Abstract
The synthetic triterpenoid 2-cyano-3, 12-dioxooleana-1, 9-dien-28-oic acid (CDDO) induces apoptosis in leukemic cells. Here we show that CDDO and its new derivative CDDO-imidazolide (CDDO-Im) trigger apoptosis in multiple myeloma (MM) cells resistant to conventional therapies including melphalan (LR-5), doxorubicin (Dox-40), and dexamethasone (MM.1R, U266, RPMI 8226) without affecting the viability of normal cells. CDDO-IM also triggers apoptosis in bone marrow stromal cells (BMSCs) and decreases interleukin-6 (IL-6) secretion induced by MM cell adhesion to BMSCs. Moreover, CDDO-Im–induced apoptosis in MM cells is not blocked by IL-6 or insulin growth factor-1 (IGF-1). Importantly, CDDO-Im and bortezomib/proteasome inhibitor PS-341 trigger synergistic apoptosis in MM cells associated with loss of mitochondrial membrane potential, superoxide generation, release of mitochondrial proteinscytochrome c/second mitochondria-derived activator of caspases (cyctochrome c/Smac), and activation of caspase-8, -9, and -3. Conversely, the pancaspase inhibitor Z-VAD-fmk abrogates the CDDO-Im + bortezomib–induced apoptosis. Low doses of CDDO-Im and bortezomib overcome the cytoprotective effects of antiapoptotic proteins Bcl2 and heat shock protein-27 (Hsp27) as well as nuclear factor–kappa B (NF-κB)–mediated growth/survival and drug resistance. Finally, combining CDDO-Im and bortezomib induces apoptosis even in bortezomib-resistant MM patient cells. Together, these findings provide the framework for clinical evaluation of CDDO-Im, either alone or in combination with bortezomib, to overcome drug resistance and improve patient outcome in MM. (Blood. 2004;103: 3158-3166)
Introduction
Multiple myeloma (MM) remains fatal despite all available therapies. Initial treatment with dexamethasone (Dex) effectively induces MM cell death; however, prolonged drug exposures result in the development of de novo chemoresistance.1,2 The development of chemoresistance is associated with these events: defective apoptotic signaling in response to drugs; overexpression of antiapoptotic proteins such as Bcl2 or inhibitors of apoptosis protein (IAPs)3,4 ; expression of multidrug resistance (MDR) gene5,6 ; the presence of growth-promoting cytokines within the bone marrow (BM) microenvironment such as interleukin-6 7 ; and insulin growth factor-1 (IGF-1).2,8,9 Novel anti-MM agents that reverse drug resistance and enhance cell death are needed.
Previous studies have shown that synthetic triterpenoid 2-cyano-3, 12-dioxooleana-1, 9-dien-28-oic acid (CDDO) triggers apoptosis in various human carcinomas and leukemic cells.10-14 CDDO-induced apoptosis is associated with these events: down-regulation of caspase-8 homolog Fas-ligand interleukin-1–converting enzyme (FLICE)–inhibitory protein (c-FLIP), cleavage of Bid, activation of caspase-8 and -3, release of mitochondrial protein cytochrome c, inhibition of de novo nitric oxide synthase (iNos), modulation of proximal proliferator-activated receptor-gamma (PPAR-γ) transcriptional activity, and inhibition of nuclear factor–kappa B (NF-κB).10-14 A recent study showed that a new derivative of CDDO, CDDO-imidazolide (CDDO-Im), is more potent than its parent compound, CDDO, both in vitro15 and in vivo against murine melanoma and leukemic cells. To date, however, the effect of triterpenoids on MM cells is undefined.
Our recent studies showed that bortezomib/proteasome inhibitor PS-341 induces apoptosis in MM cells refractory to multiple prior therapies including dexamethasone (Dex), melphalan, or thalidomide.16,17 On the basis of our preclinical and clinical studies, the Food and Drug Administration (FDA) recently approved bortezomib for the treatment of relapsed refractory MM. Although initial treatment with bortezomib triggers apoptosis in MM cells, de novo PS-341 resistance ultimately develops in some cases. To date, however, there is no therapy to overcome PS-341 resistance.
In the present study, we asked (1) whether triterpenoids affect MM cell viability, and (2) whether a combination of minimally toxic doses of CDDO-Im + bortezomib induces apoptosis in MM cells, overcomes bortezomib resistance, and enhances anti-MM activity. We show that both CDDO and CDDO-Im induce apoptosis in MM cells, with CDDO-Im 4- to 6-fold more cytotoxic than its parental compound, CDDO. Importantly, a combination of subtoxic doses of CDDO-Im and bortezomib triggers significant apoptosis even in MM cells resistant to bortezomib, via mitochondria-dependent and mitochondria-independent apoptotic signaling pathways. These preclinical studies provide the framework for clinical evaluation of triterpenoids, either as a monotherapy or in combination with less toxic doses of bortezomib, to inhibit MM cell growth and overcome drug resistance.
Materials and methods
Cell culture and reagents
Dex-sensitive MM.1S and Dex-resistant MM.1R human MM cell lines18,19 were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL). Doxorubicin (Dox)–resistant (Dox-40) and melphalan-resistant (LR-5) RPMI 8226 cells were kindly provided by Dr William Dalton (Moffit Cancer Center, Tampa, FL). The U266 MM cell line was obtained from the American Type Culture Collection (Rockville, MD). SUDHL4 (DHL4) lymphoma cells were kindly provided by Dr Margaret Shipp, Dana-Farber Cancer Institute, Boston, MA. Human B-cell lymphoma cell line RC-K8 was kindly provided by Dr Thomas Gilmore (Boston University, MA). All cell lines were grown in RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine. Drug-resistant cell lines were cultured with low doses of drugs to confirm their lack of drug sensitivity. The MM cells were freshly isolated from patients relapsing after multiple prior therapies including dexamethasone, melphalan, thalidomide, or bortezomib. Approval for these studies was obtained from the Dana-Farber Cancer Institute institutional review board. Informed consent was obtained from all patients in accordance with Declaration of Helsinki protocol. Mononuclear cells were prepared from MM patient BM samples by Ficoll-Hypaque density gradient centrifugation. Tumor cells (CD138+ 97% ± 2.0%) were isolated by CD138+ selection method19 using CD138 (syndecan-1) Micro Beads and the Auto MACS magnetic cell sorter, according to manufacturer's instructions (Miltenyi Biotec, Auburn, CA). CD138+ myeloma cells were viable (94%-97%) for 2 to 3 weeks in vitro. Cells were treated with various concentrations of CDDO, CDDO-Im, bortezomib (Millenium Pharmaceuticals, Cambridge, MA), and/or Dex (Sigma Chemical, St Louis, MO).
