Abstract
Interferon-γ (IFN-γ) production and cytolytic activity are 2 major biologic functions of natural killer (NK) cells that are important for innate immunity. We demonstrate here that these functions are compromised in human NK cells treated with peroxisome proliferator-activated-γ (PPAR-γ) ligands via both PPAR-γ-dependent and -independent pathways due to variation in PPAR-γ expression. In PPAR-γ-null NK cells, 15-deoxy-Δ12,14 prostaglandin J2 (15d-PGJ2), a natural PPAR-γ ligand, reduces IFN-γ production that can be reversed by MG132 and/or chloroquine, and it inhibits cytolytic activity of NK cells through reduction of both conjugate formation and CD69 expression. In PPARγ-positive NK cells, PPAR-γ activation by 15d-PGJ2 and ciglitazone (a synthetic ligand) leads to reduction in both mRNA and protein levels of IFN-γ. Overexpression of PPAR-γ in PPAR-γ-null NK cells reduces IFN-γ gene expression. However, PPAR-γ expression and activation has no effect on NK cell cytolytic activity. In addition, 15d-PGJ2 but not ciglitazone reduces expression of CD69 in human NK cells, whereas CD44 expression is not affected. These results reveal novel pathways regulating NK cell biologic functions and provide a basis for the design of therapeutic agents that can regulate the function of NK cells within the innate immune response. (Blood. 2004;104:3276-3284)
Introduction
Natural killer (NK) cells are bone marrow-derived cytotoxic lymphocytes with a granular morphology and account for up to 15% of peripheral blood lymphocytes.1,2 They are important cellular components of innate immune defense against infection and immune surveillance against malignancies and transplanted organs.3,4 NK cells are also implicated in the regulation of hematopoiesis and adaptive immune responses.2,5 The biologic activity of NK cells includes not only cytokine production (eg, interferon-γ [IFN-γ]) to regulate the type 1 T-cell immune response but also cytolytic activity, because NK cells can destroy target cells prior to sensitization and without restriction by major histocompatibility complex (MHC) antigens (natural killing activity). Imbalance of NK biologic functions has been implicated in the pathogenesis of autoimmune diseases such as diabetes.6 Removal of NK cells abrogates cyclophosphamide-induced diabetes in nonobese diabetic (NOD) mice and prevents streptozotocin-induced diabetes in CD-1 mice.7,8 Furthermore, IFN-γ promotes the penetration of diabetogenic cells into pancreatic islets, and depletion of IFN-γ delays the development of autoimmune diabetes in NOD mice.9,10
Whereas the role of NK cells in pathogenesis has been extensively studied, the mechanisms involved in the regulation of NK functions remain to be defined. Many agents like cytokines, prostaglandins, and drugs regulate NK cell function. Interleukin-2 (IL-2) enhances intrinsic killing activity of NK cells and also induces a rapid expression of IFN-γ.11 In contrast, interleukin-4 (IL-4) inhibits IFN-γ production by human NK cells and regulates growth factor-dependent proliferation and differentiation of these cells.12-14 Additionally, prostaglandins have been shown to inhibit NK cell cytolytic activity.15-17
Peroxisome proliferator-activated-γ (PPAR-γ) is a member of the nuclear receptor superfamily of ligand-dependent transcription factors, and its main function was originally thought to primarily regulate adipocyte differentiation and glucose homeostasis.18 While the expression of PPAR-γ is found mainly in adipocytes and is inducible by adipocyte differentiation agents, several recent studies demonstrate that PPAR-γ can be dramatically induced in monocytes/macrophages and T cells by IL-4.19-22 Several naturally occurring polyunsaturated fatty acids and their metabolites have been suggested as ligands for PPAR-γ.23,24 One of the most widely studied natural ligands with high affinity is 15-deoxy-Δ12,14 prostaglandin J2 (15d-PGJ2),25-28 although its role in adipocyte differentiation in vivo has recently been challenged.28,29 15d-PGJ2 is a dehydration product of prostaglandin D2 (PGD2), and its level fluctuates during the resolution phase of inflammation, implicating its role in anti-inflammation.30,31 15d-PGJ2 regulates immune function in activated monocytes/macrophages, T cells, B cells, and dendritic cells either in the absence of PPAR-γ or as a ligand for PPAR-γ.19,22,23,32-35 Importantly, the thiazolidinedione class of drugs for type 2 diabetes mellitus (eg, ciglitazone and troglitazone) has also been identified as ligands for PPAR-γ and functions as immune modulators.19,22,23 More recent studies suggest that PPAR-γ activation by 15d-PGJ2 and ciglitazone promotes host defense in the early phase of infection through enhancement of macrophage mannose receptor expression.36
