Abstract
In pro-B cell acute lymphoblastic leukemia (ALL), expression of the E2A-HLF fusion gene as a result of t(17;19)(q22;p13) is associated with poor prognosis, hypercalcemia, and hemorrhagic complications. We previously reported that the E2A-HLF fusion protein protects interleukin-3 (IL-3)–dependent lymphoid cells from apoptosis caused by cytokine starvation. Here, we report that annexin II, a surface phospholipid-binding protein and one of the proposed causes of the hemorrhagic complications of acute promyelocytic leukemia (APL), is also implicated in t(17;19)+ ALL. Annexin II was expressed at high levels in APL cells and in each of 4 t(17;19)+ leukemia cell lines, and annexin II expression was induced by enforced expression of E2A-HLF in leukemia cells. In IL-3–dependent cells, we found that annexin II expression was regulated by IL-3 mainly by Ras pathways, including Ras/phosphatidylinositol 3-kinase pathways. Moreover, E2A-HLF increased annexin II expression in IL-3–dependent cells in the absence of the cytokine. These findings indicate that E2A-HLF induces annexin II by substituting for cytokines that activate downstream pathways of Ras. (Blood. 2004;103:3185-3191)
Introduction
The E2A-HLF fusion transcription factor, which is generated by the t(17;19)(q22;p13) translocation, is found in some cases of pro-B cell acute lymphoblastic leukemia (ALL) that occur in older children and adolescents.1,2 In this chimeric molecule, the trans-activation domain of E2A is fused to the basic region and the leucine zipper (bZIP) domain of HLF, which mediate DNA binding and dimerization. Two distinct types of genomic rearrangements resulting in E2A-HLF fusion have been described in t(17;19)+ ALL.1-4 In type 1 rearrangements, an insertion that codes for a portion of the chimera not found in either wild-type protein occurs between E2A exon 13 and HLF exon 4. This insertion, derived from a cryptic exon spanning the 17;19 breakpoint, contains E2A intronic sequences at its 5′ end, HLF intronic sequences at its 3′ end, and various numbers of nontemplated nucleotides in the middle. The type 2 rearrangements arise from more 5′ breakpoints in E2A and result in a fusion with E2A exon 12 spliced directly to HLF exon 4. The leukemias associated with the E2A-HLF fusion protein do not respond well to intensive chemotherapy, not even the aggressive conditioning for bone marrow transplantation. Moreover, these leukemias frequently manifest with intravascular coagulopathy and hypercalcemia, which are generally rare complications in children with pro-B ALL.3,5 Table 1 6-9 summarizes the features of all reported t(17;19)+ ALLs that have been molecularly analyzed to date. Although the DNA-binding activities and the transcriptional activation properties of the type 1 and type 2 E2A-HLF fusion proteins appear to be similar, coagulopathy develops more frequently among patients with a type 1 rearrangement than among those with type 2.4,10
We previously demonstrated that E2A-HLF blocks apoptosis in cytokine-deprived murine interleukin-3 (IL-3)–dependent B precursor cells, suggesting that this fusion protein contributes to leukemogenesis by substituting for the antiapoptotic function of cytokines.11,12 IL-3 supports cell survival through 2 distinct signaling pathways. One pathway acts through the proximal portion of the common β (βc) chain, a subunit shared between the receptor for IL-3 and the receptor for granulocyte-macrophage colony-stimulating factor (GM-CSF); the βc chain proximal portion activates Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways to induce Bcl-xL.13 In the other pathway, the βc chain distal portion activates Ras pathways.14-16 E2A-HLF likely protects lymphoid progenitors from apoptosis by activating the latter because its physiologic counterpart, E4BP4/NFIL3, a related bZIP factor with antiapoptotic function, is induced by IL-3 through signals mainly from the βc chain distal portion,17 especially through Ras–phosphoinositide 3-kinase (Ras-PI3-K) and Ras-Raf–mitogen-activated protein kinase (Ras-Raf-MAPK) pathways.15 We also identified several downstream targets of E2A-HLF, namely, SLUG, a zinc finger transcription factor implicated in the antiapoptotic function of E2A-HLF18 ; groucho-related genes that suppress RUNX119 ; and annexin VIII and SRPUL (sushi-repeat protein up-regulated in leukemia), which are postulated to play paraneoplastic roles in this type of pro-B cell leukemia.20