Cell viability assays
Cell viability was assessed by 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Chemicon International, Temecula, CA) assay according to manufacturer's instructions, with some modifications. Cells were seeded in 96-well plates in RPMI 1640 medium containing 5% fetal bovine serum (FBS). CDDO, CDDO-Im, or bortezomib were added 24 hours later and incubated for 24 hours. Cells were also treated with CDDO-Im + interleukin-6 (IL-6) (10 ng/mL) or CDDO-Im + IGF-1 (50 ng/mL) and analyzed for cell viability, as previously described.19,20
Quantification of apoptosis
Cell Death Detection ELISAplus was utilized to quantitate cell death, as per manufacturer's instructions (Roche Applied Sciences, Indianapolis, IN). Apoptosis was also assessed by annexin V staining, as previously described.19 Dual fluorescence staining with DNA-binding fluorochrome Hoechst 33342 (HO) and propidium iodide (PI) was used to quantitate the percentage of apoptotic (PI-HO+) cells using flow cytometry (The Vantage, Becton Dickinson, Bedford, MA), as previously described.19
Enzyme-linked immunosorbent assays (ELISAs)
Conditioned media were generated from 12-hour cultures of MM patient–derived BMSCs, MM.1S cells, and BMSCs + MM.1S cells, either untreated or treated with CDDO-Im (200 nM) or bortezomib (4 nM); IL-6 levels were measured using enzyme-linked immunosorbent assays (ELISAs) (R&D Systems, Minneapolis, MN), as previously described.20 Briefly, 96-well plates were coated with antihuman IL-6 antibodies (Abs) overnight, washed, and then blocked with 300 μL phosphate-buffered saline (PBS), 1% bovine serum albumin (BSA), 5% sucrose, and 0.05% NaN3 for 2 hours. After washing, 100 μL sample or standards diluted in Tris (tris(hydroxymethyl)aminomethane)–Cl, 0.1% BSA, and 0.05% Tween 20 (pH 7.3) were added to the wells and incubated for 2 hours at room temperature (RT). The cells were rinsed, and biotinylated antihuman IL-6 Ab was added for 2 hours at RT. After a further washing step, the wells were incubated with streptavidin horseradish peroxidase for 20 minutes at RT and rinsed. The reaction was started by the addition of 100 μL H2O2 and tetramethylbenzidine for 30 minutes at RT. After stopping the reaction with 1 μM H2SO4, the optical density of each well was detected by means of a microtiter plate reader at 450 nm, with correction at 540 nm.
Mitochondrial membrane potential (•Ψm) and generation of superoxide (O2-) anions
Serum-starved MM.1S cells were treated with bortezomib (2 nM) alone, CDDO-Im (80 nM) alone, or bortezomib + CDDO-Im for 12 hours; incubated with chloromethyl-X-rosamine (CMXRos) for the last 20 minutes; stained with lipophilic cationic dye CMXRos (Mitotracker Red) (Molecular Probes, Eugene, OR) in PBS for 20 minutes at 37°C; and analyzed by flow cytometry to assay for alterations in •Ψm.21 Superoxide production was measured by staining cells with membrane-permeable dye dihydroethidium (HE), as previously described.22
Western blotting
Total cell lysates were prepared, and Western blot analysis was performed as previously described.23 Briefly, equal amounts of proteins were resolved by 10% or 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto nitrocellulose membranes, filters were blocked by incubation in 5% dry milk in PBST (0.05% Tween 20 in PBS), and probed with anti–cytochrome c (anti–cyto-c), anti–second mitochondria-derived activator of caspases (anti-Smac) (kindly provided by Dr Xiaodong Wang, University of Texas Southwestern Medical Center at Dallas), antitubulin (Sigma Chemical), anticaspase-8, -9, or -3 (Cell Signaling Technology, Beverly, MA), anticaspase-3 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Bcl2, or anti–heat shock protein-27 (anti-Hsp27) Abs (Transduction Laboratories). Blots were developed by enhanced chemiluminesence (ECL; Amersham, Arlington Heights, IL). The immunoblots were scanned using an LKB Produkter (Bromma, Sweden) Ultrascan XL laser densitometer and analyzed with the Gelscan software package (Bromma, Sweden). Signal intensity was determined in a linear range and normalized to that for tubulin. Preparation of cell lysates for poly(adenosine diphosphate [ADP]–ribose) polymerase (PARP) immunoblot analysis was performed as described using C-2-10 anti-PARP monoclonal antibody.24
Isobologram analysis
The interaction between anti-MM agents CDDO-Im and bortezomib was analyzed using CalcuSyn software program (Biosoft, Ferguson, MO). Data from cell viability assay (MTT) were expressed as fraction of cells with growth affected (FA) in drug-treated versus untreated cells. This program is based upon the Chou-Talalay method25 according to the following equation: CI = (D)1/(Dx)1 + (D)2/(Dx)2 + (D)1(D)2/(Dx)1(Dx)2, where (D)1 and (D)2 are the doses of drug 1 and drug 2 that have x effect when used in combination, and (Dx)1 and (Dx)2 are the doses of drug 1 and drug 2 that have the same x effect when used alone. When CI = 1, this equation represents the conservation isobologram and indicates additive effects. CI values of less than 1.0 indicate synergism.
Results and discussion
CDDO-Im inhibits growth and induces apoptosis in MM cells resistant to conventional therapy
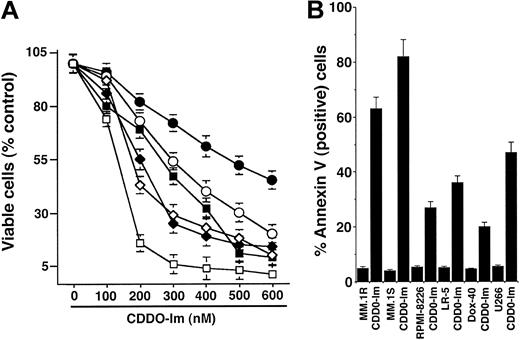
We first determined whether CDDO-Im affects the viability using MTT assay in MM cells resistant to conventional therapy, including doxorubicn (Dox-40)–, melphalan (LR-5)–, and dexamethasone (MM.1R)–resistant cells. As seen in Figure 1A, treatment of MM cells with CDDO-Im (0.05-0.6 μM) for 24 hours induces a significant (P < .0005; n = 3) decrease in cell viability in a dose-dependent manner in all cell lines. Similar results were observed in Dex-sensitive MM.1S cells. Fifty percent decrease in viable cells was noted at 0.12 to 0.5 μM. IC50 of CDDO-Im for MM.1S cells was 0.12 to 0.15 μM. We next examined whether CDDO-Im–induced cytotoxicity correlates with apoptosis in these cells. As seen in Figure 1B, CDDO-Im (200 nM) triggers significant apoptosis in MM cells, evidenced by an increase in percentage of annexin V–positive cells (P < .005; n = 3). To determine whether the parental compound of CDDO-Im shows similar anti-MM activity, MM cell lines were treated with various concentrations of CDDO and analyzed for apoptosis. CDDO induces apoptosis in MM cells at approximately 4- to 6-fold lower concentrations than CDDO-Im (data not shown). Together, these data suggest that (1) CDDO-Im inhibits growth and induces apoptosis in MM cells sensitive and resistant to conventional therapies and (2) CDDO-Im is more potent than its parental compound, CDDO (IC50 for CDDO = 0.9 to 1.0 μM versus IC50 for CDDO-Im = 0.10 to 0.15 μM). These findings are consistent with another study showing greater antitumor activity of CDDO-Im versus CDDO in murine leukemic cells.15
CDDO-Im inhibits growth and induces apoptosis in human MM cells sensitive and resistant to conventional drugs. (A) MTT assays were performed after incubation of Dex-sensitive MM.1S (□), U266 ( ), Dex-resistant MM.1R (⋄), melphalan-resistant LR-5 (▪), RPMI 8226 (○), and doxorubicin-resistant Dox-40 (•) with indicated doses of CDDO-Im for 24 hours. Results are mean ± SD from 3 independent experiments (P < .0001 for all cell lines). (B) MM cell lines were treated with CDDO-Im (200 nM) and analyzed for apoptosis by annexin V staining. Results are mean ± SD from 3 independent experiments (P < .005 for all cell lines).