Using fresh human NK cells and 2 human NK cell lines, we analyzed the role of PPAR-γ and its ligands, the natural ligand 15d-PGJ2 and the synthetic ligand ciglitazone, in the regulation of IFN-γ production and cytolytic activity of NK cells. Our results indicate that the expression of PPAR-γ in NK cells plays a permissive role in the production of IFN-γ but not the cytolytic activity. The natural ligand for PPAR-γ inhibited both IFN-γ production and cytolytic activity with or without PPAR-γ, whereas the synthetic ligand only reduced IFN-γ production upon PPAR-γ expression. Thus, selective PPAR-γ ligands may modulate distinct NK cell functions in the innate immune response.
Materials and methods
Cells and reagents
Fresh human NK cells were isolated from healthy donors after depletion of the adherent population by sequential incubation at 37°C for 1 hour on plastic flasks and nylon wool columns. The NK cells were fractioned by a 7-step Percoll gradient and negative selection methods as previously reported.37 Purity of the enriched NK cell population was determined using flow cytometry by labeling the cells with fluorochrome-conjugated anti-CD3, -CD14, -CD19, and -CD56 antibodies (Abs) as described previously. The enriched NK cell population of a typical experiment was more than 85% CD56+, less than 5% CD3+, and less than 1% CD14+ and CD19+. NK92 and NK leukemia (L) cells were maintained in RPMI 1640 medium supplemented with glutamine, a combination of penicillin and streptomycin, 10% fetal bovine serum (FBS), and IL-2 (200 U/mL)/IL-15 (10 ng/mL). K562 cells were cultured in the conditions as described by the American Type Culture Collection (Manassas, VA). Magnetic-activated cell separation (MACS) columns were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany) Fluorochrome-conjugated anti-CDs antibodies were purchased from BD Biosciences (San Jose, CA). Cytokines were purchased from PeproTech (Rocky Hill, NJ). 15d-PGJ2 and ciglitazone were obtained from Cayman (Ann Arbor, MI). Anti-PPAR-γ antibody (sc-1984) for Western blotting was obtained from Santa Cruz Biotechnology (Santa Cruz, CA); and enhanced chemiluminescence (ECL) reagents were from Pierce Biotechnology (Rockford, IL).
ELISA, multiple-probe RNAase protection assay (RPA), and RT-PCR
Immunoreactive IFN-γ was assayed in the cell culture supernatants by a double-Ab enzyme-linked immunosorbent assay (ELISA) kit using recombinant cytokines as standards (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Total RNA was extracted using a single-step phenol/chloroform extraction procedure (TRIzol; Invitrogen, Carlsbad, CA). The abundance of RNA was quantified spectrophotometrically. A total of 5 μg RNA from each treatment was used in the assays. Multicytokine templates (BD PharMingen, San Diego, CA) were used to generate 33P-labeled riboprobes. The ribonuclease protection assay including the labeling of probe, hybridization, RNAase digestion, and denaturing polyacrylamide gel electrophoresis was performed as previously described.11
One microgram of isolated RNA was utilized for cDNA synthesis by ThermoScript reverse transcriptase-polymerase chain reaction (RT-PCR) system (Invitrogen). Platinum PCR Supermix (Invitrogen) was used for semiquantitative RT-PCR at a TouchDown Thermal Cycling System (Thermo-Hybaid, Franklin, MA). Sequences of primers used are listed as follows: human PPAR-γ1: (forward) 5′-ggttgacacagagatgccattctg-3′; human PPAR-γ2: (forward) 5′-gggtgaaactctgggagattctcc-3′; human PPAR-γ1 and PPAR-γ2: (reverse) 5′-gagttggaaggctcttcatgaggc-3′; human T-bet: (forward) 5′-ttgtggacgtggtcttggt-3′ and (reverse) 5′-taagcctggggaaccacat-3′; human GAPDH: (forward) 5′-ccaaaagggtcatcatctctgc-3′ and (reverse) 5′-atttggcaggtttttctagacgg-3′. Twenty-five cycles of PCR were used under the following conditions with denaturing at 95°C for 1 minute, annealing at 56°C for 1 minute, and extension at 72°C for 1 minute.