Leukemia patients with the E2A-HLF fusion product and a tendency to bleed have laboratory data similar to those of patients with t(15;17)+ acute promyelocytic leukemia (APL).21 Major determinants for the pathogenesis of APL-associated coagulopathy have been investigated for the past decade, and factors expressed in leukemia cells that affect procoagulant or fibrinolytic activities have been identified (for a review, see Falanga et al21 ). These factors include tissue factor, inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), and members of the annexin family.22,23 One of these, annexin II, is reported to be a cause of the coagulopathy associated with APL.24 Annexin II is a 36-kDa protein that forms a complex with the annexin II light chain, a member of the S100 family. Annexin II is a calcium-regulated, phospholipid-binding protein expressed on the surfaces of endothelial cells, macrophages, and some tumor cells; it has been implicated in cell-cell adhesion and in plasminogen activation, and it may function as a cell surface receptor (for reviews, see Hajjar et al25,26 ). Surface localization of annexin II is absolutely dependent on micromolar-free Ca2+; the protein is stripped from the cell surface by EGTA (ethyleneglycotetraacetic acid).27 Annexin II is translocated to the endothelial cell surface within 16 hours of biosynthesis, and cell surface annexin II comprises approximately 4% of the total pool of annexin II in endothelial cells.27 The presence of the t(15;17) translocation is correlated with overexpression of annexin II, and annexin II mRNA expression is known to be down-regulated by treatment with all-trans retinoic acid (ATRA). Thus, the anomalous expression of annexin II on the surfaces of circulating APL cells may result in primary (annexin II–dependent) hyperfibrinolysis, thereby shifting the hemostatic balance toward excessive bleeding.24 It has not, however, been clearly demonstrated that annexin II is a downstream target for the promyelocytic leukemia–retinoic acid receptor α (PML-RARα) chimeric transcription factor that results from the t(15;17) translocation.24 Moreover, it is controversial whether annexin II is the real cause of coagulopathy associated with APL, because laboratory findings in APL, such as elevation of the D-dimer level, suggest the occurrence of secondary hyperfibrinolysis rather than annexin II–dependent primary hyperfibrinolysis.21
Here, we show that annexin II is expressed at high levels in leukemia cells expressing E2A-HLF and that enforced expression of E2A-HLF in leukemia cells increases the expression of annexin II. We also demonstrate that annexin II in IL-3–dependent cells is regulated by IL-3 mainly through Ras pathways and that ectopic expression of E2A-HLF in these cells induces it in the absence of IL-3. These findings indicate that annexin II is a downstream target of the E2A-HLF oncoprotein. However, surface expression levels of annexin II among leukemia cells with t(17;19) varied in a manner that suggests annexin II plays a role in the hypercalcemia or in the leukemic invasion rather than in the coagulopathy associated with this type of ALL.
Materials and methods
Cell culture and cell survival assay
Murine IL-3–dependent FL5.12 and Baf-3 pro-B lymphoid cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 0.3% 10T1/2-conditioned medium as a source of IL-3. Cell density was maintained below 106/mL to avoid IL-3–independent growth. Establishment of FL5.12 cells expressing zinc-inducible E2A-HLF (FL5.12/E2A-HLF) or annexin II (FL5.12/annexin II) using the pMT-CB6+ eukaryotic expression vector (a gift from Dr F. Rauscher III, Wistar Institute, Philadelphia, PA) has been described previously.11 Stable transfectants of a truncated form of the human GM-CSF (hGM-CSF) receptor (β544 cells) and Ras mutants were described previously.14,28 Transfectants were maintained in medium containing either 0.6 μg/mL G418 or 200 μg/mL hygromycin. Human ALL cell lines that express E2A-HLF (UOC-B1, HAL-O1, YCUB-2, and Endo-kun) and other leukemia cell lines (Sup-B2, RS4;11, REH, 697, Jurkat, and NB-4) were cultured in RPMI 1640 medium containing 10% FCS; 697/E2A-HLF and 697/pMT cells that were transfected with the pMT/E2A-HLF vector or the empty pMT-CB6+ vector were described previously.20 For cell survival assays, annexin II or E2A-HLF expression was induced in FL5.12 cells by adding 100 μM ZnCl2 for 16 hours before growth factor deprivation. IL-3 was removed by repeated centrifugation in fresh media, the cells were adjusted to 5 × 105/mL on day 0, and culture continued without IL-3. Viable cell numbers were determined by trypan blue dye exclusion. Wortmannin and LY294002 were purchased from Sigma-Aldrich (St Louis, MO).
