Abstract
The long pentraxin PTX3 is a soluble pattern recognition receptor produced by monocytes and endothelial cells that plays a nonredundant role in inflammation. Several pathologic conditions are characterized by local production of both PTX3 and the angiogenic fibroblast growth factor-2 (FGF2). Here, solid-phase binding assays demonstrated that PTX3 binds with high affinity to FGF2 but not to a panel of cytokines and growth factors, including FGF1, FGF4, and FGF8. Accordingly, PTX3 prevented 125I-FGF2 binding to endothelial cell receptors, leading to specific inhibition of FGF2-induced proliferation. PTX3 hampered also the motogenic activity exerted by endogenous FGF2 on a wounded endothelial cell monolayer. Moreover, PTX3 cDNA transduction in FGF2-transformed endothelial cells inhibited their autocrine FGF2-dependent proliferation and morphogenesis in vitro and their capacity to generate vascular lesions when injected in nude mice. Finally, PTX3 suppressed neovascularization triggered by FGF2 in the chick embryo chorioallantoic membrane with no effect on physiologic angiogenesis. In contrast, the short pentraxin C-reactive protein was a poor FGF2 ligand/antagonist. These results establish the selective binding of a member of the pentraxin superfamily to a growth factor. PTX3/FGF2 interaction may modulate angiogenesis in various physiopathologic conditions driven by inflammation, innate immunity, and/or neoplastic transformation.
Introduction
Angiogenesis is the process of generating new capillary blood vessels. In the adult, angiogenesis occurs under tight regulation in the female reproductive system and during wound healing. Uncontrolled neovascularization is observed during tumor growth and atherosclerosis. Also, angiogenesis plays a pivotal role in inflammation.1,2
Fibroblast growth factor-2 (FGF2) is a heparin-binding growth factor that induces cell proliferation, chemotaxis, and protease production in cultured endothelial cells (ECs)3 by interacting with high-affinity tyrosine-kinase receptors (FGFRs) and low-affinity heparan sulfate (HS) proteoglycans (HSPGs).4 FGF2 induces angiogenesis in vivo5 and modulates neovascularization during wound healing, inflammation, atherosclerosis, and tumor growth.6 Accordingly, preclinical studies demonstrate that FGF2 antagonists inhibit tumor growth and vascularization.7,8 FGF2 is produced by various tumor and normal cell types, including cells involved in inflammation and immunity, such as mononuclear phagocytes,9 T lymphocytes,10 and ECs.11 FGF2 lacks a classical signal peptide for secretion. Nevertheless, it can be released by producing cells after cell damage or by way of an alternative pathway for secretion.12 FGF2 production and release is modulated by inflammatory mediators (eg, interleukin 1 [IL-1]13 and nitric oxide14 ), hypoxia,9 and cell damage.15 Finally, endogenous FGF2 can play an autocrine role in ECs following its release and FGFR interaction.16-18
Angiogenesis is controlled by the balance between proangiogenic and antiangiogenic factors.19 Several molecules sequester FGF2 in the extracellular environment, thus preventing its interaction with EC receptors. Free heparin and HS,4 sialo-gangliosides,20 α-2 macroglobulin,21 platelet-derived growth factor BB,22 platelet factor 4,23 perlecan,24 thrombospondin,25 and FGF-binding protein26 bind FGF2 and inhibit its angiogenic activity. Many of these inhibitors are produced or released locally or systemically during wound healing, inflammation, atherosclerosis, and tumor growth, thus underlying the complex tuning of the angiogenesis process that characterizes these physiopathologic conditions.
Pentraxin 3 (PTX3) is the prototypic member of the long pentraxin family that includes also apexin,27 XL-PXN1,28 neuronal pentraxins 1 and 2,29 and Narp.30 Originally cloned from IL-1–stimulated ECs,31 PTX3 is a 45-kDa glycosylated protein predominantly assembled in 10 to 20 mer multimers that depend on interchain disulfide bonds.32 The COOH-terminal 203-amino acid pentraxin domain of PTX3 shares homology with the classic short pentraxin C-reactive protein (CRP), whereas its NH2-terminal 178-amino acid portion does not show any significant homology with any other known protein.31
Unlike CRP, which is produced by the liver in response to inflammatory mediators, PTX3 is synthesized locally at the inflammatory site by mononuclear phagocytes and ECs in response to IL-1β,31 tumor necrosis factor α,33 and bacterial components.34
PTX3 expression increases in mice during inflammation,35,36 whereas PTX3 transgenic mice show improved rates of survival to endotoxemia and sepsis.37 Also, the levels of circulating PTX3 increase in humans during vasculitides,38 acute myocardial infarction,39 rheumatoid arthritis,40 and systemic inflammatory response syndrome or septic shock.41 High levels of PTX3 are found associated to macrophages and ECs in atherosclerotic plaques.42
At the molecular level, PTX3 binds C1q, activating the complement cascade.32 Also, PTX3 binds histones, suggesting a role in the opsonization of intracellular residues during necrosis.32 Moreover, PTX3 binds to apoptotic cells regulating their clearance by dendritic cells.43 In ECs, PTX3 up-regulates tissue-factor expression, suggesting its action as a regulator of endothelium during thrombogenesis and ischemic vascular disease.44 Taken together, these data suggest that PTX3 may serve as a mechanism of amplification of inflammation and innate immunity. Accordingly, PTX3–/– mice show defective resistance against selected pathogens45 and show an alteration in seizure-induced damage in the central nervous system.36 Intriguingly, PTX3–/– female mice are also subfertile.46 Thus, available information suggests that PTX3 is a soluble pattern recognition receptor with unique nonredundant functions in various physiopathologic conditions.
In an effort to dissect the mechanisms responsible for the complex functions of PTX3, we investigated its ability to interact with cytokines and growth factors. Surprisingly, we found that human PTX3 binds specifically to FGF2. PTX3/FGF2 interaction neutralizes the capacity of the growth factor to bind FGFRs and HSPGs in ECs, thus inhibiting its mitogenic activity in vitro and angiogenic activity in vivo. PTX3 retains its FGF2-antagonist activity in vitro and in vivo also when overexpressed by ECs. Thus, PTX3 may contribute to the modulation of angiogenesis during inflammation, wound healing, atherosclerosis, and neoplasia. This represents the first demonstration that a member of the pentraxin superfamily specifically recognizes a growth factor, a finding that calls for similar studies with related long pentraxins.