51Cr-release cytotoxicity assay
After the treatment period, NK cells were harvested, washed once with phosphate-buffered saline (PBS), and used as effector cells. The cytolytic activity of NK cells was measured using the 51Cr-release assay with K562 tumor cell line as the target cell. The target cells were loaded with 51Cr for 1 hour prior to use and then washed 3 times, and approximately 5000 target cells per well were placed in a 96-well U-bottom plate. The effector cells were distributed in triplicate at an effector-target (E/T) ratio from 30:1 to 0.5:1. After the effector cells and target cells were coincubated for 4 hours, the cells were centrifuged for supernatant collection, and the radioactivity in counts per minute (cpm) for each sample was counted in a γ counter. The percentage of cytolysis was calculated by comparing the 51Cr release as follows: % specific 51Cr release = (sample cpm - spontaneous release)/(maximum release - spontaneous release) × 100%.
Measurement of effector-target conjugation by 2-color flow cytometry
NK92 (effector cells) and K562 (target cells) were washed twice in PBS and adjusted to 2 × 106/mL in the working solution of the appropriate dye. NK92 cells were incubated with 400 nM calcein acetoxymethylester (Ca-AM; Molecular Probes, Eugene, OR; fluorescence emission 520 nm) for green labeling, and K562 cells (target cells) were labeled red with 250 μM hydroethidine (HE; Molecular Probes; fluorescence emission 598 nm). Both dyes can be excited by 488 nm wavelength light from an argon laser. The labeling reaction was stopped by washing twice with complete medium after incubation for 1 hour. Next, equal volumes (100 μL) of labeled NK92 and K562 cells with concentrations ranging between 105 to 107/mL were added to the wells of 96-well U-bottom microtiter plates and incubated at room temperature for 20 minutes. After centrifugation for 5 minutes, the pellets were gently resuspended 3 times with a micropipette and kept on ice until they were analyzed in the FACSort (BD Biosciences, San Jose, CA). Conjugation measurement was done by a FACSort flow cytometer equipped with a 488-nm argon laser as described here. FL1 (Ca-AM stained) and FL2 (HE stained) data were acquired in the logarithmic mode. The percentage of effector and target cells conjugated was determined by gating the green, the red, and dual-labeled events, respectively.
Flow cytometric measurement of intracellular IFN-γ, PPAR-γ, and T-bet expression
Flow cytometric measurement of intracellular IFN-γ expression was performed with PharMingen Cytofix/Cytoperm Kit. The cells were permeabilized by a saponin-based method (BD PharMingen, San Jose, CA) and stained with phycoerythrin (PE)-conjugated anti-IFN-γ. Samples were analyzed on a FACSort flow cytometer (Becton Dickinson). NK cells were initially gated by forward scatter/side scatter; secondary gates were set on the basis of staining with isotype control, monoclonal Abs (mAbs) so that less than 1% of cells stained positive. For the measurement of intracellular PPAR-γ and T-bet expression, NK cells were gated based on allophycocyanin (APC)-conjugated anti-CD56 and stained with fluorescein isothiocyanate (FITC)-conjugated anti-PPAR-γ (sc-7273FITC) and PE-conjugated anti-T-bet (sc-21749PE) (Santa Cruz Biotechnology).
