Abstract
Mature dendritic cells (mDCs) can trigger the effector functions of natural killer (NK) cells. Knock-out, small-interfering RNA or neutralizing antibodies targeting interleukin 12 (IL-12) subunits revealed a critical role for IL-12 in NK cell interferon γ (IFN-γ) secretion promoted by mDCs. However, NK cell activation by DCs also required direct cell-to-cell contacts. DC-mediated NK cell activation involved the formation of stimulatory synapses between DCs and NK cells. The formation of DC/NK cell conjugates depended on cytoskeleton remodeling and lipid raft mobilization in DCs. Moreover, the disruption of the DC cytoskeleton using pharmacologic agents or the loss-of-function mutation of the Wiskott-Aldrich syndrome protein abolished the DC-mediated NK cell activation. Synapse formation promoted the polarized secretion of preassembled stores of IL-12 by DCs toward the NK cell. The synaptic delivery of IL-12 by DCs was required for IFN-γ secretion by NK cells, as assessed using inhibitors of cytoskeleton rearrangements and transwell experiments. Therefore, the cross-talk between DCs and NK cells is dictated by functional synapses. (Blood. 2004;104:3267-3275)
Introduction
Natural killer (NK) cells recognize and kill target cells expressing virus-encoded proteins, as well as tumor cells that have lost the expression of major histocompatibility complex (MHC) class I antigens.1-5 Activation of NK cells results from a balance between inhibitory and activating signaling pathways.6 Incompatibilities in HLA-Cw alleles between NK and target cells promote the cytolytic function of NK cells involved in the graft-versus-leukemia reaction.7 In contrast, receptor-ligand interactions between MHC class I molecules and killer inhibitory immunoglobulin-like receptor (KIR) or lectin-type inhibitory NK cell receptor can initiate a dominant inhibitory signaling cascade that blocks NK cell cytotoxicity. Recent studies of the physical interaction between NK cells and target cells have highlighted the functional impact of its synaptic organization. Thus, Lou et al8 reported that, within the NK/target cell synapse, lipid rafts polarized to the site of the cell contact in conjugates with sensitive MHC class I-negative targets but not in conjugates with resistant MHC class I-positive targets. Moreover, the negative signals between an NK cell and a target cell are transmitted by KIR at the site of membrane apposition, where inhibitory receptors become clustered with MHC class I ligands in a supramolecular structure known as an inhibitory NK immune synapse (IS).9,10 KIR signaling is critical for blocking lipid raft polarization and NK cell cytotoxicity, both depending on movements of microtubuli and actin filaments.11 The composition of adhesion, costimulatory, cytoskeletal, and signaling molecules in the supramolecular activation clusters (SMACs) of the cytolytic and noncytolytic NK cell IS revealed profound differences.12 Indeed, cytoskeleton remodeling and redistribution of NK cell signaling molecules occur mainly in cytolytic NK cell-target cell conjugates.8,13 F-actin and Wiskott-Aldrich syndrome protein (WASp) also localize in the cytolytic NK cell immune synapse. Loss-of-function mutation in WASp results in defects of both actin polymerization and cytolytic synapse formation.14,15
However, NK cell activation does not merely result from target cell recognition. Previous reports emphasized the pivotal role of dendritic cells (DCs) for NK cell triggering,16-20 a biologic phenomenon that we will henceforth refer to as “DAK” activity for DC-mediated activation of NK cells. DAK activity reportedly combats viral21 and tumor spreading16 in vivo. The precise locations of the DC/NK cell encounter remain unclear,22 but the outcome of the cross-talk critically depends on the activation state of the DCs or on the concomitant presence of inflammatory stimuli.17,18 Indeed, interferon α (IFN-α) or lipopolysaccharide (LPS)-stimulated DCs were found to trigger NK cell cytolytic activity and IFN-γ secretion. The mechanisms underlying the LPS-mediated DAK activity have been partially unraveled. Some reports involved interleukin 12 (IL-12) as a key factor promoting DC-mediated NK cell activation,21,23,24 whereas others pointed to a critical role of cell-to-cell contacts and membrane-associated molecules.16,17 Here we first confirmed the role of IL-12 in the DC-mediated NK cell activation following LPS stimulation using different approaches (small interfering RNA [siRNA], neutralizing antibody, and loss-of-function mutations), both in the mouse and in the human system. Second, we underscored that DC/NK cell contacts are critical for the formation of a synapse, which depends on remodeling of the DC cytoskeleton and raft mobilization. Third, we showed that this DC/NK cell synapse (henceforth referred to as DCNK-IS for DC/NK immunostimulatory synapse) led to IL-12 polarization in DCs toward the DC/NK cell interface. Our results thus reveal for the first time an activating IS between mature DCs (mDCs) and resting NK cells and indicate that this synapse is mandatory for IL-12 polarization in DCs and IL-12-dependent NK cell activation.
Materials and methods
Culture of peripheral blood monocytes
Human monocyte-derived DCs. Human monocyte-derived DCs (MD-DCs) were generated from monocytes of healthy volunteers after elutriation of peripheral blood according to the French Establissement Français du Sang (EFS) procedures (Pr Jacky Bernard, Jean Godinot Institute, Reims, France). Briefly, MD-DCs were cultured in 75-cm2 culture flasks for 6 days in AIMV complete medium (Gibco-BRL, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS; Pan Biotech, Aidenbach, Germany), 1000 IU/mL recombinant human granulocyte-macrophage colony-stimulating factor (rhuGM-CSF; Leucomax; Schering Plough, Dardilly, France) and 1000 IU/mL rhuIL-4 (R&D Systems, Abingdon, United Kingdom). Maturation of MD-DCs was induced (mDCs) at day 6 using LPS (2 μg/mL; Sigma-Aldrich, Saint-Quentin Fallavier, France). The MD-DC phenotype was assessed using 3-color immunostaining with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, and allophycocyanin-conjugated monoclonal anti-CD1a (HI149), anti-CD14 (M5E2), anti-CD83 (HB15e), anti-CD80 (L307,4), anti-CD86 (FUN-1), anti-CD40 (41), anti-HLA-DR (G46-6) antibodies (all purchased from PharMingen, Erembodegem, Belgium), anti-DC-SIGN monoclonal antibody (mAb; 120507; R&D Systems), and mixed lymphocyte reaction (not shown) as previously described.19
Mouse BM-DCs. Bone marrow-derived DCs (BM-DCs) were propagated from BM progenitor cells harvested from wild-type BL6- or WASp- or IL-12p35-deficient mice in culture medium supplemented with 1000 IU/mL recombinant murine GM-CSF (rmGM-CSF) and 1000 IU/mL rmIL-4 (R&D Systems) as previously described.16
Purification of NK cells
Human NK cells. Autologous peripheral blood lymphocytes from healthy volunteers were thawed and NK cells were negatively selected (Miltenyi Biotech, Paris, France) prior to coculture with freshly propagated day-7 MD-DCs. The purity of CD56+/CD3- NK cells was assessed by flow cytometry; anti-CD56 (B159) and anti-CD3 (UCHT1) mAbs were purchased from PharMingen (Erembodegem, Belgium) and ranged from 90% to 98%.
Mouse NK cells. Splenocytes were harvested from BALB/c severe combined immunodeficiency (SCID) mice. Splenic nonadherent cells were generated by subjecting red blood cell-deprived splenocytes to 3 hours of adherence at 37°C. NK cells were negatively selected from nonadherent cells (Miltenyi Biotech). Up to 80% of such splenocytes were CD3-/DX5+ cells.
Preparation of DC/NK cell cocultures
Human DC/NK cocultures. After magnetic negative selection and washing, NK cells were cultured alone (at 106/mL) or cocultured with immature DCs (iDCs) or mDCs in round-bottomed 96-well plates at a DC/NK cell ratio of 1:10 in AIMV medium for 24 hours, as described.19
Mouse DC/NK cocultures. NK cells were seeded at 1 × 105/well in round-bottomed 96-well plates for 20 hours and cocultured with BM-DCs at DC/NK cell ratios of 0.5:1, as described.16
Assessment of NK cell effector functions
Cytokine detection and quantification in supernatants. After 24 hours of BM-DC/NK coculture, supernatants were harvested, stored at -80°C, and assessed using commercial enzyme-linked immunosorbent assay (ELISA) kits (OptEIATM ELISA kit; PharMingen, San Diego, CA). The sensitivities of the murine IFN-γ kit, human IFN-γ kit, and human IL-12 kit were more than 31.5 pg/mL, more than 4.7 pg/mL, and more than 7.5 pg/mL, respectively. For intracytoplasmic detection assays in murine BM-DCs, cells were permeabilized and stained with an anti-IL-12 (p40/p70) mAb (rat IgG1, C15-6; PharMingen, Erembodegem, Belgium) or with matched control antibody. Alexa fluor 488 donkey anti-rat IgG (H+L; Molecular Probes, Leiden, the Netherlands) was used as a secondary mAb.