Materials and methods
Chemicals
Human recombinant FGF2 and PTX3 were expressed in Escherichia coli and Chinese hamster ovary cells, respectively, and purified as described.32,47 Recombinant FGF4 and FGF8 were provided by C. Basilico (New York University Medical Center, New York, NY) and M. Jalkanen (Biotie, Turku, Finland), respectively. Neutralizing anti-FGF2 antibodies were from Upstate Biotech (Lake Placid, NY). Suramin was from FBA (Bayer AG, Leverkusen, Germany). C1q, CRP, histone IIIS, and cytochrome C were from Sigma (St Louis, MO). 1,2-dioctanoyl-sn-glycerol (DAG), epidermal growth factor (EGF), 12-O-tetradecanoyl phorbol 13-acetate (TPA), and vascular endothelial growth factor (VEGF)165 isoform were from Calbiochem (La Jolla, CA). FGF1, nerve growth factor, IL-4, macrophage colony-stimulating factor, monocyte chemotactic protein 1, IL-8, and lymphotactin were from Peprotech (London, United Kingdom). Tumor necrosis factor (TNF) was from BASF (Ludwigshafen, Germany). IL-6 was from Serono (Rome, Italy). IL-10 was from Schering-Plough (Milan, Italy). IL-1 was obtained from F. Colotta (Dompè, L'Aquila, Italy). IL-12 was from Genetics Institute (Andover, MA).
Solid-phase binding assays
Enzyme-linked immunosorbent assay (ELISA) microplates were incubated for 16 hours at 4° C with 100 μL of 100 mM NaHCO3, pH 9.6 (carbonate buffer) containing different concentrations of PTX3, FGF2, or various control molecules. Then, wells were washed and incubated for 2 hours at 37° C with 1.0% bovine serum albumin (BSA) in carbonate buffer. For binding experiments, 200-μL aliquots of phosphate-buffered saline (PBS) containing 125I-FGF2 (1.6 nM) were incubated for 2 hours at 37° C onto PTX3-coated wells in the presence of different competitors. Then, wells were washed, and bound radioactivity was solubilized by incubating the wells for 30 minutes at 50° C with 2.0% sodium dodecyl sulfate (SDS) in H2O, collected, and measured in a liquid scintillation counter. Alternatively, 100-μL aliquots of PBS containing various concentrations of biotin-labeled PTX3 (bPTX3) were incubated for 30 minutes at 37° C onto FGF2-coated wells with or without different competitors. Then, wells were washed, and the amount of bound bPTX3 was evaluated as described.32
Cell cultures
Fetal bovine aortic endothelial GM 7373 cells48 were obtained from the N.I.G.M.S. Human Genetic Mutant Cell Repository (Institute for Medical Research, Camden, NJ) and grown in Eagle minimal essential medium (MEM) containing 10% fetal calf serum (FCS). Bovine aortic endothelial (BAE) cells and murine endothelial E1G11 cells (provided by A. Vecchi, Istituto Mario Negri, Milan, Italy) were grown in Dulbecco minimal essential medium (DMEM) containing 10% heat-inactivated or 20% FCS, respectively. Human umbilical vein endothelial (HUVE) cells at passage 3 (Clonetics, Biowhittaker, Walkersville, MA) were grown in complete EGM-2 medium (Clonetics). Human embryonic kidney (EcoPack2-293) packaging cells (Clontech, Palo Alto, CA) and Balb/c mouse aortic endothelial 22106 cells stably transfected with a human FGF2 cDNA (FGF2-T-MAE cells)49 were grown in DMEM containing 10% FCS plus 500 μg/mL G418 (Sigma).
PTX3 cDNA transfection
FGF2-T-MAE cells (3 × 105 cells/100-mm plates) were incubated for 48 hours with Lipofectamin (Invitrogen, San Diego, CA) and 10 μg of the expression vector PLXSH harboring the 1470-bp human PTX3 cDNA under the control of the 5′ long terminal repeat (LTR) promoter (PLX-PTX3).50 Transfected clones were selected with hygromycin B (600 μg/mL; Boehringer Mannheim GmbH, Mannheim, Germany) and tested for FGF2 and PTX3 protein expression by ELISA of the cell extract and supernatant, respectively.
PTX3 retroviral infection
The cDNAs encoding for human PTX3 and for the enhanced green fluorescent protein (EGFP) were obtained from PLX-PTX3 and pEGFP-C1 (Clontech) plasmids, respectively. The 2 fragments were cloned in the pBABE retroviral vector,51 thus generating pBABE-PTX3 and pBABE-EGFP that were used to transfect the EcoPack2-293 packaging cells in the presence of Lipofectamin. Transduced cells were selected with puromycin (1 μg/mL; Sigma) for 2 weeks. Clones with a viral titer higher than 105 cfu/mL were used for further experimentation. Confluent cultures of FGF2-T-MAE cells were incubated for 24 hours with the conditioned medium from pBABE-PTX3 and pBABE-EGFP packaging cells in the presence of polybrene (8 μg/mL; Sigma). Infected cell populations were selected for 7 days with puromycin and evaluated for FGF2 and PTX3 expression by ELISA. Observation of EGFP-infected cells by epifluorescence microscopy (Axiovert S100 microscope, ×10/0.25; Zeiss, Göttingen, Germany) showed that retroviral infection efficiency was higher than 95%.
125I-FGF2 cell-binding assay
FGF2 was iodinated at a specific radioactivity equal to 800 cpm/fmol as described.52 GM 7373 cells were incubated at 4° C in serum-free medium containing 125I-FGF2 (0.55 nM), 0.15% gelatin, 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (pH 7.5), and increasing concentrations of PTX3. After 2 hours, the amount of 125I-FGF2 bound to low- and high-affinity sites was evaluated as described.53
Cell proliferation assays
Cell proliferation assay on GM 7373 cells was performed as described.54 Briefly, 16 hours after seeding at 75 000 cells/cm2 in 24-well dishes, GM 7373 cells were incubated in fresh medium containing 0.4% FCS with or without FGF2 (0.55 nM) and increasing concentrations of PTX3 or other antagonists. After 24 hours, cells were trypsinized and counted in a Burker chamber. HUVE cells (2500 cells/well in 96-well plates) were incubated for 24 hours in complete EGM-2 medium. After washing, cells were incubated for another further 72 hours in EGM-2 medium plus 2.0% FCS without FGF2, VEGF, and heparin. Finally, cells were added with FGF2 in the presence of PTX3. After 3 days cells were trypsinized and counted in a Burker chamber. E1G11 cells seeded at 8000 cells/cm2 in 1.0% gelatin-coated 48-well plates and incubated for 24 hours in complete DMEM medium were added with fresh medium containing 0.4% FCS and FGF2 in the presence of PTX3. After 3 days of incubation, cells were trypsinized and counted in a Burker chamber.
EC monolayer wound healing assay
Confluent BAE cell cultures on 1.5% gelatin-coated wells were wounded with a rubber policeman. After extensive washing, wounded monolayers were incubated at 37° C with DMEM containing 0.1% gelatin in the absence or in the presence of anti-FGF2 antibodies or PTX3. Microphotographs were taken under an inverted microscope (Olympus 1 × 51 microscope with Camedia C-4040 digital camera, ×10/0.25; Olympus Biosystem, Munich, Germany) to follow the repair process.