Cloning of annexin II full-length cDNA and Northern blot analysis
The annexin II cDNA was cloned from UOC-B1 cells by reverse transcription–polymerase chain reaction (RT-PCR) using upstream and downstream primers (5′-TCTCAGCTCTCGGCGCACGG-3′ and 5′-TTTTCTAGACCTGTTAGCT-3′). RT-PCR was performed with a cDNA Cycle Kit (Invitrogen, Carlsbad, CA). DNA sequencing confirmed that the insert sequence was identical to that of the annexin II cDNA.29 Total cellular or poly(A)–selected RNA was isolated using RNeasy kits (Qiagen, Hilden, Germany) or Fast Track kits (Invitrogen), respectively, according to the manufacturers' instructions. One microgram of messenger RNA or 20 μg total RNA was separated by electrophoresis in 1% agarose gels containing 2.2 M formaldehyde, transferred to nylon membranes, and hybridized with an appropriate probe according to standard procedures.
Immunoblot analysis
Cells were solubilized in Nonidet P-40 lysis buffer (150 mM NaCl, 1.0% Nonidet P-40, 50 mM Tris, pH 8.0), and total cellular proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). After wet electrotransfer onto nitrocellulose membranes, the proteins were detected using appropriate antibodies and following standard procedures. Blots were then stained with primary antibodies followed by horseradish peroxidase–conjugated antirabbit immunoglobulin secondary antibodies and were subjected to chemiluminescent detection according to the manufacturer's instructions (Amersham Pharmacia Biotech, Buckinghamshire, England). Anti–annexin II polyclonal antibody was purchased from BD Transduction Laboratories (Lexington, KY). Anti-HLF(C) antibody for the detection of the E2A-HLF chimeric protein was described previously.30 For immunoblot analysis of cell surface eluates, cells (1 × 108) were washed 3 times with either Hanks balanced salt solution (HBSS; Invitrogen) alone or HBSS containing 4 mM CaCl2 (HBSS/Ca) and were treated with either HBSS alone or HBSS/Ca for 30 minutes on ice. Elutes were collected and were solubilized in Laemmli lysis buffer (10% glycerol, 2% SDS, 50 mM Tris, pH 8.0) and were separated using SDS-PAGE. Assays for eluted lactate dehydrogenase were performed as previously described.27
Results
Annexin II is induced by E2A-HLF
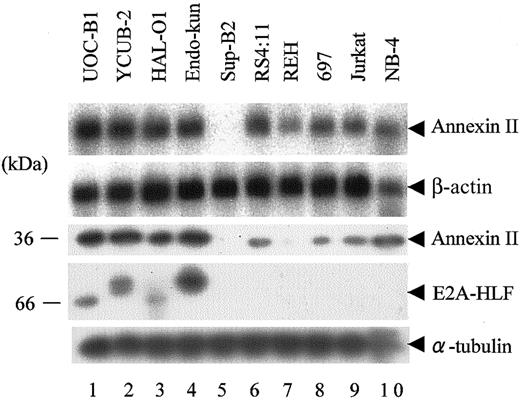
We performed Northern blot and immunoblot analyses to test the expression of annexin II in human leukemia cell lines. Because t(17;19)+ ALLs constitute only approximately 1% of childhood B-precursor ALLs,3 we used cell lines instead of primary patient samples. Four cell lines harboring the E2A-HLF chimeric protein (UOC-B1, HAL-O1, YCUB-2, and Endo-kun) uniformly expressed annexin II mRNA and protein at higher levels than NB-4, a t(15;17)-positive APL cell line used as a positive control (Figure 1, lanes 1-4, 10).
Expression of annexin II in human leukemia cell lines. (Top 2 panels) Northern blot analysis of poly(A)+ RNA (1 μg per lane) isolated from human leukemia cell lines. The blot was hybridized with an annexin II cDNA probe and then rehybridized with a β-actin probe. (Lower 3 panels) Immunoblot analysis using whole-cell lysates. Annexin II, E2A-HLF, and α-tubulin proteins were detected with specific antibodies. Lanes 1 to 4: UOC-B1, YCUB-2, HAL-O1, and Endo-kun t(17;19)–positive pro-B ALL cell lines. Lanes 5 to 8: Sup-B2, RS4;11, REH, and 697 pro-B ALL cell lines without t(17;19). Lane 9: Jurkat T-ALL cell line. Lane 10: NB-4 APL cell line.
Expression of annexin II in human leukemia cell lines. (Top 2 panels) Northern blot analysis of poly(A)+ RNA (1 μg per lane) isolated from human leukemia cell lines. The blot was hybridized with an annexin II cDNA probe and then rehybridized with a β-actin probe. (Lower 3 panels) Immunoblot analysis using whole-cell lysates. Annexin II, E2A-HLF, and α-tubulin proteins were detected with specific antibodies. Lanes 1 to 4: UOC-B1, YCUB-2, HAL-O1, and Endo-kun t(17;19)–positive pro-B ALL cell lines. Lanes 5 to 8: Sup-B2, RS4;11, REH, and 697 pro-B ALL cell lines without t(17;19). Lane 9: Jurkat T-ALL cell line. Lane 10: NB-4 APL cell line.