Cytotoxicity assays. After 18 hours of coculture of WASp-/- BM-DCs with mouse NK cells, cells were subjected to a killing assay of 51Cr YAC-1 targets.16
siRNAs and electroporation
The 21-nucleotide-long interfering RNA duplexes with two 3′-end overhang dT nucleotides in the antisense strand were synthesized as reported.25,26 The sequence of the control siRNA with random nucleotides and no known specificity was: 5′-ACG GGG GGC CCU UAA AAC AdTdT (MWG Biotec, Ebersberg, Germany). The sequence of the antisense strands targeting the p50 subunit of nuclear factor κB (NF-κB) has been previously described.27 The sequence of the antisense strands targeting the p40 subunit of IL-12 was 5′-CGC ACG CUA AUG CUG GCA UdTdT (Qiagen, Cambridge, MA). Transfection of siRNAs in human MD-DCs was performed by electroporation using an ECM 830 square wave electroporation system (BTX, San Diego, CA).27 Cells were then washed and cultured in GM-CSF and IL-4 before maturation with LPS (2 μg/mL, Sigma-Aldrich).
Slide preparation and confocal microscopy
MD-DCs (iDCs or mDCs) and autologous NK cells were resuspended in AIMV (Gibco-BRL) at 1.106 cells/mL. Then, 50 μL DCs and 100 μL NK cells were gently mixed and spread onto a slide coated with poly-l-lysine (Sigma, Paris, France). Slides were incubated for 25 minutes at 37°C. Cells were fixed in 4% paraformaldehyde and permeabilized with 0.2% sodium dodecyl sulfate (SDS). After 20 minutes of blocking in 10% fetal bovine serum (FBS) and washing, cells were stained with the appropriate mAbs or an isotype-matched control antibody in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) for 1 hour. Afterward, slides were extensively washed and incubated with the appropriate secondary antibodies for 1 hour. Finally, 0.17-mm cover glasses were mounted on the slides using a drop of vectashield hard set (Vector, Burlingame, CA). Stacks of confocal images were collected with a Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss, Paris, France) using a × 63 1.4 NA apochromat plan objective. Z-projection of slices and image analyses were performed using Zeiss LSM Image examiner software. A minimum of 30 conjugates per slide were analyzed in at least 3 independent experiments. Conjugates were studied in both 2-dimensional (x-y-axis) and 3-dimensional (x-z-axis) analyses. The relocalization of a given molecule into the synaptic area was considered significant when the fluorescence intensity of the synapse was at least 2-fold higher than that of the opposite site. Deconvolution was performed using the Huygens software (Scientific Volume Imaging, Hilversum, the Netherlands). Three-dimensional reconstitutions were performed using the Imaris 3.3 software (Bitplane, Zurich, Switzerland).
Cell labeling
Phalloidin-Texas red (Molecular Probes, Eugene, OR) was used to detect polymerized F-actin.
Primary antibodies. The anti-IL-15 (AZX04) and anti-IL-12 (24910.1) mAbs were purchased from R&D Systems. The anti-CD11a (25.3) mAb was purchased from Beckman Coulter (Villepinte, France). The anti-CD50 (TU41), the anti-CD54 (HA58), and the anti-CD45 (2D1) were purchased from PharMingen (Erembodegem, Belgium). The antifascin (55K2) mAb was purchased from Dako (Glostrup, Denmark). PT-100 (Cell Signaling Technology, Beverly, MA) and PT66-FITC (Sigma) were used to stain the intracellular phosphotyrosines.
Secondary antibodies. The Alexa fluor 488 goat anti-mouse IgG and the Alexa fluor 546 goat anti-mouse IgG were purchased from Molecular Probes (Eugene, OR).
Cholera toxin staining
DCs or NK cells were incubated with FITC-labeled cholera toxin β subunit (CTX β; Sigma, St Louis, MO) at 8 μg/mL for 1 hour on ice to visualize raft-associated GM1 gangliosides. Cells were washed twice in PBS, counted, resuspended for coculture, and prepared for microscopy as described (see “Slide preparation and confocal microscopy”).
Inhibition of cytoskeleton and raft rearrangements
mDCs were incubated for 30 minutes with 100 nM methyl β cyclodextrin (MCD) or for 1 hour with 10 μM demecolcine, 20 μM nocodazole, 20 μM cytochalasin D or B, and dimethyl sulfoxide (DMSO; as a control) at 37°C. MDC-treated mDCs were washed and exposed to 60 μg/mL water-soluble cholesterol (Sigma, St Louis, MO) for 30 minutes before coculture with NK cells to reverse the MDC effect.
Videomicroscopy and intracellular calcium concentration assessment
NK cells were labeled with 4 μM Fluo 4-am (Molecular Probes, Eugene, OR) for 45 minutes at 37°C, washed twice, and incubated in RPMI 1640 plus 10% FCS for 30 minutes before coculture with iDCs or mDCs on a glass slide. Videomicroscopy was performed for 1 hour in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-buffered medium and a temperature-controlled chamber. Pictures were acquired every 10 seconds. An increase in the fluorescence intensity of at least 50% above the background level (of resting NK cells) was considered significant for calcium influx. The data reported are representative of 2 independent experiments.
Mice
Female BALB/c (H-2d)/SCID mice and 129Sv/Ev (H-2b) were obtained from the Center d'Elevage Janvier (Le Genest St Isle, France) and maintained in the Institute Gustave Roussy animal facility according to the guidelines of the Animal Experimental Ethics Committee. WASp-deficient mice originated from S. B. Snapper (Children's Hospital, Boston, MA). Briefly, disruption of the murine WASp-encoding gene was obtained by inserting the neomycin-resistance gene into exon 7 of WASp cDNA in the reverse transcriptional orientation in TC-1 embryonic stem (ES) cells. WASp-deficient mice were bred at the Institute Gustave Roussy animal facility and used after the sixth generation of back-crosses onto 129 wild-type mice. IL-12 p35 knock-out mice were provided by Bernhard Ryffel (GEM2358, CNRS, Orleans, France). All female mice were used at 6 to 25 weeks of age.
Statistical analyses
The Student t test or the Fisher exact method were used to assess significant differences between experimental groups.
Results
IFN-γ secretion of NK cells promoted by DCs depends on IL-12
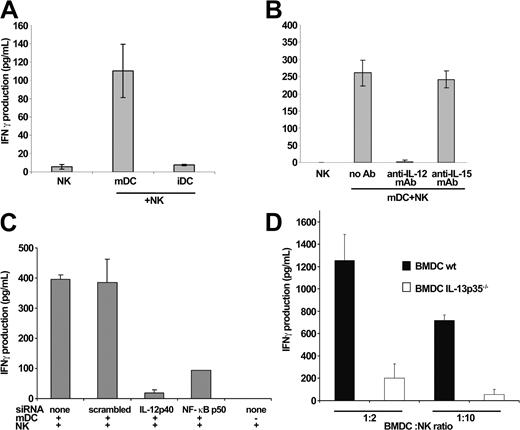
We investigated the role of IL-12 in the DC-stimulated IFN-γ production by NK cells in the murine and human systems. LPS-activated DCs (but not their immature counterparts) triggered IFN-γ secretion by NK cells (Figure 1A). However, an antibody-neutralizing IL-12p70 (Figure 1B) or siRNA specific for the IL-12p40 or the NF-κB p50 subunit (Figure 1C) completely abrogated the capacity of mDCs to induce IFN-γ secretion by resting NK cells. In contrast, anti-IL-15 mAb or control scrambled siRNA did not hamper the DC-mediated NK cell activation (Figure 1B-C). Similarly, BM-DCs propagated from IL-12p35-/- mice failed to promote IFN-γ secretion by splenic NK cells (Figure 1D) while maintaining a similar level of (T cell-mediated) allostimulation as their wild-type counterparts in vitro (not shown). As a result, mature DCs can stimulate NK cells to secrete IFN-γ, and this effect depends on IL-12.