Subcutaneous tumor growth assay
Five-week-old nu/nu female mice (Harlan Nossan, S. Pietro al Natisone, Italy) were injected subcutaneously in the dorsal scapular region with 1 × 106 parental, EGFP-infected, or PTX3-infected FGF2-T-MAE cells (8 animals per group). The tumor volume was measured daily with calipers. When killed, lesions were harvested, and FGF2 and PTX3 levels in the tumor extracts were measured by ELISA.
Three-dimensional fibrin gel assay
Aggregates of parental or PTX3-transfected FGF2-T-MAE cells, prepared on agarose-coated plates as described,18 were seeded onto fibrin-coated 48-well plates. Immediately after seeding, 250 μL calcium-free medium containing fibrinogen (2.5 mg/mL) and thrombin (250 mU/mL) were added to each well and allowed to gel for 5 minutes at 37° C. Then, 500 μL culture medium was added on the top of the gel. In all the experiments, the fibrinolytic inhibitor trasylol was added to the gel and to the culture medium at 200 KIU/mL to prevent the dissolution of the substrate.18 Formation of radially growing cell sprouts was observed during the next 1 to 2 days and evaluated by computerized image analysis.
CAM assay
Fertilized white leghorn chick eggs were incubated at 37° C. At day 8 of incubation, 1.0 mm3 sterilized gelatin sponges (Gelfoam; Upjohn Company, Kalamazoo, MI) adsorbed with FGF2 alone (0.028 nmoles/embryo) or in the presence of PTX3 (0.11 nmoles/embryo) dissolved in 5 μL PBS were implanted on the top of growing chorioallantoic membranes (CAMs).55 Sponges containing vehicle alone or PTX3 alone were used as controls. The angiogenic response was graded at day 12 under a stereomicroscope (STEMI-SR, × 2/0.12; Zeiss) on an arbitrary scale of 0 to 4+, with 0 representing no response and 4+ representing the strongest activity.
Results
PTX3/FGF2 interaction
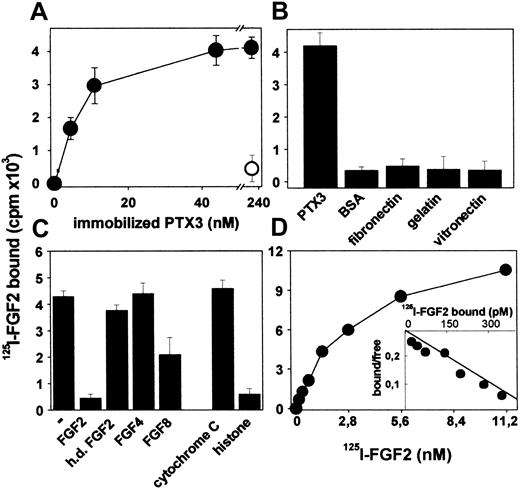
In an effort to explore new molecular targets of PTX3, we evaluated its capacity to interact with a variety of extracellular signaling polypeptides. To this purpose, a panel of cytokines, chemokines, and growth factors representative of different classes of soluble polypeptide mediators were immobilized onto plastic at 200 ng/well and compared with the PTX3 ligand C1q32 for their capacity to bind bPTX3 (22 nM). As shown in Table 1, FGF2 binds bPTX3 with high capacity, similarly to C1q. A limited binding is also exerted by immobilized VEGF, whereas no significant interaction was observed for the other polypeptides tested, including FGF1, a member of the FGF family structurally and functionally related to FGF2. Similar results were obtained when plastic was coated with higher concentrations of the proteins under test (1.0 μg/well) and when the concentration of bPTX3 was increased to 220 nM (data not shown). Scatchard plot analysis of the binding of increasing concentrations of bPTX3 (from 6.8 nM to 440 nM) to immobilized FGF2 showed that PTX3/FGF2 interaction is dose dependent and saturable (data not shown) with a Kd (dissociation constant) equal to 30 nM. This value is close to that calculated for PTX3/C1q interaction under the same experimental conditions.32
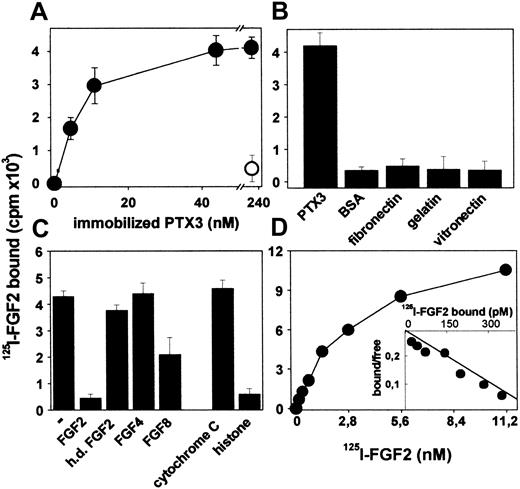
To better characterize PTX3/FGF2 interaction, PTX3 was immobilized to plastic and evaluated for its capacity to bind free 125I-FGF2. As shown in Figure 1A, immobilized PTX3 binds 125I-FGF2 in a dose-dependent manner, and the interaction is prevented by a monoclonal anti-PTX3 antibody. Under the same experimental conditions, 125I-FGF2 does not bind to immobilized BSA, fibronectin, gelatin, or laminin (Figure 1B). Unlabeled native FGF2, but not heat-denatured FGF2, competes with 125I-FGF2 for the binding to immobilized PTX3 (Figure 1C). Also, the PTX3 binder histone IIIS,32 but not cytochrome C, competes with 125I-FGF2 for the binding to immobilized PTX3 despite that both proteins share with FGF2 a similar apparent molecular mass and a highly positive charge. Specificity of the interaction was confirmed further when FGF4 and FGF8, 2 other members of the FGF family,56 were tested for their capacity to compete with 125I-FGF2 for PTX3 binding. FGF4 does not exert any competitive activity, whereas FGF8 shows only a limited effect (Figure 1C).
Binding of 125I-FGF2 to immobilized PTX3. (A) PTX3-coated wells were incubated with 125I-FGF2 in the absence (•) or in the presence (○) of anti-PTX3 antibody (5 μg/mL). (B) Wells coated with the indicated proteins (all at 440 nM) were incubated with 125I-FGF2. (C) Wells coated with PTX3 (440 nM) were incubated with 125I-FGF2 in the absence (–) or in the presence of the indicated unlabeled proteins (all at 111 nM; h.d. indicates heat denatured). (D) Increasing concentrations of 125I-FGF2 were incubated onto wells coated with PTX3 (100 nM). (D, inset) Scatchard plot regression analysis of the 125I-FGF2 binding data to immobilized PTX3. For all the experiments, bound radioactivity was measured after 2 hours at 37° C. In panels A-C, each point is the mean ± SEM of 3 to 4 determinations in duplicate, whereas the data shown in panel D represent one experiment (similar results were obtained in a second independent experiment).