Sup-B2, a non-t(17;19) pro-B ALL cell line, lacked expression of annexin II (lane 5). Although annexin II mRNA was detected in other lymphoid leukemia cell lines (RS4;11, REH, 697, and Jurkat) that lack E2A-HLF expression, annexin II protein was less abundant in these cells than in cells expressing E2A-HLF (lanes 6-9). The E2A-HLF fusion protein from each of the t(17;19)+ pro-B ALL cell lines migrated differently because the joining region at the fusion junction contains different numbers of inserted nucleotides, as described previously (fourth panel).1,2
Next, we tested whether E2A-HLF induces the expression of annexin II. For these experiments, 697 cells were transfected with a pMT-CB6+/E2A-HLF construct (see “Materials and methods”) to generate clones that express zinc-inducible E2A-HLF. Ectopic expression of E2A-HLF in 697 cells induced annexin II (Figure 2). However, 697/E2A-HLF cells grown without zinc showed a low (baseline) level of annexin II expression that was not observed in 697/pMT cells, possibly because of the leaky expression of E2A-HLF (Figure 2, lanes 1 and 7).
Induction of annexin II by enforced expression of E2A-HLF in human ALL cells. 697 cells inducibly expressing E2A-HLF (697/E2A-HLF cells) and control 697/pMT cells were cultured in medium containing 100 μM Zn2+ for the indicated times. (Top 2 panels) Northern blot analysis of poly(A)+ RNA (1 μg). The blot was hybridized with an annexin II cDNA probe and then rehybridized with a β-actin probe. (Lower 3 panels) Immunoblot analysis detecting annexin II, E2A-HLF, and α-tubulin proteins.
Induction of annexin II by enforced expression of E2A-HLF in human ALL cells. 697 cells inducibly expressing E2A-HLF (697/E2A-HLF cells) and control 697/pMT cells were cultured in medium containing 100 μM Zn2+ for the indicated times. (Top 2 panels) Northern blot analysis of poly(A)+ RNA (1 μg). The blot was hybridized with an annexin II cDNA probe and then rehybridized with a β-actin probe. (Lower 3 panels) Immunoblot analysis detecting annexin II, E2A-HLF, and α-tubulin proteins.
In control 697/pMT cells, which contain the empty vector, annexin II expression levels were unaffected by zinc, confirming that the observed changes in gene expression were induced by E2A-HLF, not by zinc (Figure 2, lanes 7-8). A comparison of the magnitude of induction of annexin II mRNA and protein suggested that E2A-HLF–induced annexin II not only by affecting mRNA levels but also through posttranscriptional mechanisms.
Annexin II is regulated by IL-3–initiated signaling pathways
We previously reported that E2A-HLF reverses apoptosis caused by cytokine starvation in murine IL-3–dependent lymphoid cells, such as Baf-3 and FL5.12 cells, indicating that the chimeric transcriptional factor at least partially substitutes for cytokine-initiated signaling.11 These findings prompted us to test whether expression of annexin II is under the control of cytokines. A marked decline in annexin II mRNA levels was observed within 8 hours or 4 hours of IL-3 deprivation in Baf-3 or FL5.12 cells, respectively (Figure 3). Similarly, annexin II protein expression declined, as shown by immunoblot analysis (Figure 3A-B, each third panel).
IL-3–dependent expression of annexin II.Baf-3 cells (A) or FL5.12 cells (B) were cultured in IL-3–free medium for the indicated times. (Top 2 panels) Northern blot analysis of total RNA (20 μg). The blot was hybridized with an annexin II cDNA probe and then rehybridized with a β-actin probe. (Lower 2 panels) Immunoblot analysis to detect annexin II and α-tubulin proteins.
IL-3–dependent expression of annexin II.Baf-3 cells (A) or FL5.12 cells (B) were cultured in IL-3–free medium for the indicated times. (Top 2 panels) Northern blot analysis of total RNA (20 μg). The blot was hybridized with an annexin II cDNA probe and then rehybridized with a β-actin probe. (Lower 2 panels) Immunoblot analysis to detect annexin II and α-tubulin proteins.
