complexes DNA is protected from nuclease degradation. Antigen-restricted stimulation of B cells by dual engagement via the B-cell receptor and TLR9 may represent the more physiologic situation of B-cell stimulation by CpG DNA.
Although polyclonal activation of B cells by CpG may be less relevant during the natural course of an infection, polyclonal versus antigen-specific B-cell activation is highly relevant for the clinical development of CpG ODNs, particularly as vaccine adjuvants. Polyclonal stimulation of B cells by the administration of CpG-C alone without specific antigen may be useful for activating memory B cells to maintain or improve serologic memory in a nonantigen-specific manner. Such an effect has been shown for activated T cells (providing CD40L) in the context of vaccination with unrelated antigens.30
In conclusion, our results establish a role of PDCs in B-cell differentiation. In addition to the premise of CpG-C as humoral vaccine adjuvant, polyclonal B-cell activation in the absence of antigen may be useful to support serologic memory in an antigen-nonspecific way. However, the potential of CpG-C to induce autoimmunity should be closely followed.
Prepublished online as Blood First Edition Paper, December 30, 2003; DOI 10.1182/blood-2003-08-2972.
Supported by a grant from the DFG HA 2780/4-1 (G.H.) and from the BMBF and Coley Pharmaceutical, Langenfeld 03-12235-6.
H.P., M.W., and J.B. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Norbert Lubenow and Prof Andreas Greinacher from the Institute of Immunology and Transfusion Medicine at the University of Greifswald, Germany, for providing blood products.
This work is part of the dissertation of H.P. and J.B. at the Ludwig-Maximilians-University (Munich, Germany).