Binding of 125I-FGF2 to immobilized PTX3. (A) PTX3-coated wells were incubated with 125I-FGF2 in the absence (•) or in the presence (○) of anti-PTX3 antibody (5 μg/mL). (B) Wells coated with the indicated proteins (all at 440 nM) were incubated with 125I-FGF2. (C) Wells coated with PTX3 (440 nM) were incubated with 125I-FGF2 in the absence (–) or in the presence of the indicated unlabeled proteins (all at 111 nM; h.d. indicates heat denatured). (D) Increasing concentrations of 125I-FGF2 were incubated onto wells coated with PTX3 (100 nM). (D, inset) Scatchard plot regression analysis of the 125I-FGF2 binding data to immobilized PTX3. For all the experiments, bound radioactivity was measured after 2 hours at 37° C. In panels A-C, each point is the mean ± SEM of 3 to 4 determinations in duplicate, whereas the data shown in panel D represent one experiment (similar results were obtained in a second independent experiment).
Finally, Scatchard plot analysis of the binding of 125I-FGF2 to immobilized PTX3 (Figure 1D) confirmed that PTX3/FGF2 interaction is a dose-dependent and saturable single component binding with a Kd value equal to 8.5 ± 7.2 nM, consistent with that calculated for the interaction of free bPTX3 with immobilized FGF2 (as described earlier).
PTX3 inhibits the mitogenic activity of FGF2 in ECs
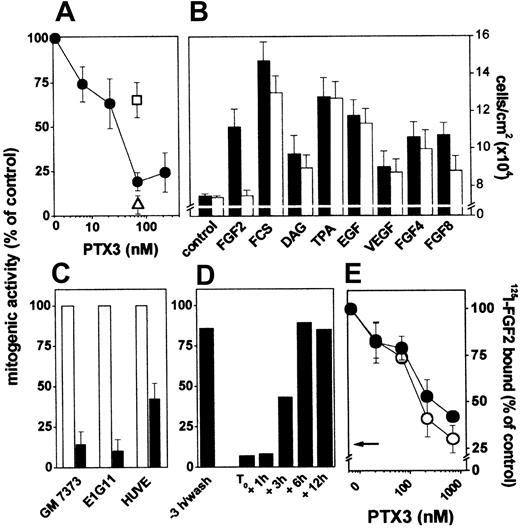
The capacity of PTX3 to bind FGF2 with high affinity prompted us to assess its effect on the mitogenic activity exerted by the growth factor on cultured ECs. PTX3 inhibits FGF2-dependent proliferation of endothelial GM 7373 cells in a dose-dependent manner, and the effect was reverted by anti-PTX3 antibody but not by irrelevant antibodies (Figure 2A). In contrast, PTX3 does not inhibit cell proliferation triggered by serum, DAG, EGF, TPA, or VEGF, in agreement with its limited capacity to bind this growth factor (Figure 2B). In keeping with its ability to interact differently with other members of the FGF family (Figure 1C), PTX3 does not affect the mitogenic activity of FGF4 and exerted a partial antagonist effect on that of FGF8 (Figure 2B).
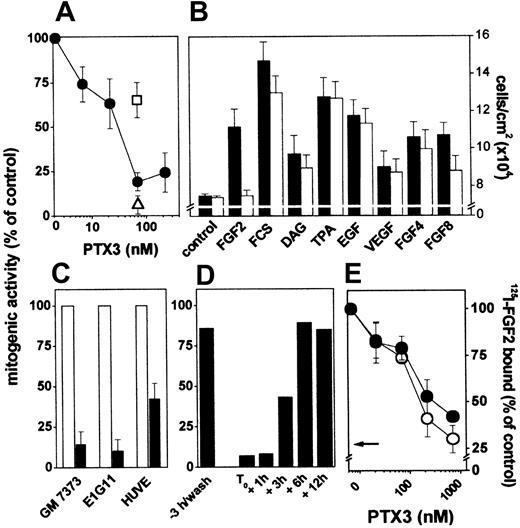
Effect of PTX3 on FGF2 receptor interaction and mitogenic activity in endothelial cells. (A) GM 7373 cells were treated with FGF2 and PTX3 in the absence (•) or in the presence of anti-PTX3 (□) or irrelevant (▵) antibodies (both at 99 μg/mL). (B) GM 7373 cells were treated with 0.4% FCS with no addition (control), FGF2 (1.66 nM), 10% FCS, DAG (15 μM), TPA (8.0 nM), EGF (0.6 nM), VEGF (0.7 nM), FGF4 (1.66 nM), or FGF8 (1.66 nM) in the absence (▪) or in the presence (□) of PTX3 (66 nM). (C) GM 7373 cells, E1G11 cells, or HUVE cells were incubated with FGF2 (0.55 nM) in the absence (□) or in the presence (▪) of PTX3 (222 nM). All mitogens induced a statistically significant increase in the proliferation rate (Student t test, P < .05). Each point is the mean ± SEM of 3 to 7 determinations in duplicate. (D) PTX3 (66 nM) was added to GM 7373 cells at the indicated periods of time before or after the beginning of FGF2 treatment (T0). When FGF2 was added after PTX3, cells were washed extensively before addition of the growth factor. Cells were counted 24 hours after the beginning of FGF2 treatment. Two additional independent experiments gave similar results. In panels A, C, and D, data are expressed as percentage of the increase in cell number relative to control cells treated with the different mitogens in the absence of PTX3. (E) GM 7373 cells were treated with 125I-FGF2 in the presence of PTX3. Then, 125I-FGF2 bound to HSPGs (○) and FGFRs (•) was evaluated and expressed as percentage of the radioactivity measured in the absence of PTX3. The arrow points to the inhibition measured in the presence of a 100-fold molar excess of unlabeled FGF2. Each point is the mean ± SEM of 3 determinations in duplicate.