To identify the signaling pathways regulating the expression of annexin II, we used Baf-3 cells expressing a truncated form (residues 1-544) of the human βc chain (β544 cells).14 In β544 cells, stimulation with hGM-CSF activates pathways emanating from the βc chain proximal region, such as JAK2/STAT5, but it does not activate signaling pathways from the βc chain distal region, including Ras pathways.14 As expected, Bcl-xL mRNA, which is known to be regulated by JAK/STAT pathways,13,15 returned rapidly to its original level after the addition of hGM-CSF (Figure 4A, bottom panel). In contrast, annexin II mRNA was barely detectable 8 hours after IL-3 deprivation (Figure 4A, upper panel, lane 2) and remained low after the addition of hGM-CSF (lanes 3-10). These results suggest that signals originating from the βc chain proximal region are not important for the stable expression of annexin II but that signals from the βc chain distal region are indispensable for annexin II gene expression.
Pathways regulating annexin II expression in IL-3–dependent cells. (A) β544 cells were cultured in cytokine-free medium for 8 hours (lane 2) and then in medium containing hGM-CSF for the indicated times (lanes 3-10). The Northern blot was hybridized with an annexin II cDNA probe and then rehybridized with a mouse Bcl-xL cDNA probe. (B-C) Baf-3 cells expressing RasG12V (B) or RasG12V/V45E (C) under the regulation of Dex were cultured in IL-3–free medium containing 10-7 M Dex in the absence (left blots) or presence (right blots) of wortmannin (0.5 μM) for the indicated times. The Northern blots were hybridized with an annexin II cDNA probe and then rehybridized with a β-actin probe. (D) FL5.12 cells inducibly expressing E2A-HLF were cultured in IL-3–free medium containing 100 μM Zn2+ for the indicated times. (Top 2 panels) The Northern blot was hybridized with an annexin II cDNA probe and then rehybridized with a β-actin probe. (Lower 2 panels) Immunoblot analysis to detect annexin II, E2A-HLF, and α-tubulin using specific antibodies.
Pathways regulating annexin II expression in IL-3–dependent cells. (A) β544 cells were cultured in cytokine-free medium for 8 hours (lane 2) and then in medium containing hGM-CSF for the indicated times (lanes 3-10). The Northern blot was hybridized with an annexin II cDNA probe and then rehybridized with a mouse Bcl-xL cDNA probe. (B-C) Baf-3 cells expressing RasG12V (B) or RasG12V/V45E (C) under the regulation of Dex were cultured in IL-3–free medium containing 10-7 M Dex in the absence (left blots) or presence (right blots) of wortmannin (0.5 μM) for the indicated times. The Northern blots were hybridized with an annexin II cDNA probe and then rehybridized with a β-actin probe. (D) FL5.12 cells inducibly expressing E2A-HLF were cultured in IL-3–free medium containing 100 μM Zn2+ for the indicated times. (Top 2 panels) The Northern blot was hybridized with an annexin II cDNA probe and then rehybridized with a β-actin probe. (Lower 2 panels) Immunoblot analysis to detect annexin II, E2A-HLF, and α-tubulin using specific antibodies.
Oncogenic RAS and E2A-HLF induce annexin II expression in mouse pro-B lymphocytes
To further identify the pathways regulating annexin II gene expression, we used Baf-3 cells with dexamethasone (Dex)–inducible expression of a constitutively active form of Ras (RasG12V).14 The level of annexin II mRNA declined by 4 hours after IL-3 deprivation and Dex treatment, but it was restored after 24 hours (Figure 4B). These effects appeared to be induced by RasG12V, not by Dex itself, because annexin II mRNA was not induced in wild-type Baf-3 cells after the addition of Dex (data not shown). Induction of annexin II by RasG12V was partially reversed by the PI3-K inhibitor wortmannin (right panel), suggesting that PI3-K pathways are important for annexin II expression.
Indeed, the RasG12V/V45E mutant, which activates PI3-K but not Raf-MAPK pathways,14 induced annexin II at levels similar to those induced by RasG12V, and this effect was almost completely reversed by wortmannin (Figure 4C). These results were confirmed by the use of another PI3-K inhibitor, LY294002 (data not shown), suggesting that Ras/PI3-K is an important pathway in the regulation of annexin II gene expression under the control of cytokines.
Next, we tested whether enforced expression of the E2A-HLF chimera can induce the expression of annexin II in FL5.12 cells in the absence of IL-3. For these experiments, we established FL5.12 cells expressing zinc-inducible E2A-HLF. Annexin II mRNA and protein expression persisted up to 32 hours after IL-3 starvation (Figure 4D), in contrast to the rapid decline in wild-type FL5.12 cells (Figure 3B), suggesting that E2A-HLF partially substitutes for the function of cytokine-initiated signaling pathways that induce annexin II expression.