), Dex-resistant MM.1R (⋄), melphalan-resistant LR-5 (▪), RPMI 8226 (○), and doxorubicin-resistant Dox-40 (•) with indicated doses of CDDO-Im for 24 hours. Results are mean ± SD from 3 independent experiments (P < .0001 for all cell lines). (B) MM cell lines were treated with CDDO-Im (200 nM) and analyzed for apoptosis by annexin V staining. Results are mean ± SD from 3 independent experiments (P < .005 for all cell lines).
CDDO-Im inhibits growth and induces apoptosis in human MM cells sensitive and resistant to conventional drugs. (A) MTT assays were performed after incubation of Dex-sensitive MM.1S (□), U266 ( ), Dex-resistant MM.1R (⋄), melphalan-resistant LR-5 (▪), RPMI 8226 (○), and doxorubicin-resistant Dox-40 (•) with indicated doses of CDDO-Im for 24 hours. Results are mean ± SD from 3 independent experiments (P < .0001 for all cell lines). (B) MM cell lines were treated with CDDO-Im (200 nM) and analyzed for apoptosis by annexin V staining. Results are mean ± SD from 3 independent experiments (P < .005 for all cell lines).
), Dex-resistant MM.1R (⋄), melphalan-resistant LR-5 (▪), RPMI 8226 (○), and doxorubicin-resistant Dox-40 (•) with indicated doses of CDDO-Im for 24 hours. Results are mean ± SD from 3 independent experiments (P < .0001 for all cell lines). (B) MM cell lines were treated with CDDO-Im (200 nM) and analyzed for apoptosis by annexin V staining. Results are mean ± SD from 3 independent experiments (P < .005 for all cell lines).
CDDO-Im does not affect viability of normal lymphocytes
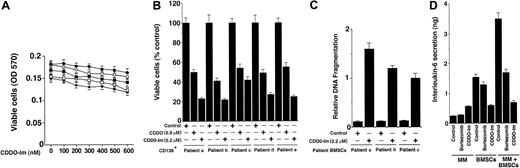
Normal lymphocytes from 5 healthy donors were treated with various doses (0.1-0.6 μM) of CDDO-Im and analyzed for both cytotoxicity and apoptosis using MTT and DNA fragmentation assays, respectively. As seen in Figure 2A and in contrast to MM cells, survival of normal lymphocytes from 5 healthy donors was not altered significantly (P = .21 from Jonchkeere-Tepstra [J-T] trend test), even at higher doses (0.5 μM) of CDDO-Im. No significant apoptosis of normal lymphocytes was observed in response to CDDO-Im (data not shown). Our data are consistent with another study demonstrating a lack of cytotoxicity of triterpenoids on normal lymphocytes.12 Taken together, these findings indicate that CDDO-Im has selective anti-MM activity.
Effects of CDDO-Im on normal lymphocytes, patient MM (CD138+), and bone marrow stromal cells (BMSCs). (A) Normal lymphocytes from 5 healthy donors were treated with CDDO-Im (0-600 nM) for 24 hours, and viability was assessed by an MTT assay. Results are the mean ± SD of 3 independent experiments, P = .25 from Jonchkeere-Tepstra (J-T) test for trend. (B) MM cells (CD138+) from 5 patients (patients a-e) were treated with either CDDO (0.9 μM) or CDDO-Im (0.2 μM) for 24 hours, followed by analysis of cell viability using an MTT assay. Values are the mean ± SD of triplicate samples, P = .05; experiments were repeated 3 times with similar results. (C) CDDO-Im triggers apoptosis in BMSCs. Patient MM-derived BMSCs (patients a-c) were treated with CDDO-Im (200 nM) for 24 hours and analyzed for apoptosis using DNA fragmentation assays. Results are mean ± SD from triplicate samples, P = .002. (D) Effect of CDDO-Im on MM cell adhesion–induced IL-6 secretion. IL-6 levels were measured using IL-6 ELISA on supernatants obtained from 24-hour cultures of MM cells, BMSCs, and BMSCs + MM cells in the presence or absence of either CDDO-Im (200 nM) or bortezomib/proteasome inhibitor PS-341 (4 nM). Results are mean ± SD of 3 independent experiments.
Effects of CDDO-Im on normal lymphocytes, patient MM (CD138+), and bone marrow stromal cells (BMSCs). (A) Normal lymphocytes from 5 healthy donors were treated with CDDO-Im (0-600 nM) for 24 hours, and viability was assessed by an MTT assay. Results are the mean ± SD of 3 independent experiments, P = .25 from Jonchkeere-Tepstra (J-T) test for trend. (B) MM cells (CD138+) from 5 patients (patients a-e) were treated with either CDDO (0.9 μM) or CDDO-Im (0.2 μM) for 24 hours, followed by analysis of cell viability using an MTT assay. Values are the mean ± SD of triplicate samples, P = .05; experiments were repeated 3 times with similar results. (C) CDDO-Im triggers apoptosis in BMSCs. Patient MM-derived BMSCs (patients a-c) were treated with CDDO-Im (200 nM) for 24 hours and analyzed for apoptosis using DNA fragmentation assays. Results are mean ± SD from triplicate samples, P = .002. (D) Effect of CDDO-Im on MM cell adhesion–induced IL-6 secretion. IL-6 levels were measured using IL-6 ELISA on supernatants obtained from 24-hour cultures of MM cells, BMSCs, and BMSCs + MM cells in the presence or absence of either CDDO-Im (200 nM) or bortezomib/proteasome inhibitor PS-341 (4 nM). Results are mean ± SD of 3 independent experiments.
CDDO-Im inhibits growth and triggers apoptosis in patient MM cells
We next determined the effects of CDDO-Im on MM cells freshly isolated from patients relapsing after multiple prior therapies including dexamethasone, melphalan, 2-methoxyestradiol, and thalidomide. Tumor cells were purified from BM aspirates by CD138+ selection using CD138 (syndecan-1) Micro Beads and Auto MACS magnetic cell sorting. As seen in Figure 2B, treatment of MM cells from 5 patients (patient nos. 1-5) with CDDO-Im (0.2 μM) for 24 hours significantly (P = .05) decreased the survival of these cells, as measured by an MTT assay. To determine whether CDDO-Im–induced growth inhibition correlates with apoptosis, 3 patient MM cells were treated with CDDO-Im and analyzed for apoptosis using PI/HO staining. CDDO-Im (0.2 μM) induces significant (P < .005; n = 3) apoptosis in patient MM cells (median percentage apoptotic [PI-HO+] cells was 63.2% ± 2.3% in CDDO-Im versus 5.3% ± 0.3% in untreated cells; P < .004). Taken together, our results suggest that CDDO-Im inhibits growth and induces apoptosis in patient MM cells.
CDDO-Im induces apoptosis in patient MM-derived bone marrow stromal cells (BMSCs)
In MM, tumor cells are predominantly localized in the BM microenvironment due to their adherence both to extracellular matrix proteins and to BMSCs. This interaction between MM cells and BMSCs triggers production of cytokines mediating autocrine and paracrine growth and survival of MM cells as well as protection against drug-induced apoptosis.2 We therefore next examined the effect of CDDO-Im on 5 patient MM-derived BMSCs. As seen in Figure 2C, treatment of BMSCs (patients a-c) with CDDO-Im (0.2 μM) for 72 hours significantly (P = .004) induces apoptosis in these cells, as evidenced by DNA fragmentation assay.