IL-12 delivery by mDCs is critical for NK cell activation. (A) mDCs promote the secretion of IFN-γ in NK cells. Purified human NK cells were cultured in the presence of iDCs or mDCs. IFN-γ was measured in the supernatants of the DC/NK cell cocultures by ELISA at 24 hours. mDCs and iDCs alone did not produce detectable amounts of IFN-γ (not shown). The depicted data represent means of triplicates ± SEM in one representative experiment of 5. (B-D) IL-12 is involved in the mDC-mediated NK cell activation. mDCs were pretreated with neutralizing anti-IL-12 or anti-IL-15 mAb prior to coculture (B) or electroporated with siRNA specific for IL-12 p40 or NF-κB p50 subunit (or control siRNA) prior to LPS stimulation and incubated with resting NK cells (at a 1:10 DC/NK cell ratio) in 96-well plates for 24 hours (C). It is noteworthy that accumulation of IL-12 in the supernatant of the 104 mDCs cultured alone or together with 105 NK cells could not be detected by ELISA (not shown). The RNA interference was functional because IL-12 was barely detectable in mDCs pretreated with siRNAp40 or siRNA NF-κBp50 using confocal microscopy and was not produced in the DC/NK cell coculture supernatants at 1:1 DC/NK cell ratios (while produced in the absence of siRNA; not shown). Mouse BM-DCs propagated for 7 days from wild-type or IL-12p35-/- mice were cocultured with wild-type NK cells at 1:2 and 1:10 DC/NK cell ratios for 18 hours. The levels of IFN-γ measured in the mDC, NK, or mDC/NK cell coculture supernatants in the human (B-C) or murine system (D) are shown as means ± SEM of triplicate wells of a representative experiment (of 3).
IL-12 delivery by mDCs is critical for NK cell activation. (A) mDCs promote the secretion of IFN-γ in NK cells. Purified human NK cells were cultured in the presence of iDCs or mDCs. IFN-γ was measured in the supernatants of the DC/NK cell cocultures by ELISA at 24 hours. mDCs and iDCs alone did not produce detectable amounts of IFN-γ (not shown). The depicted data represent means of triplicates ± SEM in one representative experiment of 5. (B-D) IL-12 is involved in the mDC-mediated NK cell activation. mDCs were pretreated with neutralizing anti-IL-12 or anti-IL-15 mAb prior to coculture (B) or electroporated with siRNA specific for IL-12 p40 or NF-κB p50 subunit (or control siRNA) prior to LPS stimulation and incubated with resting NK cells (at a 1:10 DC/NK cell ratio) in 96-well plates for 24 hours (C). It is noteworthy that accumulation of IL-12 in the supernatant of the 104 mDCs cultured alone or together with 105 NK cells could not be detected by ELISA (not shown). The RNA interference was functional because IL-12 was barely detectable in mDCs pretreated with siRNAp40 or siRNA NF-κBp50 using confocal microscopy and was not produced in the DC/NK cell coculture supernatants at 1:1 DC/NK cell ratios (while produced in the absence of siRNA; not shown). Mouse BM-DCs propagated for 7 days from wild-type or IL-12p35-/- mice were cocultured with wild-type NK cells at 1:2 and 1:10 DC/NK cell ratios for 18 hours. The levels of IFN-γ measured in the mDC, NK, or mDC/NK cell coculture supernatants in the human (B-C) or murine system (D) are shown as means ± SEM of triplicate wells of a representative experiment (of 3).
DCs polarize IL-12 toward the DC/NK cell interface
Intracellular IL-12 was readily detected in mature human or mouse DCs. In the absence of resting NK cells, the staining of LPS-matured human DCs using a mAb directed against the heterodimeric IL-12p70 revealed abundant preassembled stores of IL-12 (Figure 2A). Accordingly, up to 40% of mouse BM-DCs contained intracytoplasmic IL-12 (as assessed in flow cytometry after membrane permeabilization; Figure 2B). However, IL-12 was not detectable in the supernatants of DC/NK cell cocultures (at a 1:10 DC/NK cell ratio) using standard immunocapture assays (not shown). Moreover, secretion of IFN-γ was not triggered in NK cells incubated in the lower chamber of a transwell containing a functional DC/NK cell coculture in the upper chamber. Indeed, up to 60% of NK cells cocultured together with LPS-activated DCs without physical separation produced IFN-γ, whereas only 18% of NK cells cocultured at a distant site from DCs coincubated with NK cells released IFN-γ (Figure 2C). This result suggested that functional IL-12 is delivered to NK cells through a tight paracrine mechanism. Therefore, we assessed the temporal and spatial distribution of IL-12p70 under confocal microscopy during the interaction of DCs with resting NK cells. After 25 minutes of contact with NK cells, DCs readily redistributed IL-12 toward the interface area (Figure 2D). Following deconvolution, a 3-dimensional reconstitution of permeabilized DCs revealed a clear reduction of the cytoplasmic IL-12 granules distant from the DC/NK cell interface, yet an increase in the proximity of the DC/NK synapse (Figure 2E); 78% ± 4% of DCs interacting with NK cells relocalized their IL-12 at the cellular junction with NK cells. To assess the specificity of this observation, we investigated the distribution of IL-12p70 during T-cell activation mediated by DC MHC class I molecules. During a coculture of HLA-A2-restricted, Mart-1-specific cytotoxic T-lymphocyte (CTL) clones (LT11) with HLA-A2+ DCs pulsed with Mart-1 peptides (or Flu control peptides), no polarization of IL-12p70 was observed (Figure 2F). Priming of naive T lymphocytes with allogeneic DCs did not promote IL-12 relocalization to the DC/T-cell interface either (data not shown). Furthermore, the homotypic aggregation of LPS-activated DCs did not induce membrane redistribution of prestored IL-12 (data not shown). As a further control of specificity, staining of the DC/NK cell conjugates with an anti-IL-15 mAb revealed no signs of redistribution of IL-15-containing granules (Figure 2G). In conclusion, preassembled stores of IL-12 readily polarized within DCs at the DC/NK cell interface during interaction with resting NK cells.
The delivery of IL-12 is polarized during the DC/NK cell cross-talk. (A-B) Preassembled stores of IL-12 in activated human and mouse DCs. Confocal microscopy study of human MD-DCs stimulated by LPS for 24 hours using anti-IL-12 mAb (A). In mDCs that do not form conjugates with NK cells or mDCs cultured alone staining with anti-IL-12 mAb highlighted a dotted cytosolic and membrane-associated localization of the cytokine. Cytofluorometric analyses of day 7 mouse BM-DCs stained with anti-mouse IL-12p70 mAb or isotype control antibody (blue line; B). (C) Close contacts between DCs and NK cells are required for IL-12 bioactivity on NK cells. Human mDCs and resting NK cells were cocultured together at a 1:10 DC/NK cell ratio in the upper chamber of a transwell containing resting NK cells alone in the lower chamber. Both compartments were harvested separately at 24 hours, incubated 45 minutes with an IFN-γ capture antibody (Miltenyi Biotech), and then stained with anti-CD3 (FITC), anti-CD56 Cychrome (CyC), and anti-IFN-γ (allophycocyanin [APC]) mAbs and examined by flow cytometry. The percentage of IFN-γ staining in the CD3-/CD56+ NK cell gate is depicted for NK cells cultured without mDCs, or together with mDCs separated or not by a transwell membrane, for a representative experiment (performed twice with similar results). (D-G) Selective IL-12 polarization in DCs at the DC/NK cell interface. mDCs were admixed with autologous resting NK cells or CD8+ CTL (LT11) clones. After fixation, cells were permeabilized and stained with anti-IL-12 (D-F) or anti-IL-15 (G) mAb. In panels D, F, and G Normaski (left) and fluorescent (middle) images are shown and in panel D, fluorescence intensity is depicted (right). In mDCs forming tight conjugates with NK cells, IL-12 selectively concentrated at the DCNK-IS interface (D). A deconvoluted 3-dimensional reconstruction of the mDC in close contact with an autologous NK confirmed the mobilization of IL-12 toward the DC/NK synapse (E). A total of 78% ± 4% of DCs interacting with NK cells relocalized their IL-12 to the cellular junction with NK cells However, such an IL-12 polarization toward the intercellular junction was not seen in mDC/LT11 conjugates (F). Lack of polarization of IL-15 at the DC/NK cell surface is shown in panel G.