Induction of cell-surface annexin II by E2A-HLF
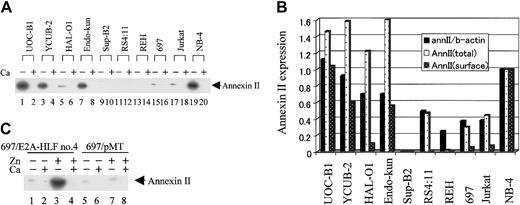
To gain insight into the roles of annexin II overexpressed in leukemia cells with t(17;19), we quantified cell-surface annexin II by eluting it with calcium-free HBSS, which disrupts the calcium-dependent binding of annexin II to the cell surface (see “Materials and methods”).31 The eluate was then tested for annexin II by immunoblot analysis. As expected, no annexin II was detected when cells were treated with HBSS containing 4 mM CaCl2 (Figure 5A, even-numbered lanes). Of 4 cell lines harboring the E2A-HLF chimeric protein, 3 showed high levels of surface annexin II, similar to that of NB-4 (lanes 1, 3, 7, and 19).24 In the third E2A-HLF–expressing cell line, HAL-O1, surface expression of annexin II was much less, though clearly, detectable (lane 5). Surface expression of annexin II was barely detectable from cells without E2A-HLF or PML-RARα (lanes 9, 11, 13, 15, and 17).
Immunodetection of annexin II from the cell surface of human leukemic cell lines. (A) Human leukemia cell lines were treated with either HBSS alone (lanes with a minus sign) or HBSS containing 4 mM CaCl2 (lanes with a plus sign) for 30 minutes. Annexin II proteins were detected with specific antibodies. (B) The levels of mRNA and total and surface annexin II were determined by the band intensity of autoradiograms from Figures 1 and 5A. Amounts shown are relative to levels in the NB-4 APL cell line. AnnII/b-actin indicates ratio of annexin II mRNA to β-actin mRNA; annII(total), total annexin II protein; and annII(surface), surface annexin II protein. (C) 697/E2A-HLF cells and 697/pMT cells were cultured in medium with or without 100 μM Zn2+ as indicated for 16 hours before treatment with either HBSS alone (lanes with a Ca2+ minus sign) or HBSS/Ca (lanes with a Ca2+ plus sign). Annexin II proteins were detected using specific antibodies.
Immunodetection of annexin II from the cell surface of human leukemic cell lines. (A) Human leukemia cell lines were treated with either HBSS alone (lanes with a minus sign) or HBSS containing 4 mM CaCl2 (lanes with a plus sign) for 30 minutes. Annexin II proteins were detected with specific antibodies. (B) The levels of mRNA and total and surface annexin II were determined by the band intensity of autoradiograms from Figures 1 and 5A. Amounts shown are relative to levels in the NB-4 APL cell line. AnnII/b-actin indicates ratio of annexin II mRNA to β-actin mRNA; annII(total), total annexin II protein; and annII(surface), surface annexin II protein. (C) 697/E2A-HLF cells and 697/pMT cells were cultured in medium with or without 100 μM Zn2+ as indicated for 16 hours before treatment with either HBSS alone (lanes with a Ca2+ minus sign) or HBSS/Ca (lanes with a Ca2+ plus sign). Annexin II proteins were detected using specific antibodies.
As a control, the eluates were also assayed for lactate dehydrogenase (LDH) activity. LDH activity was uniformly low in calcium-free and calcium-containing eluates (data not shown), suggesting that annexin II protein detected in this assay originated from the cell surface, not from the cytoplasm. Two cell lines with high levels of surface annexin II, YCUB-2 and Endo-kun, were derived from patients without hemorrhagic complications at onset, whereas HAL-O1, which expressed less surface annexin II, was established from a patient with coagulopathy (Table 1). These results thus suggested that levels of surface annexin II are not related to coagulopathy.
Annexin II mRNA and protein were quantified by densitometry; Figure 5B shows levels of mRNA and total and surface protein relative to those of NB-4 cells as positive control. The magnitude of total annexin II protein induction was greater than that of mRNA, suggesting that E2A-HLF induces annexin II not only at the mRNA level but also through posttranscriptional mechanisms, in accordance with the results from enforced expression of E2A-HLF (Figure 2). In contrast, a comparison of the magnitude of induction of total annexin II protein with surface annexin II suggests that E2A-HLF dose not selectively induce surface annexin II.