Effect of PTX3 on FGF2 receptor interaction and mitogenic activity in endothelial cells. (A) GM 7373 cells were treated with FGF2 and PTX3 in the absence (•) or in the presence of anti-PTX3 (□) or irrelevant (▵) antibodies (both at 99 μg/mL). (B) GM 7373 cells were treated with 0.4% FCS with no addition (control), FGF2 (1.66 nM), 10% FCS, DAG (15 μM), TPA (8.0 nM), EGF (0.6 nM), VEGF (0.7 nM), FGF4 (1.66 nM), or FGF8 (1.66 nM) in the absence (▪) or in the presence (□) of PTX3 (66 nM). (C) GM 7373 cells, E1G11 cells, or HUVE cells were incubated with FGF2 (0.55 nM) in the absence (□) or in the presence (▪) of PTX3 (222 nM). All mitogens induced a statistically significant increase in the proliferation rate (Student t test, P < .05). Each point is the mean ± SEM of 3 to 7 determinations in duplicate. (D) PTX3 (66 nM) was added to GM 7373 cells at the indicated periods of time before or after the beginning of FGF2 treatment (T0). When FGF2 was added after PTX3, cells were washed extensively before addition of the growth factor. Cells were counted 24 hours after the beginning of FGF2 treatment. Two additional independent experiments gave similar results. In panels A, C, and D, data are expressed as percentage of the increase in cell number relative to control cells treated with the different mitogens in the absence of PTX3. (E) GM 7373 cells were treated with 125I-FGF2 in the presence of PTX3. Then, 125I-FGF2 bound to HSPGs (○) and FGFRs (•) was evaluated and expressed as percentage of the radioactivity measured in the absence of PTX3. The arrow points to the inhibition measured in the presence of a 100-fold molar excess of unlabeled FGF2. Each point is the mean ± SEM of 3 determinations in duplicate.
The FGF2 antagonist activity of PTX3 was not restricted to GM 7373 cells. Indeed, PTX3 inhibits also cell proliferation driven by FGF2 in mouse microvascular endothelial E1G11 cells and in HUVE cells (Figure 2C).
The time dependency of the FGF2-antagonist activity of PTX3 was investigated. As shown in Figure 2D, 3-hour pretreatment with PTX3 followed by its removal before FGF2 administration did not affect the mitogenic activity of the growth factor. In contrast, PTX3 retained its inhibitory capacity when added to the culture medium within 1 to 3 hours after the beginning of FGF2 treatment. Delayed administration of PTX3 (≥ 6 hours) was instead ineffective. These data indicate that PTX3 affects early event(s) exerted by FGF2 in ECs, possibly related to the interaction of FGF2 with its cell surface receptors.
To assess this possibility, PTX3 was evaluated for its capacity to affect the binding of FGF2 to HSPGs and FGFRs present on EC surface. As shown in Figure 2E, PTX3 inhibits the binding of 125I-FGF2 to both HSPGs and FGFRs expressed by endothelial GM 7373 cells in a dose-dependent manner.
In conclusion, PTX3 can sequester free FGF2, thus preventing its binding to EC surface receptors and leading to the inhibition of its mitogenic activity.
Interaction of CRP with FGF2
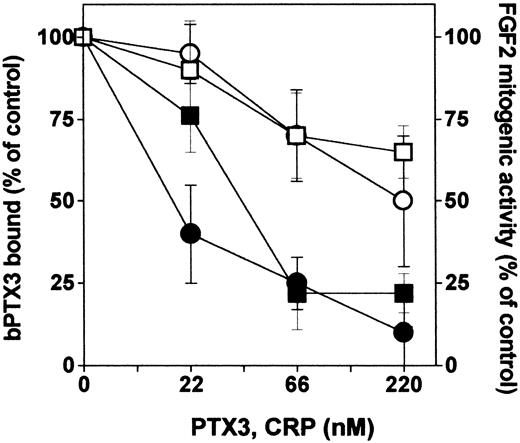
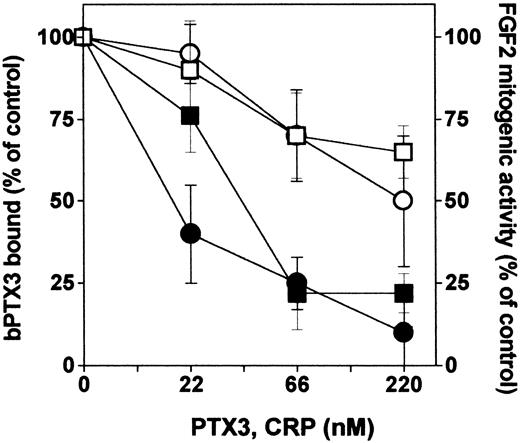
The COOH-terminal portion of PTX3 shares high homology with the entire sequence of the classic short pentraxin CRP. To assess whether also short pentraxins might interact with FGF2, we compared unlabeled PTX3 and CRP for the capacity to compete with bPTX3 for the binding to immobilized FGF2. As shown in Figure 3, CRP exerted only a partial competition when compared with unlabeled PTX3. Accordingly, CRP exerts only a limited inhibition on the mitogenic activity triggered by FGF2 in GM 7373 cells (Figure 3).
Interaction of short pentraxin CRP with FGF2. Wells coated with FGF2 (270 nM) were incubated with bPTX3 (22 nM) in the absence or in the presence of increasing concentrations of unlabeled PTX3 (•) or CRP (○). The amount of bPTX3 bound to immobilized FGF2 was then measured and data were expressed as percentage of binding measured in the absence of any competitor. In parallel, GM 7373 cells were treated with FGF2 in the absence or in the presence of increasing concentrations of PTX3 (▪) or CRP (□). Cells were then counted and data were expressed as percentage of the increase in cell proliferation in respect to cells treated with FGF2 alone. Each point is the mean ± SEM of 3 determinations in duplicate.
Interaction of short pentraxin CRP with FGF2. Wells coated with FGF2 (270 nM) were incubated with bPTX3 (22 nM) in the absence or in the presence of increasing concentrations of unlabeled PTX3 (•) or CRP (○). The amount of bPTX3 bound to immobilized FGF2 was then measured and data were expressed as percentage of binding measured in the absence of any competitor. In parallel, GM 7373 cells were treated with FGF2 in the absence or in the presence of increasing concentrations of PTX3 (▪) or CRP (□). Cells were then counted and data were expressed as percentage of the increase in cell proliferation in respect to cells treated with FGF2 alone. Each point is the mean ± SEM of 3 determinations in duplicate.