The delivery of IL-12 is polarized during the DC/NK cell cross-talk. (A-B) Preassembled stores of IL-12 in activated human and mouse DCs. Confocal microscopy study of human MD-DCs stimulated by LPS for 24 hours using anti-IL-12 mAb (A). In mDCs that do not form conjugates with NK cells or mDCs cultured alone staining with anti-IL-12 mAb highlighted a dotted cytosolic and membrane-associated localization of the cytokine. Cytofluorometric analyses of day 7 mouse BM-DCs stained with anti-mouse IL-12p70 mAb or isotype control antibody (blue line; B). (C) Close contacts between DCs and NK cells are required for IL-12 bioactivity on NK cells. Human mDCs and resting NK cells were cocultured together at a 1:10 DC/NK cell ratio in the upper chamber of a transwell containing resting NK cells alone in the lower chamber. Both compartments were harvested separately at 24 hours, incubated 45 minutes with an IFN-γ capture antibody (Miltenyi Biotech), and then stained with anti-CD3 (FITC), anti-CD56 Cychrome (CyC), and anti-IFN-γ (allophycocyanin [APC]) mAbs and examined by flow cytometry. The percentage of IFN-γ staining in the CD3-/CD56+ NK cell gate is depicted for NK cells cultured without mDCs, or together with mDCs separated or not by a transwell membrane, for a representative experiment (performed twice with similar results). (D-G) Selective IL-12 polarization in DCs at the DC/NK cell interface. mDCs were admixed with autologous resting NK cells or CD8+ CTL (LT11) clones. After fixation, cells were permeabilized and stained with anti-IL-12 (D-F) or anti-IL-15 (G) mAb. In panels D, F, and G Normaski (left) and fluorescent (middle) images are shown and in panel D, fluorescence intensity is depicted (right). In mDCs forming tight conjugates with NK cells, IL-12 selectively concentrated at the DCNK-IS interface (D). A deconvoluted 3-dimensional reconstruction of the mDC in close contact with an autologous NK confirmed the mobilization of IL-12 toward the DC/NK synapse (E). A total of 78% ± 4% of DCs interacting with NK cells relocalized their IL-12 to the cellular junction with NK cells However, such an IL-12 polarization toward the intercellular junction was not seen in mDC/LT11 conjugates (F). Lack of polarization of IL-15 at the DC/NK cell surface is shown in panel G.
DC and NK cells form productive conjugates in vitro
To further characterize the DC/NK cell interface, we compared the ability of human iDCs versus LPS-activated iDCs (mDCs) to promote the formation of DC/NK cell conjugates during coculture with autologous resting NK cells. The observation of DC/NK cell cocultures in transmission light microscopy revealed flattened intercellular junctions at the mDC/NK cell interface leading to the formation of stable cellular conjugates (Figure 3A). Up to 30.3% ± 4% of mDCs formed conjugates with a single resting NK cell, whereas only 3.6% ± 2.1% of iDCs could do so (P = .002; Figure 3B). The propagation of productive signals was determined by immunostaining for phosphotyrosine (pTyr). Resting NK cells were incubated with mDCs for 25 minutes before fixation and staining. Almost all mDC/NK cell conjugates (85% ± 5.2%) displayed pTyr accumulation at the DC/NK cell interface (Figure 3C), whereas this event was rarely observed in iDC/NK cell conjugates (10% ± 1.3%, P < .01 between iDC versus mDC cocultures) or in DCs or NK cells cultured alone.
The formation of DC/NK cell conjugates is associated with Ca2+ influx and tyrosine phosphorylation. (A-B) mDCs form conjugates with resting NK cells. iDCs versus LPS-matured DCs (mDCs) were admixed with resting NK cells (at a 1:10 DC/NK cell ratio) and analyzed in transmission light microscopy as described in “Materials and methods.” Representative pictures of various fields are shown in panel A. More than 500 DCs were examined for their capacity to bind to resting NK cells. The percentages of DCs forming conjugates with NK cells in transmission light microscopy are shown as a mean ± SEM of 3 independent experiments (B). (C) The cell-to-cell contacts between mDCs and resting NK cells are associated with intracellular tyrosine phosphorylation. mDCs were admixed with resting NK cells. After fixation, cells were permeabilized and stained with anti-pTyr antibodies. The numbers of conjugates associated with intracellular phosphotyrosines were counted and reported as the total number of conjugates. There was an accumulation of pTyr at the DC/NK cell contact area in 85% ± 5.2% of the mDC/NK cell conjugates and in less than 10% ± 1.3% of the iDC/NK conjugates. Similar confocal images were acquired in 5 independent experiments. A representative picture of a resting NK cell interacting with an mDC (lower cell) at a contact area displaying accumulation of pTyr is shown. A more distant resting NK cell (upper cell) does not exhibit detectable pTyr. (D) Ca2+ influx is triggered in resting NK cells encountering mDCs. mDCs were mixed with resting autologous NK cells labeled with 4 μM Fluo4-AM and observed for 60 minutes by videomicroscopy at 37°C. One picture was acquired every 10 seconds. An increase in the intensity of Fluo-4 fluorescence of at least 50% above the level of resting NK cells was considered significant. Representative pictures of conjugate formation between mDCs and resting NK cells are shown. Intracellular Ca2+ raises (bottom panels) were monitored in NK cells 50 seconds after interaction with mDCs. The fluorescence intensity scale (unit of fluorescence intensity [UFI]) is shown on a gray level scale ranging from 0 to 256. The percentages of iDCs or mDCs (of 200) forming productive interactions leading to significant Ca2+ increases in NK cells are indicated as a mean ± SEM of 2 independent experiments (E, left graph). The mean duration of productive (with Ca2+) and nonproductive (without Ca2+) mDC/NK cell conjugates is reported in the right graph (E).
The formation of DC/NK cell conjugates is associated with Ca2+ influx and tyrosine phosphorylation. (A-B) mDCs form conjugates with resting NK cells. iDCs versus LPS-matured DCs (mDCs) were admixed with resting NK cells (at a 1:10 DC/NK cell ratio) and analyzed in transmission light microscopy as described in “Materials and methods.” Representative pictures of various fields are shown in panel A. More than 500 DCs were examined for their capacity to bind to resting NK cells. The percentages of DCs forming conjugates with NK cells in transmission light microscopy are shown as a mean ± SEM of 3 independent experiments (B). (C) The cell-to-cell contacts between mDCs and resting NK cells are associated with intracellular tyrosine phosphorylation. mDCs were admixed with resting NK cells. After fixation, cells were permeabilized and stained with anti-pTyr antibodies. The numbers of conjugates associated with intracellular phosphotyrosines were counted and reported as the total number of conjugates. There was an accumulation of pTyr at the DC/NK cell contact area in 85% ± 5.2% of the mDC/NK cell conjugates and in less than 10% ± 1.3% of the iDC/NK conjugates. Similar confocal images were acquired in 5 independent experiments. A representative picture of a resting NK cell interacting with an mDC (lower cell) at a contact area displaying accumulation of pTyr is shown. A more distant resting NK cell (upper cell) does not exhibit detectable pTyr. (D) Ca2+ influx is triggered in resting NK cells encountering mDCs. mDCs were mixed with resting autologous NK cells labeled with 4 μM Fluo4-AM and observed for 60 minutes by videomicroscopy at 37°C. One picture was acquired every 10 seconds. An increase in the intensity of Fluo-4 fluorescence of at least 50% above the level of resting NK cells was considered significant. Representative pictures of conjugate formation between mDCs and resting NK cells are shown. Intracellular Ca2+ raises (bottom panels) were monitored in NK cells 50 seconds after interaction with mDCs. The fluorescence intensity scale (unit of fluorescence intensity [UFI]) is shown on a gray level scale ranging from 0 to 256. The percentages of iDCs or mDCs (of 200) forming productive interactions leading to significant Ca2+ increases in NK cells are indicated as a mean ± SEM of 2 independent experiments (E, left graph). The mean duration of productive (with Ca2+) and nonproductive (without Ca2+) mDC/NK cell conjugates is reported in the right graph (E).