Next, we tested whether ectopic expression of E2A-HLF induces the surface expression of annexin II. Following zinc-induced overexpression of E2A-HLF in 697/E2A-HLF cells, levels of surface annexin II were increased (Figure 5C, lanes 1-4). Surface annexin II levels were unaffected by zinc in control 697/pMT cells, confirming that the observed changes in gene expression were induced by E2A-HLF and not by zinc (lanes 5-8).
Lack of antiapoptotic activity of annexin II
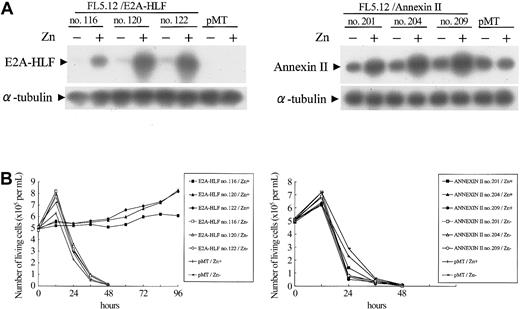
Because E2A-HLF protects IL-3–dependent lymphoid cells from apoptosis caused by IL-3 deprivation,11 we tested whether annexin II is involved in the regulation of cell survival (Figure 6A). FL5.12 cells expressing E2A-HLF survived more than 4 days in IL-3–free medium, as we previously reported (Figure 6B).11 In contrast, cells with zinc-induced overexpression of annexin II did not survive without the cytokine (Figure 6B), suggesting that annexin II does not contribute to the survival of hematopoietic progenitors.
Effect of E2A-HLF and annexin II on the survival of FL 5.12 cells deprived of IL-3. (A) Immunoblot analysis of 3 independently established clones of G418-resistant FL5.12 cells stably transfected with pMT/E2A-HLF (left blot) or pMT/annexin II (right blot) and control cells that received the empty pMT vector. Cells were cultured in the presence or absence of zinc. E2A-HLF (left blot, top row) and annexin II (right blot, top row) and α-tubulin (both blots, bottom row) proteins were detected using specific antibodies. (B) Survival of the transfected FL5.12 cells in the absence of IL-3. Cells growing exponentially in IL-3–containing medium in the presence or absence of 100 μM Zn2+ for 16 hours were adjusted to 5 × 105 cells/mL at hour 0, and viable cell numbers were determined at the indicated times after the removal of IL-3.
Effect of E2A-HLF and annexin II on the survival of FL 5.12 cells deprived of IL-3. (A) Immunoblot analysis of 3 independently established clones of G418-resistant FL5.12 cells stably transfected with pMT/E2A-HLF (left blot) or pMT/annexin II (right blot) and control cells that received the empty pMT vector. Cells were cultured in the presence or absence of zinc. E2A-HLF (left blot, top row) and annexin II (right blot, top row) and α-tubulin (both blots, bottom row) proteins were detected using specific antibodies. (B) Survival of the transfected FL5.12 cells in the absence of IL-3. Cells growing exponentially in IL-3–containing medium in the presence or absence of 100 μM Zn2+ for 16 hours were adjusted to 5 × 105 cells/mL at hour 0, and viable cell numbers were determined at the indicated times after the removal of IL-3.
Discussion
In this study, we demonstrated that annexin II expression is regulated by IL-3 in murine IL-3–dependent Baf-3 and FL5.12 cells (Figure 3). Using β544 cells and cells expressing constitutively active Ras mutants, Ras pathways, including Ras/PI3-K pathways, were shown to be major regulators of annexin II expression (Figure 4). On the other hand, enforced expression of E2A-HLF induced annexin II in these lymphoid cells in the absence of IL-3 (Figure 4D) and in human leukemia cell lines (Figure 2), indicating that annexin II is a downstream target of E2A-HLF. E2A-HLF induced annexin II not only by affecting mRNA levels but also through posttranscriptional mechanisms (Figures 2, 5B).
