Abstract
We delivered the homologous erythropoietin (Epo) cDNA driven from a doxycycline-regulated promoter via recombinant adeno-associated virus in skeletal muscle of 9 cynomolgus macaques. Upon induction, rapid supraphysiologic levels of Epo were obtained. Unexpectedly, some individuals developed a profound anemia that correlated with the appearance of neutralizing antibodies against the endogenous Epo. Both the endogenous erythropoietin and vector sequences were identical. This is the first example of the inadvertent development of an autoimmune disease in primates as a result of gene transfer of a gene expressing a self-antigen. It raises some concerns when a therapeutic protein is produced at high levels from an ectopic site. (Blood. 2004;103:3303-3304)
Introduction
Phase 1/2 gene therapy trials for hemophilia B patients have exploited the easy access to well-vascularized skeletal muscle for gene transfer via a simple intramuscular injection of recombinant adeno-associated-virus (rAAV) vector to achieve systemic delivery of factor IX, normally synthesized in and secreted from the liver.1 Erythropoietin (Epo) is another factor that can be secreted from skeletal muscle, instead of the kidney, after gene transfer resulting in phenotypic correction of the β-thalassemic mouse. Indeed, it is generally believed that supraphysiologic doses stimulate the synthesis of fetal β-globin and can partly compensate for deficient adult β-globin synthesis. We, and others, evaluated gene transfer of the Epo cDNA to a variety of ectopic tissues in nonhuman primates using the rapamycin or the doxycycline (Dox)-regulatable system, to allow safe drug-controlled Epo secretion.2-4 Herein, we show evidence that overexpression of the homologous Epo following rAAV-mediated gene transfer to skeletal muscle in nonhuman primates can result in severe autoimmune anemia.
Study design
The AAV vector5 encoding for the cynomolgus macaque Epo cDNA (cmEpo) under the control of the reverse Dox-dependent transactivator M26 was packaged in serotype-2 (macaque [Mac] 9 through 12), -1 (Mac 13 through 16), and -5 (Mac 17) AAV capsids. Captive-bred cynomolgus macaques received rAAV-1, -2, and -5 vector at the indicated doses (Figure 1B) expressed as vector genome (vg)/kg into the anterior tibialis muscle. Epo induction started 2 months after vector administration and consisted of a 5-day Dox pulse given intravenously and repeated once every month (indicated as “Induction peaks,” Figure 1A). Serum Epo was measured by enzyme-linked immunosorbent assay (ELISA). The protocol was approved by the Institutional Animal Care and Use Committee of the University of Nantes, France.
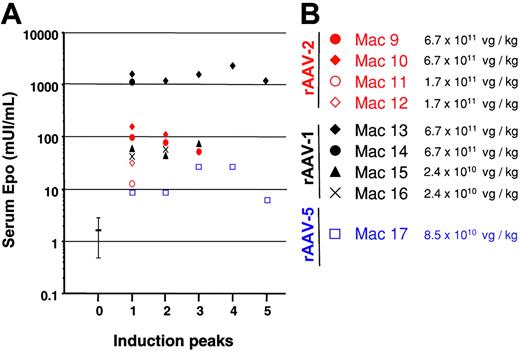
Maximum serum Epo concentration for each Dox induction cycle. (A) Dox-induced Epo secretion measured at the peak level in the serum of Mac 9 through Mac 17 after rAAV-2-mediated (red), rAAV-1-mediated (black), and rAAV-5-mediated (blue) gene transfer. Each induction (referred to as “Induction peaks ”) lasted 5 days and was repeated every month for 5 months. (B) Amounts of rAAV-2 (red), rAAV-1 (black), and rAAV-5 (blue) vector administered expressed as vector genome/kg determined by dot blot. Mean Epo levels before vector injection are represented by error bars.
Maximum serum Epo concentration for each Dox induction cycle. (A) Dox-induced Epo secretion measured at the peak level in the serum of Mac 9 through Mac 17 after rAAV-2-mediated (red), rAAV-1-mediated (black), and rAAV-5-mediated (blue) gene transfer. Each induction (referred to as “Induction peaks ”) lasted 5 days and was repeated every month for 5 months. (B) Amounts of rAAV-2 (red), rAAV-1 (black), and rAAV-5 (blue) vector administered expressed as vector genome/kg determined by dot blot. Mean Epo levels before vector injection are represented by error bars.