PTX3 hampers the autocrine activity of endogenous FGF2 in ECs
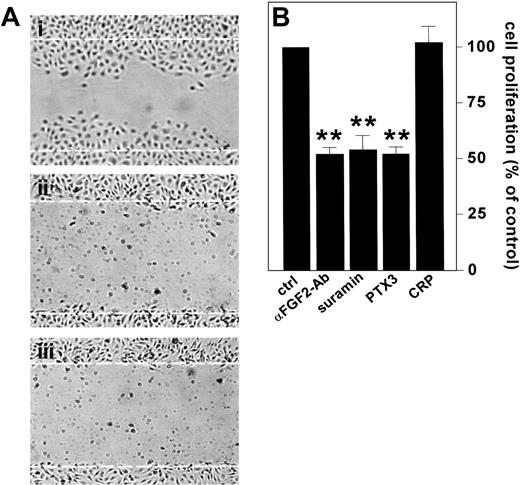
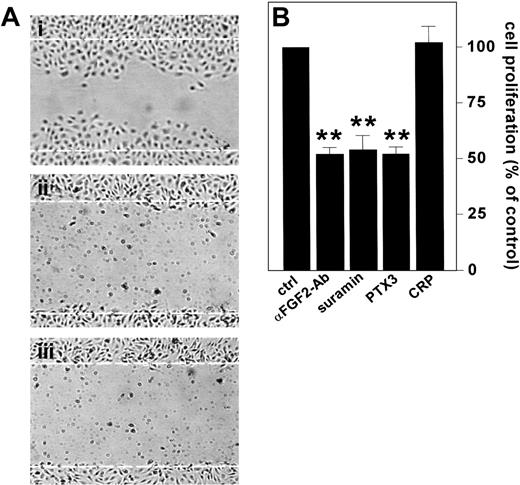
ECs express FGF2 in vitro and in vivo (see Gualandris et al18 and references therein). Endogenous FGF2 plays an autocrine role in ECs following its release by way of an alternative secretion pathway or cell damage.12 To assess the capacity of PTX3 to inhibit also the activity of endogenous FGF2 in ECs, confluent BAE cell cultures, which express significant levels of FGF2,57 were scrape-wounded, and the effect of PTX3 or neutralizing anti-FGF2 antibodies on the repair of the wounded monolayer was followed. In keeping with previous findings,16,17 anti-FGF2 antibodies prevented EC repair. This was similarly suppressed by PTX3 (Figure 4A).
Effect of PTX3 on the autocrine activity exerted by endogenous FGF2 in ECs. (A) Wounded BAE cell monolayers were grown in the absence (i) or in the presence (ii) of neutralizing anti-FGF2 antibodies (200 μg/mL) or PTX3 (666 nM) (iii). Microphotographs (original magnification, × 10) were taken 16 hours after wounding. White dotted lines mark the edge of the wound at the beginning of the experiment. (B) FGF2-T-MAE cells were seeded at 10 000 cells/cm2 in 24-well plates. After 24 hours, the medium was replaced without (ctrl) or with the addition of neutralizing anti-FGF2 antibody (αFGF2-Ab) (10 μg/mL), suramin (300 μM), PTX3 (222 nM), or CRP (222 nM). After 3 days the cells were counted and data were expressed as percentage of cell proliferation with respect to untreated controls. Each point is the mean ± SEM of 4 determinations in duplicate. Student t test, **P < .01.
Effect of PTX3 on the autocrine activity exerted by endogenous FGF2 in ECs. (A) Wounded BAE cell monolayers were grown in the absence (i) or in the presence (ii) of neutralizing anti-FGF2 antibodies (200 μg/mL) or PTX3 (666 nM) (iii). Microphotographs (original magnification, × 10) were taken 16 hours after wounding. White dotted lines mark the edge of the wound at the beginning of the experiment. (B) FGF2-T-MAE cells were seeded at 10 000 cells/cm2 in 24-well plates. After 24 hours, the medium was replaced without (ctrl) or with the addition of neutralizing anti-FGF2 antibody (αFGF2-Ab) (10 μg/mL), suramin (300 μM), PTX3 (222 nM), or CRP (222 nM). After 3 days the cells were counted and data were expressed as percentage of cell proliferation with respect to untreated controls. Each point is the mean ± SEM of 4 determinations in duplicate. Student t test, **P < .01.
To confirm these observations, we took advantage of a FGF2-overexpressing mouse aortic endothelial cell line (FGF2-T-MAE cells) generated in our laboratory.18,58 These cells are characterized by a sustained rate of growth in vitro and by the capacity to generate opportunistic vascular lesions in nude mice as the consequence of an autocrine loop of stimulation triggered by the release of the overexpressed growth factor.49 Accordingly, neutralizing anti-FGF2 antibodies or suramin, a well-known FGF2-binder/antagonist,58 caused a 50% decrease in the rate of growth of FGF2-T-MAE cells under basal culture conditions (Figure 4B). Exogenously added PTX3 caused a similar inhibitory effect, whereas CRP was ineffective (Figure 4B).
Taken together, these data indicate that exogenous PTX3 inhibits the autocrine motogenic and mitogenic activity exerted by endogenous FGF2 on ECs.
Endogenous PTX3 inhibits EC responsiveness to FGF2 in vitro and in vivo
Because ECs represent a major local source of PTX3 during inflammation,38 we investigated the FGF2 antagonist capacity of PTX3 also when produced endogenously by FGF2-activated ECs. To this purpose, FGF2-T-MAE cells were stably transfected with an expression vector harboring the full-length human PTX3 cDNA. Control cells were transfected with the empty vector. Mock and PTX3 transfectants characterized by levels of FGF2 production similar to those observed in parental FGF2-T-MAE cells (approximately 100 ng FGF2/μg protein) were screened for PTX3 secretion by ELISA. Four PTX3-producing clones, named B4, B8, B9, and B20 cells, and 2 mock clones, named BVV6 and BVV10, were selected and characterized further.
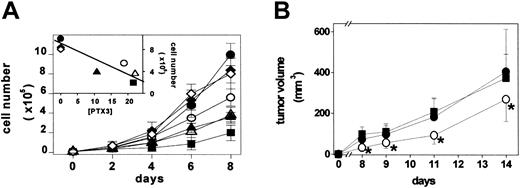
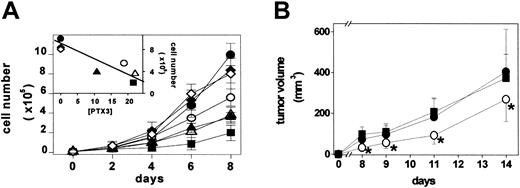
B4, B8, B9, and B20 PTX3 transfectants secrete 10.5, 22.0, 21.5, and 19.0 ng PTX3/5 × 106 cells/48 hours, respectively, whereas parental cells, BVV6 cells, and BVV10 cells do not release detectable amounts of PTX3. Mock and PTX3 transfectants were then compared with parental FGF2-T-MAE cells for their rate of growth under basal culture conditions. As shown in Figure 5A, parental cells, BVV6 cells, and BVV10 cells are characterized by a similar rate of growth. In contrast, PTX3-producing cells showed a rate of growth significantly slower than that of FGF2-T-MAE cells and inversely related to the amount of released PTX3 (inset to Figure 5A). Similar results were observed when PTX3 overexpression (45 ng PTX3/5 × 106 cells/48 hours) was triggered in FGF2-T-MAE cells by infection with a retrovirus harboring the human PTX3 cDNA (data not shown).