NK cells were also loaded with the ratiometric Ca2+ indicator Fura-4 AM prior to coculture with mDCs or iDCs. Cell-to-cell contacts were monitored for 1 hour at 37°C and image recording was performed on living cells every 10 seconds by confocal microscopy. In Figure 3D, a single mature DC was seen in the center of the frame, interacting with an NK cell and triggering a Ca2+ response. Six minutes after initiation of coculture, a tight conjugate between the cells occurred (0 seconds). Within 50 seconds after initial contact with mDCs, a raise in intracellular Ca2+ concentration was observed in the NK cell (Figure 3D, colored panels). Then, within minutes, the NK cell flattened against the mDC, thereby increasing the contact area of the interface. The overall percentage of “productive” DC/NK cell conjugates leading to Ca2+ mobilization in NK cells was 74% ± 4.7% for mDCs and less than 3% ± 2% for iDCs (P < .01; Figure 3E left graph). Moreover, the mean duration of the mDC/NK cell conjugates associated with a calcium response was 1836 seconds (210-3350 seconds) versus 128 seconds (40-360 seconds) in the nonproductive mDC/NK cell conjugates (ie, without a calcium response; Figure 3E right graph).
Altogether, these data indicate that DAK activity was associated with a prolonged mDC/NK cell interaction leading to Ca2+ influx in NK cells and pTyr accumulation at the intercellular junction.
DC/NK cell conjugates are functional synapses
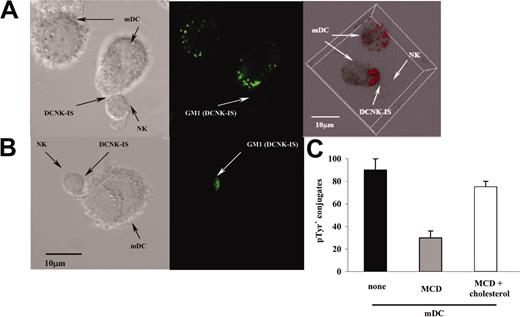
To investigate whether conjugates between mDCs and NK cells were active immunologic synapses, we analyzed lipid rafts and cytoskeleton rearrangements. Lipid rafts are cholesterol and sphingolipid-rich membrane subdomains sequestering costimulatory and adaptor molecules required to mediate signal transduction.28-30 The coalescence of rafts on the edges of the mDC/NK cell interface was investigated using FITC-labeled cholera toxin that binds GM1 gangliosides. Cholera toxin-labeled DCs or NK cells were cocultured with unlabeled NK cells (Figure 4A) or DCs (Figure 4B), respectively, for 25 minutes, fixed, and analyzed by confocal microscopy. Lipid rafts coalesced intensively at both edges of the intercellular junction (Figure 4A-B). A 3-dimensional reconstitution of mDCs revealed the redistribution of the FITC-labeled cholera toxin toward the DC/NK cell interface, whereas an evenly sparse membrane staining was observed in unconjugated mDCs (Figure 4A, right panel). Next, we pretreated mDCs with MCD to deplete cholesterol from their membranes, a manipulation that precludes the formation of membrane rafts. Whereas 85% ± 3.5% of mDCs were capable of promoting NK cell intracellular tyrosine phosphorylation, only 30% ± 2.4% of the MCD-treated mDCs induced productive conjugates with NK cells (P < .01; Figure 4C). Of note, addition of exogenous cholesterol to MCD-exposed mDCs restored their capacity to elicit phosphotyrosylation in NK cells (Figure 4C).
Lipid rafts polarized in DC and NK cells during the DC/NK cell interaction. (A-B) Confocal microscopy on mDC/NK cell cocultures stained with cholera toxin. Lipid rafts (GM1) of the mDCs (A) or of the resting NK cells (B) were stained using FITC-labeled cholera toxin. The lipid rafts of mDCs appeared as small fluorescent patches scattered on the plasma membrane in the absence of contacts (A, top left corner). In contrast, mDC lipid rafts became clustered at the mDC/NK cell interface after conjugate formation (A; DCNK-IS). A 3-dimensional reconstitution confirmed the membrane distribution of lipid rafts in the single mDC, whereas a skewed polarization was observed following DCNK-IS formation (A, right panel). The mean percentages of conjugates polarizing rafts from the DCs were 85% ± 6% in 3 independent experiments. A similar observation was made in labeled NK cells admixed with mDCs where a polarization of FITC-labeled cholera toxin was seen at the NK cell interface with mDCs. (C) Inhibitors of lipid raft mobilization prevent accumulation of phosphotyrosines at the DCNK cell interface. mDCs were pretreated with the indicated combination of MCD or cholesterol (or both) and then cocultured with NK cells (at a DC/NK cell ratio of 1:10) on slides coated with poly-L-lysine for 25 minutes, fixed, permeabilized, and stained. Results are expressed as the percentage ± SEM of conjugates associated with intracellular phosphotyrosine clustering at the DCNK-IS. One representative experiment of 3 is shown.
Lipid rafts polarized in DC and NK cells during the DC/NK cell interaction. (A-B) Confocal microscopy on mDC/NK cell cocultures stained with cholera toxin. Lipid rafts (GM1) of the mDCs (A) or of the resting NK cells (B) were stained using FITC-labeled cholera toxin. The lipid rafts of mDCs appeared as small fluorescent patches scattered on the plasma membrane in the absence of contacts (A, top left corner). In contrast, mDC lipid rafts became clustered at the mDC/NK cell interface after conjugate formation (A; DCNK-IS). A 3-dimensional reconstitution confirmed the membrane distribution of lipid rafts in the single mDC, whereas a skewed polarization was observed following DCNK-IS formation (A, right panel). The mean percentages of conjugates polarizing rafts from the DCs were 85% ± 6% in 3 independent experiments. A similar observation was made in labeled NK cells admixed with mDCs where a polarization of FITC-labeled cholera toxin was seen at the NK cell interface with mDCs. (C) Inhibitors of lipid raft mobilization prevent accumulation of phosphotyrosines at the DCNK cell interface. mDCs were pretreated with the indicated combination of MCD or cholesterol (or both) and then cocultured with NK cells (at a DC/NK cell ratio of 1:10) on slides coated with poly-L-lysine for 25 minutes, fixed, permeabilized, and stained. Results are expressed as the percentage ± SEM of conjugates associated with intracellular phosphotyrosine clustering at the DCNK-IS. One representative experiment of 3 is shown.
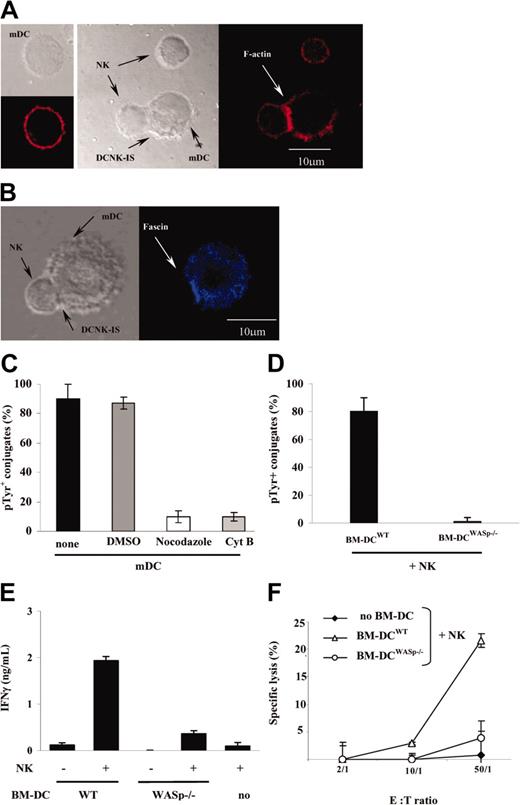
Next, the cytoskeleton remodeling of mDCs forming conjugates with resting NK cells was studied. mDC/NK cell cocultures (25 minutes) were fixed, permeabilized, and stained with a phalloidin-based probe specific for F-actin. Images of mDC/NK cell conjugates were captured by confocal laser scanning microscopy. The mDC or NK cell cytoskeleton was scored as polarized if the intensity of F-actin was at least 2-fold brighter at the DC/NK cell interface (Figure 5A, DCNK-IS) than in the rest of the cell or in noninteracting cells (Figure 5A left panel for mDCs; upper cell in the second panel for NK cells). Using 3-dimensional imaging (x-z plane), we visualized a synaptic increase of F-actin in both interacting partners (not shown). Up to 89% ± 5% of mDC/NK cell conjugates relocalized F-actin toward the synapse. To specifically assess cytoskeleton remodeling in mDCs, we investigated the distribution of the actin-bundling protein fascin,31,32 a protein expressed in mDCs (but not in NK cells). Fascin clustered at the mDC/NK cell interface (Figure 5B), supporting the involvement of the DC cytoskeleton in synapse formation with NK cells. Accordingly, pretreatment of mDCs with cytochalasin B reduced the number of mDC/NK cell conjugates (not shown) and prevented active clustering of pTyr at the DC/NK cell interface by 80% ± 3% (P < .05; Figure 5C). Similar results were obtained using cytochalasin D (not shown). To further assess the relevance of cytoskeletal remodeling for DAK activity, we used the microtubular depolymerizing agents nocodazole (Figure 5C) or demecolcine (not shown) before measuring accumulation of pTyr at the mDC/NK cell interface. Preincubation of DCs with either of the 2 inhibitors (but not their solvent DMSO alone) prevented pTyr accumulation at the DC/NK cell interface.
