Comment on Pardanani et al, page 3038
The diagnosis of hypereosinophilic syndrome (HES) has been largely a process of exclusion and by definition therefore, unsatisfactory. Frustrated hematologists were comforted by the knowledge that a better understanding of the molecular pathogenesis would eventually resolve their difficulties. This better understanding is now beginning to emerge.
Cools et al provided at least part of the explanation with the identification of the FIP1L1-PDGFRA fusion gene in a substantial proportion of cases.1 The clinical significance of this observation is the prompt and remarkable response of such patients to imatinib. In the past year a number of groups have attempted to further classify the syndrome using associated clinical and pathologic features in fusion-positive patients. Klion et al were able to identify features that separated 9 of 15 cases of HES and were associated with the presence of the fusion gene and/or response to imatinib.2 A prominent feature was a moderately raised serum tryptase, leading them to adopt the terminology HES-tryptase. Subsequently, Pardanani et al challenged this view on the basis of their observations of 5 patients thought to have systemic mast cell disease (SMCD), which suggested that FIP1LI-PDGFRA was more likely to be found in SMCD associated with eosinophilia (SMCD-eos).3
In this issue of Blood, Pardanani and colleagues provide important new information on the prevalence and characteristics of the FIP1L1-PDGFRA syndrome. They extended their observations to 57 patients with HES and 19 with SMCD-eos. Of the 11 patients positive for FIP1L1-PDGFRA, 10 had increased numbers of mast cells. The search criteria ensured that all study patients had eosinophilia, and whether there are patients with this fusion gene who do not have eosinophilia remains to be established. The authors considered that these 10 patients met the WHO criteria for SMCD4 and proposed that they should form a subgroup, namely SMCD-eos. So is this the end of the story? Were the original diagnoses of HES incorrect? Well, the devil may be in the detail. In fact, 7 of the 10 fusion-positive patients were originally diagnosed as HES and only after the fusion gene was detected were they reviewed and reclassified as SMCD-eos. It is not clear, however, that the 57 patients with HES were subjected to the same level of scrutiny, so some doubt must remain as to whether similar patterns of macrocytosis can be present without the fusion gene.
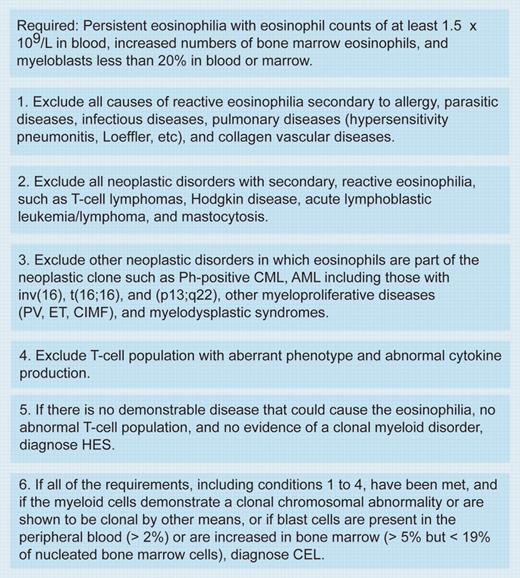
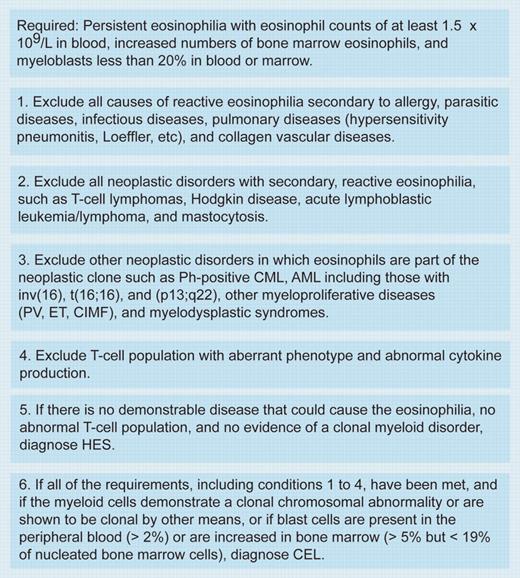
So, is the FIP1L1-PDGFRA syndrome SMCD or HES or chronic eosinophilic leukemia (CEL)? Although an interesting intellectual argument, it may have little relevance. Within the context of the WHO classification of chronic myeloproliferative disorders, patients positive for FIP1L1-PDGFRA would be classified as chronic eosinophilic leukemia (CEL) rather than HES (see figure).4 If FIP1L1-PDGFRA positivity were always associated with a diffuse mast cell infiltrate and a moderately raised serum tryptase, then further clarification regarding malignant mast cell involvement must come from the identification of the fusion gene in these cells. Rewriting the WHO classification now may be premature, but in all likelihood, the FIP1L1-PDGFRA fusion may simply define a separate entity of the myeloproliferative disorders.FIG1
Diagnosis of chronic eosinophilic leukemia and hypereosinophilic syndrome. Table was adapted with permission from Jaffe et al.4 Ph indicates Philadelphia chromosome; CML, chronic myelogenous leukemia; AML, acute myeloid leukemia; PV, polycythemia vera; ET, essential thrombocythemia; and CIMF, chronic idiopathic myelofibrosis.
Diagnosis of chronic eosinophilic leukemia and hypereosinophilic syndrome. Table was adapted with permission from Jaffe et al.4 Ph indicates Philadelphia chromosome; CML, chronic myelogenous leukemia; AML, acute myeloid leukemia; PV, polycythemia vera; ET, essential thrombocythemia; and CIMF, chronic idiopathic myelofibrosis.
The take-home message must be that all patients with persistent eosinophilia must be investigated thoroughly for all other reactive and clonal causes of eosinophilia, and such tests should include histochemical staining for mast-cell tryptase and serum tryptase levels, studies for c-kit mutations, and, of course, a search for the FIP1L1-PDGFRA fusion gene. In this respect, it is worth noting that the current reverse transcriptase–polymerase chain reaction (RT-PCR) test may lack some sensitivity, and, if negative, should always be followed by fluorescence in situ hybridization (FISH) and nested PCR analyses (European LeukemiaNet; Andreas Reiter, personal written communication 9 August 2004). Simultaneously, further observations on the clinical features of these patients, perhaps by the development of multinational registries, may help predict those patients most likely to be fusion gene positive and to benefit from imatinib.