Effect of PTX3 overexpression on endothelial FGF2-T-MAE cell growth in vitro and in vivo. (A) Parental FGF2-T-MAE cells (•), PTX3-overexpressing clones B4 (▴), B8 (▵), B9 (▪), and B20 (○), and mock-transfected clones BVV6 (♦) and BVV10 (⋄) seeded at 10 000 cells/cm2 in 24-well plates were grown and counted at the indicated periods of time. Each point is the mean of 2 determinations in duplicate. (Inset) Linear regression of the number of cells measured at day 8 versus the levels of PTX3 production (ng PTX3/5 × 106 cells/48 hours) (r = 0.88; P < .01). (B) Parental (•), EGFP-infected (▪), and PTX3-infected FGF2-T-MAE cells (○) were injected subcutaneously in nude mice, and the size of the growing tumors was measured. Each point is the mean ± SD of 8 animals. *Statistically different from parental plus EGFP-infected FGF2-T-MAE lesions (Student t test, P < .05).
Effect of PTX3 overexpression on endothelial FGF2-T-MAE cell growth in vitro and in vivo. (A) Parental FGF2-T-MAE cells (•), PTX3-overexpressing clones B4 (▴), B8 (▵), B9 (▪), and B20 (○), and mock-transfected clones BVV6 (♦) and BVV10 (⋄) seeded at 10 000 cells/cm2 in 24-well plates were grown and counted at the indicated periods of time. Each point is the mean of 2 determinations in duplicate. (Inset) Linear regression of the number of cells measured at day 8 versus the levels of PTX3 production (ng PTX3/5 × 106 cells/48 hours) (r = 0.88; P < .01). (B) Parental (•), EGFP-infected (▪), and PTX3-infected FGF2-T-MAE cells (○) were injected subcutaneously in nude mice, and the size of the growing tumors was measured. Each point is the mean ± SD of 8 animals. *Statistically different from parental plus EGFP-infected FGF2-T-MAE lesions (Student t test, P < .05).
In keeping with the in vitro data, vascular lesions originated by the subcutaneous injection in nude mice of human PTX3-infected FGF2-T-MAE cells showed a reduced rate of growth when compared with tumors originated by parental or EGFP-infected cells (Figure 5B). All xenografts expressed significant amounts of FGF2 (6.8 ± 0.3 ng/mg protein), whereas human PTX3 expression was detectable only in PTX3-transduced lesions (15.0 ± 2.6 ng/mg protein). No major morphologic differences were observed among the different lesions that retained the characteristics of hemangioendothelioma-like tumors.49
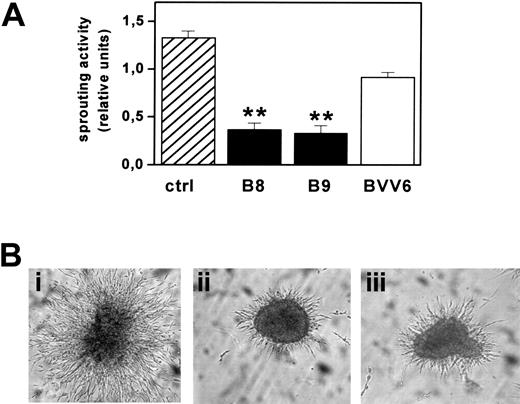
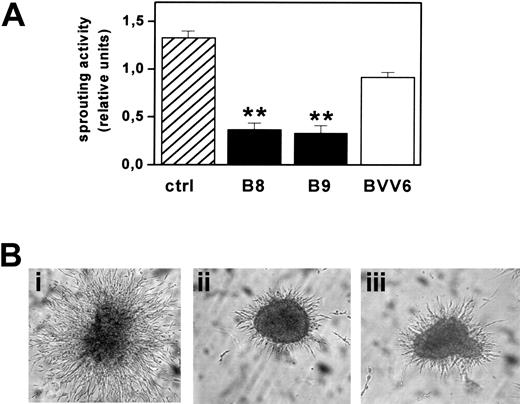
FGF2-T-MAE cell aggregates have an invasive behavior when embedded into 3D fibrin gels and form solid sprouts after 1 to 2 days in culture.18 Accordingly, mock-transfected BVV6 cells formed radially growing sprouts in fibrin gel with an efficiency similar to that of parental cells. In contrast, sprouting was strongly inhibited in PTX3-overexpressing B8 and B9 clones (Figure 6).
Effect of PTX3 overexpression on FGF2-T-MAE cell morphogenesis. (A) Aggregates of parental FGF2-T-MAE cells (ctrl), PTX3-overexpressing clones B8 and B9, and mock-transfected clone BVV6 were seeded within 3D-fibrin gels. After 24 hours, aggregates were photographed and sprouts quantified by computerized image analysis. Each point is the mean ± SEM of 4 determinations in duplicate. Student t test, **P < .01. (B) Representative images of aggregates of FGF2-T-MAE (i), B8 (ii), and B9 (iii) cells. Original magnification, × 40.
Effect of PTX3 overexpression on FGF2-T-MAE cell morphogenesis. (A) Aggregates of parental FGF2-T-MAE cells (ctrl), PTX3-overexpressing clones B8 and B9, and mock-transfected clone BVV6 were seeded within 3D-fibrin gels. After 24 hours, aggregates were photographed and sprouts quantified by computerized image analysis. Each point is the mean ± SEM of 4 determinations in duplicate. Student t test, **P < .01. (B) Representative images of aggregates of FGF2-T-MAE (i), B8 (ii), and B9 (iii) cells. Original magnification, × 40.
These data indicate that also endogenously produced PTX3 inhibits the autocrine loop of stimulation exerted by FGF2 in FGF2-T-MAE cells hampering EC proliferation and morphogenesis in vitro and tumorigenesis in vivo.
PTX3 inhibits the angiogenic activity of FGF2
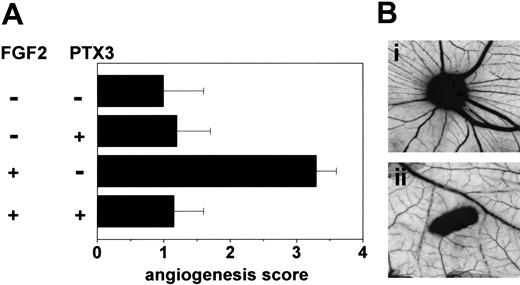
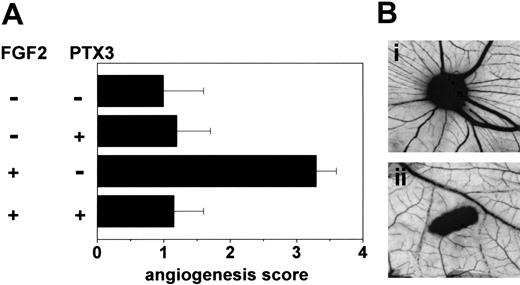
To assess the capacity of PTX3 to affect FGF2-induced neovascularization in vivo, gelatin sponges adsorbed with FGF2 alone or added with PTX3 were implanted on the top of 8-day-old chick embryo CAMs.55 Sponges adsorbed with vehicle or PTX3 alone were used as controls. As shown in Figure 7, PTX3 caused a significant inhibition of FGF2-induced angiogenesis, whereas it did not affect basal physiologic vascularization of the CAM.