Cytoskeleton remodeling in DCs is critical for synapse formation and NK cell activation. (A) F-actin relocalized at the mDC/NK cell interface. Phalloidin-PE was used to stain the F-actin cytoskeleton of either mDCs or resting NK cells, cultured alone or together (25 minutes). Whereas phalloidin staining was uniform on cell membranes of mDCs (left panel) or resting NK cells alone (upper cell of the middle panel), it clustered at the mDC/NK cell interface in cocultures (as indicated with an arrow at the DCNK-IS). (B) Fascin relocalized at the mDC/NK cell interface. Up to 89% ± 5% of mDC/NK cell conjugates observed after a 25-minute coculture relocalized fascin toward the synapse. (C) Inhibitors of cytoskeleton remodeling impaired accumulation of phosphotyrosines at the DCNK-IS. Prior to coculture with NK cells, mDCs were pretreated with cytochalasin B (CytB) or with nocodazole. The percentages ± SEM of mDC/NK cell conjugates associated with pTyr clustering at the DCNK-IS is reported. DMSO used to solubilize nocodazole and CytB was also used as a negative control. The mean percentage ± SEM of 3 independent experiments is depicted. (D-F) DCs from WASp-mutant mice failed to promote productive DC/NK cell conjugates leading to NK cell activation. Mouse WASp-/- or wild-type BM-DCs were cocultured for 25 minutes with wild-type mouse NK cells on a slide coated with poly-L-lysine (D) or for 18 hours in 96-well plates (E-F). The percentages of conjugates associated with clustering of phosphotyrosines at the BM-DC/NK cell interface are reported (D). Confocal microscopy confirmed that WASp-/- BM-DCs failed to form conjugates with NK cells (not shown). The levels of IFN-γ released in the coculture supernatants were measured using ELISA (E) and YAC-1 target cell lysis was determined by a chromium release assay (F). One representative experiment of 3 is depicted. E:T indicates effector-target ratio.
Cytoskeleton remodeling in DCs is critical for synapse formation and NK cell activation. (A) F-actin relocalized at the mDC/NK cell interface. Phalloidin-PE was used to stain the F-actin cytoskeleton of either mDCs or resting NK cells, cultured alone or together (25 minutes). Whereas phalloidin staining was uniform on cell membranes of mDCs (left panel) or resting NK cells alone (upper cell of the middle panel), it clustered at the mDC/NK cell interface in cocultures (as indicated with an arrow at the DCNK-IS). (B) Fascin relocalized at the mDC/NK cell interface. Up to 89% ± 5% of mDC/NK cell conjugates observed after a 25-minute coculture relocalized fascin toward the synapse. (C) Inhibitors of cytoskeleton remodeling impaired accumulation of phosphotyrosines at the DCNK-IS. Prior to coculture with NK cells, mDCs were pretreated with cytochalasin B (CytB) or with nocodazole. The percentages ± SEM of mDC/NK cell conjugates associated with pTyr clustering at the DCNK-IS is reported. DMSO used to solubilize nocodazole and CytB was also used as a negative control. The mean percentage ± SEM of 3 independent experiments is depicted. (D-F) DCs from WASp-mutant mice failed to promote productive DC/NK cell conjugates leading to NK cell activation. Mouse WASp-/- or wild-type BM-DCs were cocultured for 25 minutes with wild-type mouse NK cells on a slide coated with poly-L-lysine (D) or for 18 hours in 96-well plates (E-F). The percentages of conjugates associated with clustering of phosphotyrosines at the BM-DC/NK cell interface are reported (D). Confocal microscopy confirmed that WASp-/- BM-DCs failed to form conjugates with NK cells (not shown). The levels of IFN-γ released in the coculture supernatants were measured using ELISA (E) and YAC-1 target cell lysis was determined by a chromium release assay (F). One representative experiment of 3 is depicted. E:T indicates effector-target ratio.
To further substantiate the role of dynamic changes in the DC cytoskeleton for the DCNK-IS and for DAK activity, we analyzed the impact of a mutated WASp on DC function. WASp-/- BM-DCs33 exhibited a cell surface phenotype and allostimulatory capacities comparable to that of wild-type control BM-DCs (not shown). However, as compared to isogenic controls, mature WASp-/- BM-DCs were deficient in promoting NK cell pTyr recruitment following conjugate formation (Figure 5D), in inducing NK cell IFN-γ production (Figure 5E), and in enhancing NK cell killing of YAC-1 targets (Figure 5F). Altogether, these data imply that the formation of functional DC/NK cell synapses require the reorganization of the DC cytoskeleton as well as raft mobilization.
Redistribution of adhesion molecules to or from the DCNK-IS
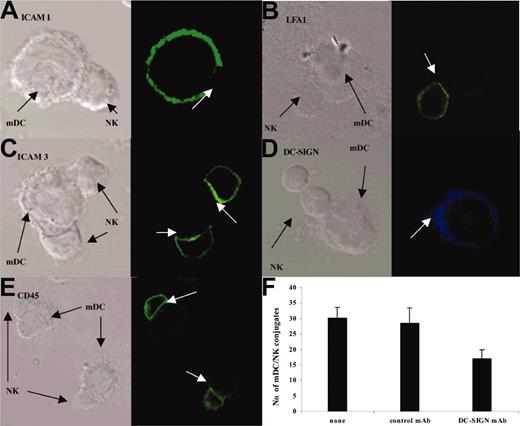
Cell-to-cell recognition often requires the formation of a highly organized pattern of molecules in the intercellular synaptic junction. Intercellular adhesion molecule 1 (ICAM-1; expressed on mDCs; Figure 6A) were excluded from 61% ± 6% of mDC/NK conjugates. In contrast, leukocyte function-associated molecule 1 (LFA-1) molecules accumulated in 74% ± 7% of mDC/NK conjugates (Figure 6B). In half of these conjugates, LFA-1 preferentially located at the banks of the interface area (not shown). ICAM-3 was expressed on NK cells and was redistributed in 86% ± 4% of mDC/NK conjugates (Figure 6C). DC-SIGN also concentrated in the mDC/NK cell junction (Figure 6D). DC-SIGN/ICAM-3 interactions might play a particular role at the early steps of mDC/NK conjugate formation because blocking DC-SIGN using neutralizing antibodies (Figure 6F) or mannan (not shown) decreased the number of conjugates (30% versus 17%; P = .037). We also investigated the distribution of CD45 molecules, which has been reported to concentrate in the pSMAC of the T/antigen-presenting cell (APC) interface and to be excluded from inhibitory but not from activating NK cell/target cell IS.34 In the DCNK-IS, CD45 staining was highly expressed on NK cells with no privileged concentration toward the synapse (Figure 6E). In conclusion, DCNK-IS formation is an active process associated with redistribution of adhesion molecules.
Redistribution of adhesion molecules in the DCNK-IS. mDC and NK cell cocultures were stained with either anti-ICAM-1 (A), anti-LFA-1 (B), anti-ICAM-3 (C), anti-DC-SIGN (D), or anti-CD45 mAb (E). Confocal microscopy analyses at 25 minutes highlighted a differential expression of these molecules in DCNK-IS with an exclusion of the ICAM-1 on mDCs and an enrichment of LFA-1 and ICAM-3 on NK cells. DC-SIGN molecules relocate from the mDC cell surface toward the mDC/NK cell synapse (D). CD45 was expressed on the whole NK cell membrane and was not specifically redistributed in the DCNK-IS (E). (A-E) White arrows depict DCNK-IS. (F) Blocking DC-SIGN using neutralizing DC-SIGN mAb decreased the early steps of mDC/NK cell conjugate formation (30% versus 17%; P = .037). Error bars represent SEM.