To compare the effects of CDDO-Im on BMSCs from MM patients versus normal donor lymphocytes, we treated these cells from 3 MM patients and 3 healthy donors with CDDO-Im (300 nM), followed by analysis for apoptosis by annexin V staining. CDDO-Im triggered apoptosis in MM patient BMSCs (66% ± 2.1% apoptotic cells, mean ± SD; n = 3) but not in normal donor lymphocytes (8.6% apoptotic cells, mean ± SD; n = 3). These findings are consistent with results obtained from either MTT (Figure 2A) or DNA fragmentation assays (Figure 2C). Together, these results suggest that CDDO-Im acts not only directly on MM cells but also affects the BM microenvironment.
CDDO-Im inhibits interleukin-6 (IL-6) secretion in BMSCs
Adhesion of MM cells to BMSCs induces IL-6 secretion from BMSCs, which not only regulates the growth of MM cells but also protects against chemotherapy.7,26,27 We therefore next examined the effect of CDDO-Im on IL-6 production in the BM microenvironment. As seen in Figure 2D, CDDO-Im (0.2 μM) significantly (P < .005) inhibits IL-6 secretion in MM patient BMSCs triggered by MM cell adhesion. Reports that high serum levels of IL-6 contribute to clinical chemoresistance and treatment failure,28 coupled with our demonstration that CDDO-Im decreases the MM adhesion-induced IL-6 secretion from BMSCs, suggest that CDDO-Im may overcome drug resistance in patients with advanced MM. Importantly, these findings also show that CDDO-Im is more potent than bortezomib at inhibiting IL-6 secretion and confirm that either agent alone blocks IL-6–mediated survival or antiapoptotic effects.
CDDO-Im overcomes the prosurvival/antiapoptotic effects of human recombinant IL-6 (hrIL-6) and insulin growth factor-1 (IGF-1)
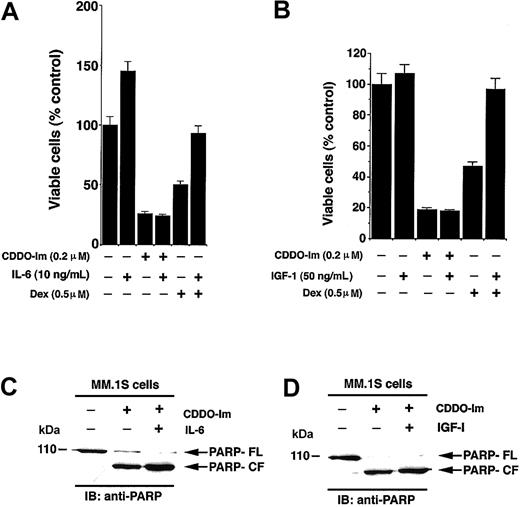
Given that both IL-6 and IGF-1 are major growth/survival factors present within the BM microenvironment and protect against Dex-induced apoptosis,29-32 we examined their effects on CDDO-Im–induced apoptosis. MM.1S cells were treated with CDDO-Im (0.2 μM) or Dex (0.5 μM) in the presence and absence of IL-6 (10 ng/mL) or IGF (50 ng/mL). As seen in Figure 3A, the median viability was 24% ± 2.1% with CDDO-Im alone and 28% ± 2.2% with CDDO-Im + IL-6 (P = .23, Wilcoxon test), whereas median viability was 43% ± 2.1% for Dex and 86% ± 5.4% for Dex + IL-6 (P = .05, as determined by 1-sided Wilcoxon rank sum test). Similar results were obtained using IGF-1: Median viability was 19.3% with CDDO-Im alone and 18.1% with CDDO-Im + IGF-1 (P = .27) (Figure 3B). These findings suggest that neither IL-6 nor IGF-1 blocks the effects of CDDO-Im on MM.1S cells. In contrast and as in our prior studies,8,33 both IL-6 and IGF-1 block Dex-induced decreased MM.1S cell viability. Furthermore, as seen in Figure 3C-D, CDDO-Im induces apoptosis in MM.1S cells even in the presence of IL-6 or IGF-1, as evidenced by proteolytic cleavage of poly(ADP-ribose) polymerase (PARP), a signature event during apoptosis.34 Taken together, our data suggest that CDDO-Im overcomes the growth and protective effects of IL-6 and IGF-1 on MM cells and indicate distinct mechanisms of actions for CDDO-Im and Dex against MM cells.
Interleukin-6 (IL-6) or insulin growth factor-1 (IGF-1) does not block CDDO-Im–induced cytotoxicity in MM cells. (A) MM.1S cells were treated with CDDO-Im (200 nM) or Dex (0.5 μM) in the presence or absence of IL-6 (10 ng/mL). At 24 hours cells were harvested and viability analyzed by MTT assays. Results are mean ± SD of 3 independent experiments (P < .005). (B) MM.1S cells were treated with CDDO-Im (200 nM) or Dex (0.5 μM) in the presence or absence of IGF-1 (50 ng/mL). At 24 hours cells were harvested and viability analyzed by MTT assays. Results are mean ± SD of 3 independent experiments (P < .005). (C-D) MM.1S cells were treated with CDDO-Im (200 nM) in the presence or absence of IL-6 (C) or IGF-1 (D). At 24 hours cells were harvested, and total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP Abs. FL indicates full length, and CF denotes cleaved fragment.
Interleukin-6 (IL-6) or insulin growth factor-1 (IGF-1) does not block CDDO-Im–induced cytotoxicity in MM cells. (A) MM.1S cells were treated with CDDO-Im (200 nM) or Dex (0.5 μM) in the presence or absence of IL-6 (10 ng/mL). At 24 hours cells were harvested and viability analyzed by MTT assays. Results are mean ± SD of 3 independent experiments (P < .005). (B) MM.1S cells were treated with CDDO-Im (200 nM) or Dex (0.5 μM) in the presence or absence of IGF-1 (50 ng/mL). At 24 hours cells were harvested and viability analyzed by MTT assays. Results are mean ± SD of 3 independent experiments (P < .005). (C-D) MM.1S cells were treated with CDDO-Im (200 nM) in the presence or absence of IL-6 (C) or IGF-1 (D). At 24 hours cells were harvested, and total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP Abs. FL indicates full length, and CF denotes cleaved fragment.
Synergistic anti-MM activity of CDDO-Im and bortezomib/proteasome inhibitor PS-341
Our recent studies showed that bortezomib induces apoptosis in refractory MM cells16,17 ; however, continuous exposure to bortezomib is associated with increased toxicity and development of de novo bortezomib resistance. We therefore next determined whether the combination of minimally toxic concentrations of CDDO-Im and bortezomib affects MM cell viability.