In earlier studies, we reported that inhibition of the DNA-binding ability of E2A-HLF by a dominant-negative form of this chimeric transcription factor induces apoptosis in UOC-B1 cells but does not affect the cell cycle.11 We also demonstrated that E2A-HLF protects Baf-3 and FL5.12 cells from apoptosis caused by IL-3 starvation,11,12 suggesting that E2A-HLF contributes to leukemogenesis through dysregulation of the cytokine-initiated cell survival system in hematopoietic progenitors. Consequently, we have postulated that a transcription factor acts as a physiologic counterpart of E2A-HLF in these IL-3–initiated cell survival systems. We identified a related bZIP factor, E4BP4/NFIL3, as a candidate, because E4BP4 avidly binds to the consensus DNA-binding sequence of E2A-HLF and because E4BP4 is induced by IL-3 through signals mainly from the βc chain distal portion,17 especially through Ras-PI3-K and Ras-Raf-MAPK pathways.15 Moreover, the enforced expression of E4BP4 in IL-3–starved Baf-3 and FL5.12 cells delays apoptosis.17
Annexin II expression is unlikely to be controlled by antiapoptotic pathways regulated by E4BP4 because the overexpression of annexin II did not protect FL5.12 cells from apoptosis caused by IL-3 starvation (Figure 6B) and because we observed that the enforced expression of E4BP4 in IL-3–deprived FL5.12 cells did not induce annexin II (data not shown). Obviously, downstream targets of E4BP4 are not the only pathway that E2A-HLF aberrantly activates in B-precursor cells. E2A-HLF almost completely blocks apoptosis caused by cytokine deprivation of FL5.12 cells (Figure 6B), but E4BP4 has limited antiapoptotic effects.15,17 Therefore, annexin II appears to be regulated by another unidentified pathway under the control of IL-3 through Ras pathways in B-progenitor cells, and E2A-HLF constitutively activates this pathway to induce annexin II in t(17;19)–positive leukemia cells.
The proportion of annexin II on the cell surface compared with the total cellular expression levels varied among the cell lines. For instance, the total annexin II level in RS4;11 cells was nearly half that in NB-4 (Figure 1), but the cell surface annexin II of RS4;11 was barely detectable (Figure 5A), suggesting that translocation of this protein from the cytosol to the cell surface may be regulated in a manner dependent on cell lineage or maturation stage. The cell surface annexin II levels of 4 cell lines expressing E2A-HLF also diverged, in spite of the similar total annexin II levels of these 4 cell lines (Figures 1 and 5A-B). These results might be explained by the differences in E2A-HLF expressed in these 4 cell lines—that is, UOC-B1 expresses type 1, YCUB-2 and Endo-kun express type 2, and HAL-O1 expresses type 1 with a mutation in the leucine zipper region of HLF that alters the fusion protein's DNA-binding properties.10
The surface expression of annexin II is unlikely to be related to coagulopathy as an initial symptom, because HAL-O1 cells, which have low surface expression of annexin II, were derived from a patient with coagulopathy, whereas YCUB-2 and Endo-kun showed high expression of surface annexin II but derive from patients without coagulopathy (Table 1; Figure 5A). Surface annexin II could be correlated with hypercalcemia at onset, the other rare complication in pro-B ALL, because HAL-O1 is the only t(17;19)+ cell line that was derived from a patient without hypercalcemia. The biologic significance of cytokine-dependent annexin II expression in lymphoid cells is unclear, but it was recently reported that in rat adrenal pheochromocytoma (PC-12) cells, nerve growth factor (NGF) induces annexin II, which contributes to NGF-induced neuritogenesis in the differentiating PC-12 cells through the generation of plasmin.32 On the other hand, annexin II has been implicated in the proliferation of hepatocytes and neurons33,34 and in the invasion and metastasis of various tumors, including glioblastoma multiforme, pancreatic cancer, lung cancer, and gastric cancer.34-37 Annexin II interacts with procathepsin B on the surfaces of tumor cells and is involved in extracellular proteolysis, facilitating tumor invasion and metastasis.38 It has also been suggested that annexin II may play a critical role in the tissue plasminogen activator–dependent, plasmin-mediated invasion of malignant glioma cells.39 Although overexpressed annexin II lacked antiapoptotic activity in IL-3–dependent cells (Figure 6B), E2A-HLF–positive leukemia is characterized by bone invasion and hypercalcemia, which are paraneoplastic syndromes that are rare complications in other types of childhood acute B-lineage leukemia.7,11 Based on the results of this study, we postulate that annexin II overexpression is a general feature of E2A-HLF–positive pro-B cell ALL and that it may have a causative role in one or more of the unique paraneoplastic syndromes associated with the expression of this oncogenic transcription factor.
Prepublished online as Blood First Edition Paper, December 11, 2003; DOI 10.1182/blood-2003-09-3022.
Supported by Grants-in-Aid for Scientific Research (C), Japan Society for the Promotion of Science.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. Eguchi for helpful discussions, support, and encouragement throughout this study. We thank F. J. Rauscher III for providing the pMT-CB6+ expression vector, A. Manabe for providing samples and information on patient 12, K. Harada and H. Aoyama for excellent technical assistance, and K. Ohyashiki and K. Toyama for the HAL-O1 cell lines.