Redistribution of adhesion molecules in the DCNK-IS. mDC and NK cell cocultures were stained with either anti-ICAM-1 (A), anti-LFA-1 (B), anti-ICAM-3 (C), anti-DC-SIGN (D), or anti-CD45 mAb (E). Confocal microscopy analyses at 25 minutes highlighted a differential expression of these molecules in DCNK-IS with an exclusion of the ICAM-1 on mDCs and an enrichment of LFA-1 and ICAM-3 on NK cells. DC-SIGN molecules relocate from the mDC cell surface toward the mDC/NK cell synapse (D). CD45 was expressed on the whole NK cell membrane and was not specifically redistributed in the DCNK-IS (E). (A-E) White arrows depict DCNK-IS. (F) Blocking DC-SIGN using neutralizing DC-SIGN mAb decreased the early steps of mDC/NK cell conjugate formation (30% versus 17%; P = .037). Error bars represent SEM.
IL-12 polarization in DCs depends on the mDC/NK cell synapse formation
To address the role of synapse formation in IL-12 polarization, we assessed the effects of actin polymerization and raft inhibitors on IL-12 relocalization toward the DC/NK cell interface in the cocultures. Such a polarization of preassembled cytoplasmic stores of IL-12 toward the DCNK-IS area was observed in 78% ± 3.7% of untreated mDC/NK cell conjugates (Figure 7). However, polarization was significantly reduced when mDCs were pretreated with microtubule depolymerizing agents (nocodazole), inhibitors of F-actin polymerization (cytochalasin B [cytB]), or agents that disrupt lipid rafts (MCD), when compared to untreated (Figure 7) or solvent-only-treated (DMSO) controls (data not shown). In line with this observation, WASP-/- BM-DCs, which produced IL-12 at a comparable level to that of their wild-type counterparts, did not polarize IL-12 nor form synapses with wild-type NK cells (not shown). In conclusion, delivery of bioactive IL-12 in DCs toward resting NK cells relies on the active formation of the DCNK-IS.
IL-12 polarization to DCNK-IS depends on cytoskeleton and raft mobilization. mDCs were pretreated with inhibitors of cytoskeleton remodeling (nocodazole, CytB) or of raft mobilization (MCD) prior to coculture with NK cells. The percentages of mDC/NK cell conjugates exhibiting IL-12 relocalization to the synapse in confocal microscopy are reported. The mean percentages ± SEM of 3 independent experiments are shown.
IL-12 polarization to DCNK-IS depends on cytoskeleton and raft mobilization. mDCs were pretreated with inhibitors of cytoskeleton remodeling (nocodazole, CytB) or of raft mobilization (MCD) prior to coculture with NK cells. The percentages of mDC/NK cell conjugates exhibiting IL-12 relocalization to the synapse in confocal microscopy are reported. The mean percentages ± SEM of 3 independent experiments are shown.
Discussion
DCs and NK cells are critical players at the interface between the innate and cognate immune responses. However, the molecular mechanisms underlying the DC-mediated NK cell activation have been largely elusive. Most of the previous reports alluded to the contribution of cell-to-cell contacts without excluding a role for IL-12.16-21,23,24 The present study, which addressed the spatiotemporal features of the mDC/NK cell dialogue, may reconcile the conflicting results on the role of IL-12 in the DC/NK cell cross-talk. Our data outline the following scenario. First, the interaction between mDCs and NK cells leads to the formation of conjugates that fulfill the criteria of bioactive synapses (DCNK-IS) in that they involve cytoskeleton remodeling and raft mobilization. Cytoskeleton remodeling and raft mobilization in DCs are indeed required for the subsequent elicitation of signals on the side of the NK cells (calcium influx and accumulation of phosphotyrosines at the synapse). Moreover, our data suggest that the synaptic polarization of prestored IL-12 supports the activation of NK cells by mDCs.
Here we provide evidence that cytoskeleton reorganization on the DC side is critical for DCNK-IS formation and NK cell triggering. Productive conjugate formation did not occur when DCs were immature or when mDCs were affected by microtubule depolymerizing agents, by the actin cytoskeleton inhibitor cytochalasin B (or D), or by mutations impairing F-actin polymerization (WASp-/-). Similar data have been reported for the DC/T-cell but not B/T-cell interface.35,36 The cytoskeleton may be involved in the stabilization of DC/NK cell conjugates, in line with the observation that productive conjugates have a mean duration time of 30 minutes compared with 5 minutes for nonproductive conjugates (P < .05). Moreover, fascin, the actin-bundling protein regulating the interaction between the cytoskeleton and the cell membrane in response to extracellular signals,31 also contributes to the DCNK-IS organization. The differential expression of fascin in mDCs (and not in iDCs) might account for the selective capacity of mDCs to promote synapse formation leading to NK cell activation. Similarly, Al-Alwan et al also reported that fascin is required for optimal antigen presentation by mDCs to T cells.31
Lipid rafts are polarized in the cytolytic NK cell-target cell IS and are required for NK-mediated target cell lysis.8,11 Here, we show that lipid rafts are polarized in the mDC/NK cell synapses and play a critical role in the mobilization of IL-12 to the DC/NK cell interface (Figure 7). Other molecular rearrangements, such as the redistribution of key adhesion molecules, accompany DCNK-IS formation. ICAM-1 is excluded from the center of the synapse, whereas the NK cell ICAM-3 is selectively relocalized at the DCNK-IS in the conjugates observed at 25 minutes. ICAM-3 has been described to play a pivotal role in T-cell-APC conjugate formation before antigen recognition in early cytoskeleton remodeling and intracellular signaling.37 The clustering of ICAM-3 in the contact zone influenced the T-cell receptor (TCR)-mediated T-cell activation.37 In addition, ICAM-3 transduced early signals leading to the reorientation of the microtubule-organizing center (MTOC) toward the activating interface.37 Indeed, in the DCNK-IS, LFA-1 and DC-SIGN relocalized to the synapse on the DC side. The precise role of DC-SIGN/ICAM-3 interaction in the kinetics of synapse formation and NK cell effector functions remains to be elucidated. Intriguingly, neutralization of DC-SIGN decreased the number of conjugates in short-term cocultures (Figure 6F) and reduced the synapse-phosphotyrosylation in short-term cocultures (15-25 minutes), suggesting that DC-SIGN increases the affinity of the mDC/NK interaction or participates in the initiation or stabilization of DCNK-IS or both. However, the number of productive conjugates with phosphotyrosine recruitment was not affected by a DC-SIGN-neutralizing mAb in cocultures observed at 60 minutes (not shown).
The DCNK-IS formation dictated IL-12 polarization in DCs. Our data support the idea that the synapse-dependent IL-12 delivery by DCs promotes NK cell IFN-γ production. IL-12 has been first described as the natural killer stimulatory factor (NKSF) capable of triggering NK cell IFN-γ production.38 IL-12 enhances the generation of lymphokine-activated killer (LAK) cells and augments the cytotoxic activity of NK cells, in part by up-regulating perforin, granzymes, and adhesion molecules.39 DCs and phagocytes produce IL-12 in response to infectious pathogens. IL-12 is also produced by DCs in contact with LPS and resting NK cells,17 and IL-12 has been reported to be critical for the mDC-mediated NK cell triggering in vitro.23 Moreover, IL-12 (along with IL-18) has been involved in the DC-mediated NK cell activation preventing the replication of murine cytomegalovirus (MCMV).21 In our experimental setting, where a few DCs were used to promote NK cell activation (1:10 DC/NK cell ratio), IL-12 was not produced at detectable levels during the mDC/NK cell coculture. However, IL-12 was biologically active in the mDC/NK cell cross-talk because blockade of IL-12 by several methods (antibody-mediated neutralization, siRNA, knock-out) abrogated NK cell IFN-γ production. We reconcile this apparent discrepancy by showing that the DC-preassembled stores of IL-12 readily (by 25 minutes) polarize toward the mDC/NK cell interface, in a cytoskeleton- and raft-dependent manner. It has been the prevailing paradigm that IL-12 production by APCs would be transcriptionally regulated.40-42 However, bioactive IL-12p70, available for rapid release after contact with intracellular parasites, has been described.43 This readily mobilizable source of IL-12 is independent of the transcription of IL-12p40. Such a release of preformed stores of IL-12 depends on the contact between DCs43 or neutrophils44 and parasites and is independent of phagocytosis. The rapid release of IL-12 from myeloid cells may contribute to amplify the DC response or to activate NK cells in the immediate vicinity as a first line of defense.