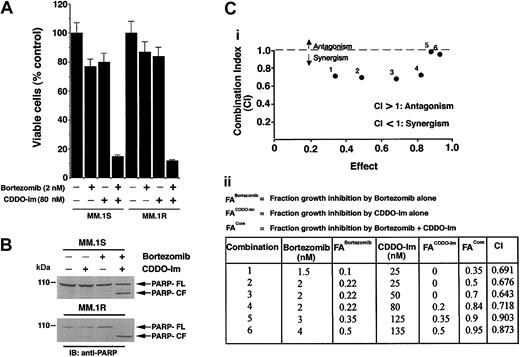
As seen in Figure 4A, treatment of MM.1S or MM.1R MM cells with CDDO-Im + bortezomib for 24 hours induces synergistic growth inhibition (median viability in MM.1S cells: bortezomib [2 nM] + CDDO-Im [80 nM] = 13% ± 1.3%; bortezomib alone = 77% ± 2.4%; and CDDO-Im alone = 80% ± 5.1% [P = .05, 1-sided Wilcoxon rank sum test]; and median viability in MM.1R cells: bortezomib [2 nM] + CDDO-Im [80 nM] = 11% ± 0.8%; bortezomib alone = 85% ± 4.4%; and CDDO-Im alone = 87% ± 6.1% [P = .04, 1-sided Wilcoxon rank sum test]). Examination of PARP cleavage demonstrated similar results. As seen in Figure 4B, treatment of MM.1S with low doses of CDDO-Im (80 nM) and bortezomib (2 nM) induces a marked proteolytic cleavage of PARP, whereas neither agent alone induces PARP cleavage at these concentrations. In addition, the combination of CDDO-Im and bortezomib also triggers apoptosis in other drug-resistant MM cells, including Dox- and melphlan-resistant RPMI 8226 MM cells; no cross-resistance to drugs was observed (data not shown). Together, these findings show that synergy between CDDO-Im and bortezomib overcomes conventional drug resistance in MM cells.
Low doses of CDDO-Im and bortezomib/proteasome inhibitor PS-341 trigger synergistic anti-MM activity in MM cell lines and patient MM cells. (A) Dex-sensitive (MM.1S) and Dex-resistant (MM.1R) cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours and assessed for viability using MTT assays. Results are mean ± SD of 3 independent experiments (P < .006). (B) MM.1S and MM.1R cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours; total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP Abs. FL indicates full length, and CF denotes cleaved fragment. (C) Isobologram analysis showing the synergistic cytotoxic effect of CDDO-Im and bortezomib in MM.1S cells. The graph (i) is derived from the values given in the table (ii). CI indicates combination index.
Low doses of CDDO-Im and bortezomib/proteasome inhibitor PS-341 trigger synergistic anti-MM activity in MM cell lines and patient MM cells. (A) Dex-sensitive (MM.1S) and Dex-resistant (MM.1R) cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours and assessed for viability using MTT assays. Results are mean ± SD of 3 independent experiments (P < .006). (B) MM.1S and MM.1R cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours; total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP Abs. FL indicates full length, and CF denotes cleaved fragment. (C) Isobologram analysis showing the synergistic cytotoxic effect of CDDO-Im and bortezomib in MM.1S cells. The graph (i) is derived from the values given in the table (ii). CI indicates combination index.
We confirmed the synergism between CDDO-Im and bortezomib using isobologram analysis. There is a synergy with a combination index (CI) less than 1.0, additive effect when CI equals 1.0, and antagonism when CI is more than 1.0. As seen in Figure 4C, isobologram analysis confirmed a synergistic anti-MM activity of CDDO-Im with bortezomib (CI less than 1.0).
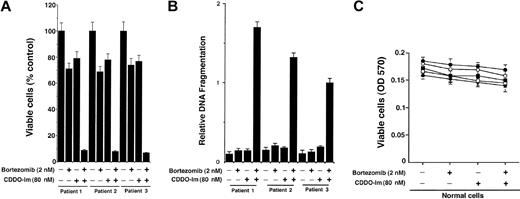
We next determined whether CDDO-Im + bortezomib also affects MM patient cells refractory to bortezomib (patient nos. 1 and 2) and thalidomide/dexamethasone (patient no. 3). Cells were treated with CDDO-Im + bortezomib and analyzed for cell viability and apoptosis. As seen in Figure 5A-B, CDDO-Im + bortezomib markedly decreases the patient MM cell viability as well as induced apoptosis in these cells. We further examined whether low-dose CDDO-Im + bortezomib similarly increases lethality in normal cells. Cells from 4 healthy donors were treated with CDDO-Im (80 nM) + bortezomib (2 nM) for 24 hours and analyzed for viability using MTT assay. As seen in Figure 5C and in contrast to MM cells, survival of normal lymphocytes was not altered significantly (P = .31 from J-T trend test) at these doses of CDDO-Im + bortezomib. No significant apoptosis of normal lymphocytes was observed in response to CDDO-Im + bortezomib (data not shown). Taken together, these findings indicate that low doses of CDDO-Im + bortezomib have selective anti-MM activity.
Low doses of CDDO-Im and bortezomib/proteasome inhibitor PS-341 trigger synergistic anti-MM activity in patient MM cells. (A) CD138+ MM patient cells (patient nos. 1-3) were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours and assessed for viability using MTT assays. Values are mean ± SD of triplicate samples, P = .05; experiments were repeated 2 times with similar results. (B) CD138+ MM patient cells (patient nos. 1-3) were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours and assessed for apoptosis by DNA fragmentation assays. Values are the mean ± SD of triplicate samples, P = .06; experiments were repeated 3 times with similar results. (C) Normal lymphocytes from 4 healthy donors were treated with CDDO-Im (80 nM) + bortezomib (2 nM) for 24 hours, and viability was assessed by an MTT assay. Results are the mean ± SD of 3 independent experiments, with P = .31 using Jonchkeere-Tepstra (J-T) test for trend.
Low doses of CDDO-Im and bortezomib/proteasome inhibitor PS-341 trigger synergistic anti-MM activity in patient MM cells. (A) CD138+ MM patient cells (patient nos. 1-3) were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours and assessed for viability using MTT assays. Values are mean ± SD of triplicate samples, P = .05; experiments were repeated 2 times with similar results. (B) CD138+ MM patient cells (patient nos. 1-3) were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours and assessed for apoptosis by DNA fragmentation assays. Values are the mean ± SD of triplicate samples, P = .06; experiments were repeated 3 times with similar results. (C) Normal lymphocytes from 4 healthy donors were treated with CDDO-Im (80 nM) + bortezomib (2 nM) for 24 hours, and viability was assessed by an MTT assay. Results are the mean ± SD of 3 independent experiments, with P = .31 using Jonchkeere-Tepstra (J-T) test for trend.
To exclude the possibility that this event is specific to bortezomib, we performed experiments using another proteasome inhibitor, MG-132. MM.1S cell were treated with CDDO-Im (80 nM) and MG-132 (50 nM) for 24 hours and then analyzed for apoptosis by dual staining with PI and HO. Treatment of MM.1S cells with either CDDO-Im or MG-132 alone triggered minimal apoptosis in these cells, whereas CDDO-Im + MG-132 induced a significant increase in apoptotic (PI-HO+) cells (median apoptotic cells: CDDO-Im alone, 10%; MG-132 alone, 9.4%; P < .004; CDDO-Im + MG-132 = 86% ± 4.2%, P < .005) (data not shown).