Results and discussion
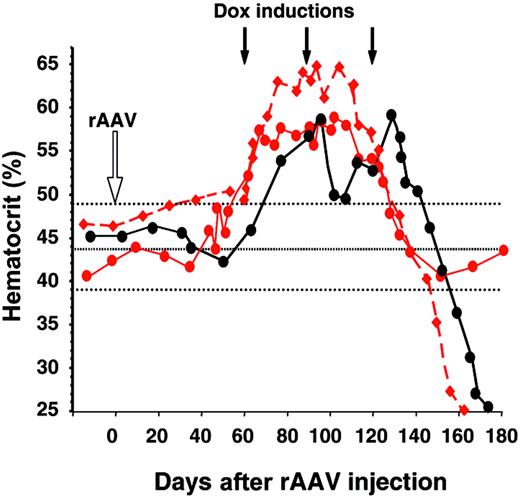
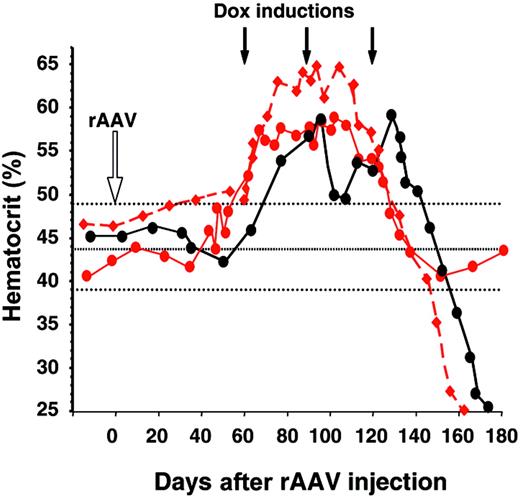
After 3 to 4 induction cycles, most animals mounted a humoral and cytotoxic T-cell immune response against the Dox-dependent transactivator-expressing cells resulting in the loss of Epo secretion from genetically modified muscles3 (Figure 1A). Animals that secreted less than 100 mU/mL Epo initially had normal hematocrit levels when inductions were discontinued (not shown and see 4 additional macaques in Favre et al3 ). However, with the exception of Mac 13, both Mac 10 and Mac 14, which exhibited the highest Epo levels (162 and 1159 mU/mL, respectively, at peak 1), exhibited a severe anemia with hematocrit levels of 0.25 (25%) (Figure 2) at the time of necropsy (Mac 10). Mac 14 recovered spontaneously in 4 weeks and remains stable. The mechanism suspected was the development of antibodies against the transgene and endogenous-derived Epo. Sera from anemic individuals precipitated 1.2 × 105 (Mac 10) and 6.7 × 104 (Mac 14) cpm of 125I-labeled recombinant human Epo (rhuEpo), whereas sera from the same animals (obtained prior to rAAV administration) or from unaffected animals showed no precipitation. Western blot of rhuEpo showed that the protein was recognized by Mac 10 and Mac 14 sera (not shown). Interestingly, Mac 9, with 100 mU/mL Epo secreted at the initial induction cycle (Figure 1A), raised low levels of anti-Epo antibodies (1.1 × 103 cpm), which likely were not enough to induce anemia (Figure 2). In addition, while the 2 animals were developing anemia, we found cellular infiltrates consisting of macrophages and T/B lymphocytes in the rAAV-injected muscles. Both Mac 10 and Mac 14 endogenous Epo were sequenced and found to be identical to the vector Epo, and the Epo protein secreted from in vitro rAAV-transduced cells had a similar molecular weight as the rhuEpo (not shown).
Hematocrit levels from Mac 9 and Mac 10 (red circles and diamonds, respectively), and Mac 14 (black circles) from the time of rAAV-2 (red) and rAAV-1 (black) injections (open arrow), until anemia occurred. Black arrows correspond to the Dox induction cycles. Dotted lines are normal hematocrit levels ± SD. Symbols in this figure are identical to those in Figure 1.
Hematocrit levels from Mac 9 and Mac 10 (red circles and diamonds, respectively), and Mac 14 (black circles) from the time of rAAV-2 (red) and rAAV-1 (black) injections (open arrow), until anemia occurred. Black arrows correspond to the Dox induction cycles. Dotted lines are normal hematocrit levels ± SD. Symbols in this figure are identical to those in Figure 1.
Gao et al7 reports a similar result with rAAV serotype-1, -5, and -8, constitutively expressing homologous Epo cDNA injected in muscle or aerosolized in lung of macaques (see the accompanying article by Gao et al,7 beginning on page 3300). The fact that an autoimmune anemia can occasionally arise using 4 different rAAV serotypes (rAAV-1, -2, -5, and -8) in 2 different ectopic organs (skeletal muscle and lung) with respect to normal Epo synthesis and secretion suggests an Epo-specific adverse effect. The partial correlation that we observed between the amount of Epo secreted at peak 1 and the occurrence of anti-Epo antibodies also suggests that posttranslational modifications could take place as previously suggested for highly expressed myotube-derived factor IX,8 thus breaking tolerance to self-antigens in an haplotype-dependent manner (see Mac 13 that, despite high levels of Epo synthesis, remained healthy). An aggravating factor could be related to the previously published observation that rAAV serotype-2 spreads efficiently to draining lymph nodes after intramuscular delivery and is associated with long-term peripheral blood monocyte cell transduction.5 Whether rAAV serotype-1, -5, and -8 have similar transduction patterns in nonhuman primates after intramuscular administration remains to be determined. In any case, the occurrence of a life-threatening autoimmune response in nonhuman primates following in vivo transfer of a homologous cDNA underscores the need for extensive preclinical studies to understand the mechanism(s) involved, in particular when a therapeutic protein is produced at high levels from an ectopic site.
Prepublished online as Blood First Edition Paper, January 22, 2004; DOI 10.1182/blood-2003-11-3845.
Supported by the INSERM and the Association Française contre les Myopathies (AFM).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the vector core at the University Hospital of Nantes and supported by the AFM. We also thank Sébastien Küry for sequencing the macaque Epo gene. We thank Joelle Nataf for work detecting anti-Epo antibodies. The authors declare that they have no competing financial interests.