A putative DC/NK cell cross-talk has been evoked in early reports demonstrating that DCs initiate the early defense against viruses and tumors.16,21 The question remains where this interaction could occur. In allergen-induced atopic dermatitis, NK cells have been found in close interaction with DCs in the inflamed dermis.45 The repertoire of chemokine receptors expressed on circulating CD56dimCD16+ blood NK cells suggests that they could efficiently home to sites of peripheral inflammation.46 Recruited NK cells could either kill iDCs or trigger their maturation or polarization, thereby shaping the adaptive immunity.47 Alternatively, the DC/NK cell cross-talk could occur in secondary lymphoid organs where the CD56brightCD16- NK cells have been found in abundance. These cells could act as regulatory cells by virtue of their ability to secrete cytokines (such as IFN-γ, tumor necrosis factor β, GM-CSF) in response to DCs or, perhaps, as immature effector cells prone to acquire cytolytic functions at the early phases of adaptive immune responses.48 However, the precise dynamics of the DCNK-IS in vivo as well as the initial events leading to DCNK-IS formation for synaptic-dependent IL-12 mobilization remain to be determined.
Prepublished online as Blood First Edition Paper, July 8, 2004; DOI 10.1182/blood-2004-01-0380.
Supported by grants from LNC (“Equipe labelisée”) and INSERM (L.Z.). C.B. received a fellowship from the Association de la Recherche contre le Cancer.
A. Caignard and L.Z. contributed equally to this work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr A. Trautmann for careful reading of the manuscript and Pr D. Olive for helpful discussions.

![Figure 2. The delivery of IL-12 is polarized during the DC/NK cell cross-talk. (A-B) Preassembled stores of IL-12 in activated human and mouse DCs. Confocal microscopy study of human MD-DCs stimulated by LPS for 24 hours using anti-IL-12 mAb (A). In mDCs that do not form conjugates with NK cells or mDCs cultured alone staining with anti-IL-12 mAb highlighted a dotted cytosolic and membrane-associated localization of the cytokine. Cytofluorometric analyses of day 7 mouse BM-DCs stained with anti-mouse IL-12p70 mAb or isotype control antibody (blue line; B). (C) Close contacts between DCs and NK cells are required for IL-12 bioactivity on NK cells. Human mDCs and resting NK cells were cocultured together at a 1:10 DC/NK cell ratio in the upper chamber of a transwell containing resting NK cells alone in the lower chamber. Both compartments were harvested separately at 24 hours, incubated 45 minutes with an IFN-γ capture antibody (Miltenyi Biotech), and then stained with anti-CD3 (FITC), anti-CD56 Cychrome (CyC), and anti-IFN-γ (allophycocyanin [APC]) mAbs and examined by flow cytometry. The percentage of IFN-γ staining in the CD3-/CD56+ NK cell gate is depicted for NK cells cultured without mDCs, or together with mDCs separated or not by a transwell membrane, for a representative experiment (performed twice with similar results). (D-G) Selective IL-12 polarization in DCs at the DC/NK cell interface. mDCs were admixed with autologous resting NK cells or CD8+ CTL (LT11) clones. After fixation, cells were permeabilized and stained with anti-IL-12 (D-F) or anti-IL-15 (G) mAb. In panels D, F, and G Normaski (left) and fluorescent (middle) images are shown and in panel D, fluorescence intensity is depicted (right). In mDCs forming tight conjugates with NK cells, IL-12 selectively concentrated at the DCNK-IS interface (D). A deconvoluted 3-dimensional reconstruction of the mDC in close contact with an autologous NK confirmed the mobilization of IL-12 toward the DC/NK synapse (E). A total of 78% ± 4% of DCs interacting with NK cells relocalized their IL-12 to the cellular junction with NK cells However, such an IL-12 polarization toward the intercellular junction was not seen in mDC/LT11 conjugates (F). Lack of polarization of IL-15 at the DC/NK cell surface is shown in panel G.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-01-0380/6/m_zh80220469130002.jpeg?Expires=1766426850&Signature=NyYqhf7pD3djBp8dTKGc45irFCuyCgb0Lc5JTPkYJNbXhPpvoqtGhi4BZihrREjdc7Il3OdKDGdjmEJ~mZ0td0dFmk49yxVyhNR8vFlCxZyOX~vmakzszleh7oDDvOetBNu9aN3Up1yFdcroB~85-iCXTbiIn1XQ3fYXGEqR89Cu79-E9JSCy11zaYl3SD96--5w~r67h2XvWVPyeYHL1JVun7~GZ-rPc52~j28a3sNsMDHPIQgi45c~Nh5oqMIOjveQUWs1pN53uLBJupRHpkxfdYGVqXvf2Tx6gLbYXKlUZDvQ1-UbVCjL5DQyFcBUSmFj0Jcj7XOvhbQ6dQ9fCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. The formation of DC/NK cell conjugates is associated with Ca2+ influx and tyrosine phosphorylation. (A-B) mDCs form conjugates with resting NK cells. iDCs versus LPS-matured DCs (mDCs) were admixed with resting NK cells (at a 1:10 DC/NK cell ratio) and analyzed in transmission light microscopy as described in “Materials and methods.” Representative pictures of various fields are shown in panel A. More than 500 DCs were examined for their capacity to bind to resting NK cells. The percentages of DCs forming conjugates with NK cells in transmission light microscopy are shown as a mean ± SEM of 3 independent experiments (B). (C) The cell-to-cell contacts between mDCs and resting NK cells are associated with intracellular tyrosine phosphorylation. mDCs were admixed with resting NK cells. After fixation, cells were permeabilized and stained with anti-pTyr antibodies. The numbers of conjugates associated with intracellular phosphotyrosines were counted and reported as the total number of conjugates. There was an accumulation of pTyr at the DC/NK cell contact area in 85% ± 5.2% of the mDC/NK cell conjugates and in less than 10% ± 1.3% of the iDC/NK conjugates. Similar confocal images were acquired in 5 independent experiments. A representative picture of a resting NK cell interacting with an mDC (lower cell) at a contact area displaying accumulation of pTyr is shown. A more distant resting NK cell (upper cell) does not exhibit detectable pTyr. (D) Ca2+ influx is triggered in resting NK cells encountering mDCs. mDCs were mixed with resting autologous NK cells labeled with 4 μM Fluo4-AM and observed for 60 minutes by videomicroscopy at 37°C. One picture was acquired every 10 seconds. An increase in the intensity of Fluo-4 fluorescence of at least 50% above the level of resting NK cells was considered significant. Representative pictures of conjugate formation between mDCs and resting NK cells are shown. Intracellular Ca2+ raises (bottom panels) were monitored in NK cells 50 seconds after interaction with mDCs. The fluorescence intensity scale (unit of fluorescence intensity [UFI]) is shown on a gray level scale ranging from 0 to 256. The percentages of iDCs or mDCs (of 200) forming productive interactions leading to significant Ca2+ increases in NK cells are indicated as a mean ± SEM of 2 independent experiments (E, left graph). The mean duration of productive (with Ca2+) and nonproductive (without Ca2+) mDC/NK cell conjugates is reported in the right graph (E).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/10/10.1182_blood-2004-01-0380/6/m_zh80220469130003.jpeg?Expires=1766426850&Signature=NklzIgA~Fz~qdoyl5I4kges-qLKLCFBlaxClPwkybxX5fGt86LJHYvcwbjLsTSGeMrq11NDXIik2nTybTwumwRkax9U~eZlTy3taKGD6oO8rIvUD3IywRe7a8LXCXcqiIGMQkb9BqwUTErEaABaBHgZHWIQ9GsqDgFBNWIaF82k1ecfds15dvbDmqSvqQvTCMKgtyuffMlzp98kV8AZk5uCzRTkNla6ac7SxH4g1DXPXE50MyKZE~QddD8xU5SAbOhZCyHjP5LD01Y2-HIjsLdo8Uz6Lm4p0mzdrQE8lyNoAx0SRhFZdVNhib8ctPDA1qPuqJ017JrmB8ukKeB8~ww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)