CDDO-Im ± bortezomib alters mitochondrial membrane potential (ΔΨm), generation of superoxide (O2-), release of mitochondrial proteins cytochrome c and Smac, and activation of caspases
We next examined the molecular mechanism whereby low-dose combinations of CDDO-Im and bortezomib trigger apoptosis in MM cells. Stress-induced apoptosis correlates with mitochondria-related events: loss of ΔΨm and generation of reactive oxygen species (ROS), including O2-; release of cyto-c and Smac (second mitochondria-derived activator of caspases) from mitochondria to cytosol; and activation of downstream capase-9 and -3.4 In addition, mitochondria-independent apoptosis can occur via death receptor–mediated caspase-8–caspase-3 pathway.35 In this context, prior studies have shown that CDDO, a parental compound of CDDO-Im, induces apoptosis predominantly by caspase-8 pathway12 ; however, CDDO-Im–triggered apoptotic signaling is not defined. Moreover, we and others have shown that bortezomib triggers both caspase-8– and caspase-9–mediated apoptotic signaling, albeit at high concentrations (10-20 nM).12,36-38 Given the synergism between CDDO-Im and bortezomib in mediating anti-MM activity, we next examined the signaling mechanisms triggered in response to these agents in MM cells.
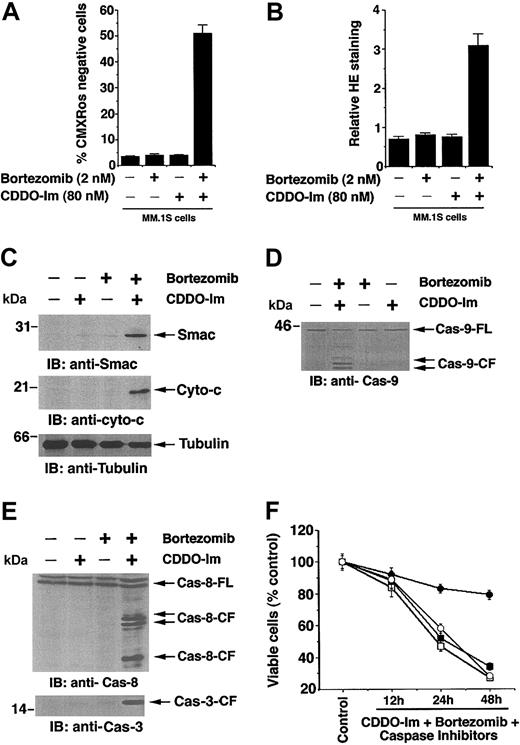
Mitochondria play a critical role in apoptosis induction during stress.35 We therefore first determined whether CDDO-Im + bortezomib induces a loss in mitochondrial membrane potential (ΔΨm). MM.1S cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 12 hours; stained with CMXRos; and analyzed by flow cytometry, as previously described.39 As seen in Figure 6A, neither CDDO-Im nor bortezomib alone triggers any significant decrease in ΔΨm, whereas CDDO-Im + bortezomib induces a significant decrease in ΔΨm in MM.1S cells, as measured by an increase in number of CMXRos-negative cells (P < .005; n = 3).
CDDO-Im + bortezomib alters mitochondrial membrane potential (ΔΨm), generation of superoxide (O2-), release of mitochondrial proteins cytochrome c and Smac, and activation of caspases. (A) MM.1S cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 12 hours; incubated with CMXRos for the last 20 minutes; and analyzed by flow cytometry to assay for alterations in ΔΨm. Increase in the number of CMXRos-negative cells indicates loss in ΔΨm. Results are mean ± SD of 3 independent experiments (P < .003). (B) MM.1S cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 12 hours; harvested; stained with membrane-permeable dye dihydroethidium (HE) for the last 15 minutes; and analyzed by flow cytometry. Results are mean ± SD of 3 independent experiments (P < .005). Superoxide anions oxidize HE to fluorescent ethidium, permitting analysis by flow cytometry. (C) MM.1S cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours and harvested; cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anti-Smac (top blot) or anti–cyto-c (middle blot) Abs. As a control for equal loading of proteins, filters were also reprobed with antitubulin Ab (bottom blot). Densitometric analysis of the immunoblot demonstrated that CDDO-Im + bortezomib induces a 6- to 7-fold increase in cytosolic cyto-c and Smac levels compared with untreated cells. Blots are representative of 3 independent experiments. (D) MM.1S cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours and harvested; cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anticaspase-9 Abs. Blots are representative of 3 independent experiments. FL indicates full length, and CF denotes cleaved fragment. (E) MM.1S cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours; cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anticaspase-8 and anticaspase-3 Abs. Blots are representative of 3 independent experiments. (F) MM.1S cells were treated with CDDO-Im + bortezomib alone (□) or in the presence of caspase-8 inhibitor (○), caspase-9 inhibitor (▪), or pancaspase inhibitor ( ) for 24 hours and harvested and assessed for viability using MTT assays. Results are mean ± SD of 3 independent experiments (P < .005).
) for 24 hours and harvested and assessed for viability using MTT assays. Results are mean ± SD of 3 independent experiments (P < .005).
CDDO-Im + bortezomib alters mitochondrial membrane potential (ΔΨm), generation of superoxide (O2-), release of mitochondrial proteins cytochrome c and Smac, and activation of caspases. (A) MM.1S cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 12 hours; incubated with CMXRos for the last 20 minutes; and analyzed by flow cytometry to assay for alterations in ΔΨm. Increase in the number of CMXRos-negative cells indicates loss in ΔΨm. Results are mean ± SD of 3 independent experiments (P < .003). (B) MM.1S cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 12 hours; harvested; stained with membrane-permeable dye dihydroethidium (HE) for the last 15 minutes; and analyzed by flow cytometry. Results are mean ± SD of 3 independent experiments (P < .005). Superoxide anions oxidize HE to fluorescent ethidium, permitting analysis by flow cytometry. (C) MM.1S cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours and harvested; cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anti-Smac (top blot) or anti–cyto-c (middle blot) Abs. As a control for equal loading of proteins, filters were also reprobed with antitubulin Ab (bottom blot). Densitometric analysis of the immunoblot demonstrated that CDDO-Im + bortezomib induces a 6- to 7-fold increase in cytosolic cyto-c and Smac levels compared with untreated cells. Blots are representative of 3 independent experiments. (D) MM.1S cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours and harvested; cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anticaspase-9 Abs. Blots are representative of 3 independent experiments. FL indicates full length, and CF denotes cleaved fragment. (E) MM.1S cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 24 hours; cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anticaspase-8 and anticaspase-3 Abs. Blots are representative of 3 independent experiments. (F) MM.1S cells were treated with CDDO-Im + bortezomib alone (□) or in the presence of caspase-8 inhibitor (○), caspase-9 inhibitor (▪), or pancaspase inhibitor ( ) for 24 hours and harvested and assessed for viability using MTT assays. Results are mean ± SD of 3 independent experiments (P < .005).
) for 24 hours and harvested and assessed for viability using MTT assays. Results are mean ± SD of 3 independent experiments (P < .005).
Similar decreases in ΔΨm were observed in Dex-resistant MM.1R cells (data not shown). Because loss of ΔΨm is associated with O2- production,40,41 we next determined whether CDDO-Im + bortezomib also affects O2- generation. MM.1S cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib for 12 hours; stained with dihydroethidium (HE); and analyzed by flow cytometry. In contrast to treatment with either agent alone, the combination of CDDO-Im + bortezomib induces generation of O2- in these cells (Figure 6B). Similar increases in O2- generation were observed in response to higher doses of each agent alone (CDDO-Im = 0.2 μM and bortezomib = 10 nM). These data suggest that combinations of subtoxic concentrations of CDDO-Im and bortezomib trigger mitochondrial perturbations sufficient to initiate apoptotic signaling and that this strategy may be useful to avoid dose-related toxic effects of either drug when used alone at higher concentrations.