Effect of PTX3 on FGF2-induced neovascularization. (A) Chick embryo CAMs were implanted with gelatin sponges adsorbed with vehicle, FGF2 (0.028 nmoles/embryo), PTX3 (0.11 nmoles/embryo), or both. At day 12, the angiogenic response in each egg was graded. Bars represent the mean ± SEM of 10 to 15 embryos per group. (B) Representative CAMs implanted with gelatin sponges adsorbed with FGF2 alone (i) or FGF2 plus PTX3 (ii). Original magnification, × 5.
Effect of PTX3 on FGF2-induced neovascularization. (A) Chick embryo CAMs were implanted with gelatin sponges adsorbed with vehicle, FGF2 (0.028 nmoles/embryo), PTX3 (0.11 nmoles/embryo), or both. At day 12, the angiogenic response in each egg was graded. Bars represent the mean ± SEM of 10 to 15 embryos per group. (B) Representative CAMs implanted with gelatin sponges adsorbed with FGF2 alone (i) or FGF2 plus PTX3 (ii). Original magnification, × 5.
Discussion
Here, we demonstrate that PTX3 binds FGF2 with high affinity and selectivity. PTX3/FGF2 interaction prevents the binding of the growth factor to FGFRs and HSPGs on EC surface, thus inhibiting its angiogenic activity in vitro and in vivo and the capacity of FGF2-transformed ECs to generate vascular lesions in nude mice.
PTX3 binds to FGF2 but it does not interact with a variety of cytokines, chemokines, and growth factors (Table 1). In particular, PTX3 does not bind or binds poorly to other members of the FGF family, including FGF1, FGF4, and FGF8, indicating a high degree of specificity for PTX3/FGF2 interaction.
X-ray crystallography has identified a “basic pocket” in the FGF2 molecule that mediates the interaction of the growth factor with heparin.59 However, a 100-molar excess of heparin does not compete with bPTX3 for the binding to immobilized FGF2 (data not shown), ruling out the possibility that the basic domain of FGF2 is involved in PTX3 interaction. In keeping with this hypothesis is the overmentioned incapacity of other heparin-binding members of the FGF family and chemokines to bind PTX3. Also, the observation that PTX3 does not recognize heat-denatured FGF2 or the cationic cytochrome C indicates that the overall basic charge of FGF2 is not sufficient for the interaction that requires a correct 3D conformation of the growth factor. These data, together with the incapacity of PTX3 to bind heparin (M.R., unpublished observations, December 14, 2002), suggest that PTX3 prevents the interaction of FGF2 with cell-associated HSPGs by steric hindrance.
The short pentraxin CRP is an inefficient FGF2 binder/antagonist despite its sequence homology with the 203-amino acid COOH-terminal portion of PTX3.32 Similar to CRP, PTX3 multimers bind C1q by way of the pentraxin domain.32 However, differences in binding properties of CRP and PTX3 have been described.32 The capacity of PTX3, but not of CRP, to bind FGF2 suggests that the 178-amino acid NH2-terminal portion of PTX3, absent in short pentraxins, mediates the binding to FGF2. Further experiments are required to map the domains in PTX3 and FGF2 molecules responsible for the interaction.
PTX3 inhibits the mitogenic activity exerted by FGF2 in bovine, murine, and human ECs in vitro. This appears to be due to the capacity of PTX3 to prevent FGF2 binding to FGFRs rather than to a direct action of PTX3 on ECs. Indeed, PTX3 pretreatment does not affect EC responsiveness to FGF2. Also, PTX3 does not inhibit EC proliferation triggered by various mitogens. It must be pointed out that PTX3 interacts with and inhibits the biologic activity of FGF2 at doses comparable to those measured in the blood of patients affected by inflammatory diseases.38,39 Moreover, the local concentration of PTX3 at the site of inflammation can be significantly higher than that measured in the bloodstream, supporting the possibility that PTX3/FGF2 interaction may occur and be biologically relevant in vivo. In keeping with this hypothesis is the capacity of retroviral vector–transduced PTX3 to inhibit the growth of vascular lesions caused by subcutaneous injection of FGF2-activated endothelial FGF2-T-MAE cells in nude mice.
PTX3 is produced by macrophages,34 fibroblasts,33 myoblasts,60 microglia,35 and ECs,31 indicating that it may exert paracrine and autocrine functions on endothelium. Similarly, various stimuli, including inflammatory mediators IL-1 and nitric oxide14,61 induce FGF2 expression in ECs that undergo an autocrine loop of stimulation. Thus, ECs and other cell types can express both PTX3 and FGF2. The capacity of PTX3 to inhibit the repair driven by endogenous FGF2 in wounded BAE cell monolayers, together with the inhibitory effect exerted by PTX3 cDNA overexpression in FGF2-T-MAE cells, indicates that both exogenous and endogenous PTX3 can suppress the autocrine loop of stimulation triggered by FGF2 in ECs, leading to a significant inhibition of cell migration, proliferation, morphogenesis, and tumor growth. Thus, PTX3 produced by inflammatory cells or by ECs themselves may affect the autocrine and paracrine activity exerted by FGF2 on endothelium in vitro and in vivo. This should allow a fine tuning of neovascularization by way of the production of both angiogenesis inhibitors and stimulators.
FGF2 is a pleiotropic growth factor that stimulates various cell types of endodermal and mesodermal origin.62 Therefore, the role exerted by FGF2 in various physiopathologic conditions may not be limited to its angiogenic activity. For instance, FGF2 stimulates the migration and proliferation of fibroblasts during wound healing and of smooth muscle cells during atherosclerosis10,63 and restenosis.64 Also, it may favor neuronal cell survival and glia cell proliferation in the injured central nervous system.65 In all these conditions, the concomitant production of PTX336,42 may modulate the activity exerted by FGF2 on these cells. Indeed, preliminary experiments showed the capacity of PTX3 to inhibit FGF2-dependent proliferation in smooth muscle cells (M.C., unpublished data, March 2003).
In conclusion, we demonstrate for the first time that PTX3, a member of the pentraxin superfamily released locally by inflammatory cells, binds the angiogenic growth factor FGF2 with high affinity and specificity, acting as a natural angiogenesis inhibitor. Thus, PTX3 may play an important role in modulating the cross-talk between inflammatory cells and endothelium in various physiopathologic settings.
Prepublished online as Blood First Edition Paper, March 18, 2004; DOI 10.1182/blood-2003-10-3433.
Supported partially by grants from Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR; Centro di Eccellenza “IDET,” FIRB, Cofin 2002), Associazione Italiana per la Ricerca sul Cancro (M.P.), and by Istituto Superiore di Sanità (AIDS Project) and Cofin 2003 (M.R.).
M.R. and M.C. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.