Alterations in the ΔΨm are associated with the release of mitochondrial proteins cyto-c and Smac to cytosol, thereby triggering caspase-9 and caspase-3.42,43 Having shown that CDDO-Im + bortezomib–triggered apoptosis is associated with loss of ΔΨm and an increase in O2- production, we next asked whether cyto-c or Smac release is similarly affected by these agents. MM.1S cells were treated with CDDO-Im (80 nM), bortezomib (2 nM), or CDDO-Im + bortezomib; cytosolic extracts were then prepared and subjected to immunoblot analysis with anti-Smac or anti–cyto-c Abs. As seen in Figure 6C (upper and middle panels), treatment of MM.1S cells with CDDO-Im + bortezomib triggers the release of both Smac and cyto-c. In contrast, neither agent alone induced significant release of cyto-c and Smac at these concentrations. Reprobing the immunoblots with antitubulin Abs confirms equal protein loading (Figure 6C, lower panel). These findings show that O2- generation is associated with cyto-c- or Smac-mediated apoptotic signaling triggered in response to CDDO-Im + bortezomib in MM cells.
Release of mitochondrial apoptogenic proteins cyto-c and Smac/DIABLO (Smac/direct IAP binding protein with low pI) induces activation of caspase-9 and -3.42,43 We next determined whether CDDO-Im + bortezomib–induced apoptosis correlates with activation of caspase-9. As seen in Figure 6D, treatment of MM.1S cells with CDDO-Im + bortezomib, but not CDDO-Im or bortezomib alone, induces proteolytic cleavage of caspase-9. Prior studies have shown that CDDO, a parental compound of CDDO-Im, induces caspase-8 activation12 ; we therefore next examined whether CDDO-Im–induced apoptosis involves caspase-8 activation. As seen in Figure 6E, CDDO-Im + bortezomib, but not either agent alone, triggered marked caspase-8 cleavage at these concentrations. Both caspase-9 (mitochondria dependent) and caspase-8 (mitochondria independent) are known to proteolytically cleave and activate a common downstream effector procapsase-3,44 and our data further show that CDDO-Im + bortezomib triggers caspase-3 cleavage (Figure 6E, lower panel).
We next determined the requirement of caspase-8 versus caspase-9 during CDDO-Im + bortezomib–induced apoptosis. MM.1S cells were treated with caspase-9 inhibitor (LEHD-fmk), caspase-8 inhibitor (IETD-fmk), or pancaspase inhibitor, Z-Val-Ala-Asp-fluoromethylketone (z-VAD-fmk). As seen in Figure 6F, pancaspase inhibitor, but not caspase-8 or caspase-9 inhibitor, abrogates CDDO-Im + bortezomib–induced apoptosis. To examine whether CDDO-Im + bortezomib–induced apoptosis requires both caspase-8 and -9, cells were incubated with capsase-8 and -9 inhibitors, treated with CDDO-Im + bortezomib, and then analyzed for apoptosis. Blockade of caspase-8 and -9 led to a 60% ± 2.9% decrease in CDDO-Im + bortezomib–triggered cell death (P < .005; n = 3). Together, these findings suggest that CDDO-Im + bortezomib–induced apoptosis involves activation of both caspase-8 and -9 and is not completely dependent on either of these caspases. These data further suggest that other upstream pathways that trigger caspase-3 activation other than caspase-8 and -9 are also induced during CDDO-Im + bortezomib–induced apoptosis. Our ongoing studies are focused on establishing a stable MM cell line resistant to either CDDO-Im or bortezomib that will provide more insight into the mechanisms of synergism of CDDO-Im with bortezomib.
CDDO-Im + bortezomib overcomes Bcl2-mediated protective effects
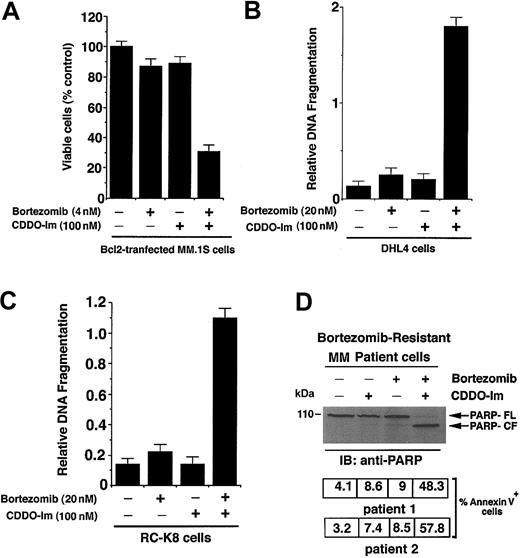
Overexpression of Bcl2 confers resistance to conventional therapies in cancer cells, including MM.45-47 Our prior study showed that Bcl2 can modestly attenuate bortezomib-induced apoptosis.37 We therefore next directly examined whether ectopic expression of Bcl2 in MM.1S cells affects responsiveness to CDDO-Im + bortezomib. MM.1S cells were stably transfected with Bcl2 construct and analyzed for alterations in cell viability by an MTT assay. As seen in Figure 7A, treatment of cells with CDDO-Im (100 nM) + bortezomib (4 nM) significantly decreases cell viability in Bcl2-transfected MM.1S cells (P < .005). In contrast, neither agent alone reduced survival in Bcl2-transfected cells. Examination of the effects of CDDO-Im + bortezomib in control vector–transfected MM.1S cells versus Bcl2-tranfected MM.1S cells showed 17% ± 1.4% less cell death in Bcl2-transfected cells compared with empty vector–transfected MM.1S cells (data not shown). Taken together, these findings suggest that CDDO-Im + bortezomib at high synergistic doses can overcome Bcl2-mediated protection.
CDDO-Im + bortezomib triggers apoptosis in bortezomib-resistant cancer cells. (A) CDDO-Im and bortezomib decrease survival in Bcl2-overexpressing MM.1S cells. MM.1S cells were stably transfected with Bcl2 construct; treated with CDDO-Im, bortezomib, or CDDO-Im + bortezomib for 24 hours; and assessed for viability using MTT assays. Results are mean ± SD of 4 independent experiments (P < .004). (B) CDDO-Im and bortezomib induce apoptosis in SUDHL4 (DHL4) lymphoma cells expressing high levels of heat shock protein-27 (Hsp27). DHL4 cells were treated with CDDO-Im, bortezomib, or CDDO-Im + bortezomib for 24 hours and assessed for apoptosis by DNA fragmentation assays. Results are mean ± SD of 4 independent experiments (P < .005). (C) CDDO-Im and bortezomib induce apoptosis in RC-K8 lymphoma cells with genetically inactivated IκB-α protein and high intrinsic activation of NF-κB. RC-K8 cells were treated with CDDO-Im, bortezomib, or CDDO-Im + bortezomib for 24 hours and assessed for apoptosis by DNA fragmentation assays. Results are mean ± SD of 4 independent experiments (P < .005). (D) CDDO-Im + bortezomib induces apoptosis in bortezomib-resistant patient MM cells. CD138+ cells were freshly isolated from an MM patient (patient no. 1) refractory to bortezomib; treated with CDDO-Im, bortezomib, or CDDO-Im + bortezomib for 24 hours; and cytosolic proteins separated by 12.5% SDS-PAGE and analyzed for apoptosis by immunoblotting with anti-PARP Abs (top panel). Blots are representative of 3 independent experiments. FL indicates full length, and CF denotes cleaved fragment. Apoptosis was also assessed by annexin V staining in CDDO-Im + bortezomib-treated MM cells from patient no. 1 (middle panel) and patient no. 2 (bottom panel). Percent positive annexin V cells shown are mean ± SD of triplicate samples.
CDDO-Im + bortezomib triggers apoptosis in bortezomib-resistant cancer cells. (A) CDDO-Im and bortezomib decrease survival in Bcl2-overexpressing MM.1S cells. MM.1S cells were stably transfected with Bcl2 construct; treated with CDDO-Im, bortezomib, or CDDO-Im + bortezomib for 24 hours; and assessed for viability using MTT assays. Results are mean ± SD of 4 independent experiments (P < .004). (B) CDDO-Im and bortezomib induce apoptosis in SUDHL4 (DHL4) lymphoma cells expressing high levels of heat shock protein-27 (Hsp27). DHL4 cells were treated with CDDO-Im, bortezomib, or CDDO-Im + bortezomib for 24 hours and assessed for apoptosis by DNA fragmentation assays. Results are mean ± SD of 4 independent experiments (P < .005). (C) CDDO-Im and bortezomib induce apoptosis in RC-K8 lymphoma cells with genetically inactivated IκB-α protein and high intrinsic activation of NF-κB. RC-K8 cells were treated with CDDO-Im, bortezomib, or CDDO-Im + bortezomib for 24 hours and assessed for apoptosis by DNA fragmentation assays. Results are mean ± SD of 4 independent experiments (P < .005). (D) CDDO-Im + bortezomib induces apoptosis in bortezomib-resistant patient MM cells. CD138+ cells were freshly isolated from an MM patient (patient no. 1) refractory to bortezomib; treated with CDDO-Im, bortezomib, or CDDO-Im + bortezomib for 24 hours; and cytosolic proteins separated by 12.5% SDS-PAGE and analyzed for apoptosis by immunoblotting with anti-PARP Abs (top panel). Blots are representative of 3 independent experiments. FL indicates full length, and CF denotes cleaved fragment. Apoptosis was also assessed by annexin V staining in CDDO-Im + bortezomib-treated MM cells from patient no. 1 (middle panel) and patient no. 2 (bottom panel). Percent positive annexin V cells shown are mean ± SD of triplicate samples.
CDDO-Im + bortezomib overcomes bortezomib resistance in SUDHL4 (DHL4) lymphoma cells
Although resistance to another proteasome inhibitor, MG-132, has been reported in Burkitt lymphoma cells,48 the mechanism of PS-341 resistance in any cell type is unknown. Our recent study showed that treatment with bortezomib failed to induce apoptosis in SUDHL4 (DHL4) lymphoma cells.49 Having shown that a low-dose combination of CDDO-Im and bortezomib is able to overcome chemoresistance in MM cells, we next determined whether the use of these agents together can overcome bortezomib resistance in DHL4 cells. As seen in Figure 7B, CDDO-Im (100 nM) + bortezomib (20 nM) triggered a significant decrease in DHL4 cell viability without any similar effects when these agents were used alone. The mechanisms mediating bortezomib resistance are unclear. In this context, our previous study showed that overexpression of heat shock protein-27 (Hsp27) confers bortezomib resistance in DHL4 cells.49 It is well established that Hsp27, like Bcl2,50,51 confers resistance to chemotherapy. Whether Hsp27 confers bortezomib resistance in MM cells remains to be examined. Nevertheless, our data suggest that the combination of CDDO-Im and bortezomib overcomes the cytoprotective effects of both Bcl2 and Hsp27.
CDDO-Im + bortezomib triggers apoptosis in cells with constitutively mutated I-kappa B-α (IκB-α)/activated nuclear factor–kappa B (NF-κB)
Intrinsic activation of NF-κB is associated with growth and survival of cancer cells, including MM cells.52 In addition, adhesion of MM cells to BMSCs triggers NF-κB–mediated transcription and secretion of IL-6.7,53 We therefore next examined whether combinations of low doses of CDDO-Im + bortezomib modulate NF-κB activation. For these studies, we selected RC-K8 lymphoma cells with mutated IκB-α gene resulting in constitutively increased expression of several Rel/NF-κB target genes required for growth and survival.54 As seen in Figure 7C, low doses of CDDO-Im + bortezomib, but not either agent alone, induce DNA fragmentation even in RC-K8 cells. These findings suggest potential utility of combining CDDO-Im and bortezomib to overcome NF-κB–mediated growth, survival, and drug resistance in cancer cells.
CDDO-Im + bortezomib triggers apoptosis in bortezomib-resistant patient MM cells
Although treatment with bortezomib triggers apoptosis in MM cells, bortezomib resistance can develop after prolonged drug exposures. We therefore next examined whether CDDO-Im + bortezomib alters bortezomib resistance in patient MM cells. As seen in Figure 7D, treatment with low-dose CDDO-Im and bortezomib, but not with either agent alone, triggers significant apoptosis in bortezomib-resistant MM patient cells, as evidenced by proteolytic cleavage of PARP (Patient no. 1; Figure 7D, upper panel) and marked increase in the percentage of apoptotic cells (annexin V–positive) (patient no. 1; Figure 7D, middle panel). Similar results were observed using other bortezomib-resistant patient MM cells (patient no. 2) (Figure 7D, lower panel). Ongoing efforts in our laboratory are focused on establishing MM cell lines resistant to either CDDO-Im or bortezomib to determine the mechanisms of synergism between CDDO-Im and bortezomib. Nevertheless, the present findings in patient MM cells confirm that combination therapy with CDDO-Im and bortezomib can overcome bortezomib resistance.
Collectively, our study shows the following: (1) CDDO-Im, a novel derivative of triterpenoid CDDO, induces apoptosis in MM cells resistant to conventional therapies without affecting normal cell viability; (2) CDDO-Im inhibits BMSC growth and related IL-6 secretion; (3) exogenous hIL-6 or hIGF-1 fails to abrogate CDDO-Im–induced apoptosis; (4) the combination of low-dose CDDO-Im and the proteasome inhibitor bortezomib triggers synergistic anti-MM activity in MM cell lines and patient MM cells; (5) CDDO-Im + bortezomib–induced apoptosis requires caspase activation and occurs via both mitochondria-dependent (loss of ΔΨm, increase in O2- production, release of cyto-c/Smac, and activation of 9/3) and mitochondria-independent (caspase-8– caspase-3) mechanisms; (6) the combination of CDDO-Im and bortezomib overcomes the cytoprotective effects of antiapoptotic proteins Bcl2 and Hsp27 as well as NF-κB–mediated growth, survival, and drug resistance; and (7) CDDO-Im + bortezomib induces apoptosis even in bortezomib-resistant tumor cells. Our study therefore suggests that the combination of bortezomib with CDDO-Im will enhance clinical efficacy, reduce toxicity, and overcome drug resistance in patients with relapsed refractory MM.
Prepublished online as Blood First Edition Paper, December 11, 2003; DOI 10.1182/blood-2003-08-2873.
Supported by National Institutes of Health (NIH) grants 50947, CA 78373, CA 78814, CA 78814, SPORE P50 CA100707-01, and P01 CA078378-06; a Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.); The Myeloma Research Fund; The Cure Myeloma Fund; and The National Foundation of Cancer Research
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.