Retroviral infection of NK92 cells
Phoenix retrovirus packaging cell lines were cultured in DMEM plus 10% FBS. Recombinant retrovirus was produced following the previously described protocol with minor modifications.38 Briefly, Phoenix cells were transfected with viral DNA at 50% confluence with Fugene 6 transfection reagent (Roche Diagnostics, Indianapolis, IN). After 36 hours of transfection, the cells were placed in fresh medium. After a further 16-hour culture, virus-containing supernatant was harvested and filtered. NK92 cells were infected 3 times with virus-containing supernatant in the presence of 8 μg/mL polybrene (hexadimethrine bromide; Sigma, St. Louis, MO) by centrifugation at 1800 rpm for 1 hour followed by overnight incubation in the presence of standard culture medium for NK92 cells as described above. The following day, the cells were split and subjected to puromycin (Sigma) selection (2 μg/mL). Approximately 5 days later, the surviving cells were selected and pooled for further experiments. Expression of PPAR-γ was confirmed by RT-PCR. Constructs of pBABEpuro and pBABEpuro-PPAR-γ2 were obtained from B. M. Spiegelman (Harvard Medical School, Boston, MA).
Results
PPAR-γ is expressed by human NK cells
PPAR-γ has been found in T cells, B cells, dendritic cells, and monocytes/macrophages, but its expression in NK cells has not been analyzed.19,22 We assessed PPAR-γ expression in freshly isolated human NK cells. A specific transcript for PPAR-γ was detected by RT-PCR in NK cells, which was confirmed at the protein level by Western blotting (Figure 1A-B). We examined the expression of PPAR-γ by flow cytometry through intracellular staining and confirmed that PPAR-γ expression was present in the CD56+ gated NK cells (Figure 1C). In addition, a key transcription factor for IFN-γ gene expression, T-bet, could also be detected (Figure 1A,C). While T-bet expression was homogeneous, PPAR-γ levels partially varied among different donors as measured by intracellular staining (Figure 1C). Previous studies suggested that PPAR-γ was regulated by selective exogenous stimuli.39,40 Indeed, we found that IL-4 could up-regulate PPAR-γ in NK cells, whereas IL-12 had no effect (Figure 1A). There are 2 major isoforms of the PPAR-γ transcript: PPAR-γ1 and PPAR-γ2. As determined by specific primers for each isoform, we found that PPAR-γ1 was the only isoform expressed by human NK cells (data not shown), and the additional bands in the Western blot were either nonspecific or PPAR-γ2 from contaminated monocytes/macrophages (Figure 1B). Our data indicate that both PPAR-γ mRNA and protein are expressed by human NK cells.
PPAR-γ expression in freshly isolated human NK cells. (A) RT-PCR detection of PPAR-γ mRNA and T-bet. Freshly isolated human NK cells were treated with IL-2, IL-4, IL-12, and IL-2 plus IL-12 for 24 hours. RT-PCR was performed to detect PPAR-γ, T-bet, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Densitometry analysis was performed to quantitate the expression level of PPAR-γ as indicated. (B) Identification of PPAR-γ protein by Western blotting. Immunodetection of PPAR-γ in cell lysates of freshly isolated human NK cells, NK92 cells, NKL cells, THP-1 cells, and U937 cells. NKL cells were treated with IL-4 for 24 hours. Both THP-1 and U937 cells were treated with phorbol-12-myristate-13-acetate (PMA) for 24 hours. (C) Detection of PPAR-γ and T-bet by flow cytometry in human NK cells. CD56+ cells were gated on human NK cell population, and expression of PPAR-γ and T-bet was detected by flow cytometry through intracellular staining. Shaded histograms are the Ab isotype control; open histograms are anti-T-bet and anti-PPAR-γ, respectively. Brackets represent the percentage of positive cells relative to the control. Numbers above the peaks refer to the mean fluorescence intensity (MFI). (D) Relative expression levels of PPAR-γ and T-bet in NK cells from 7 donors. MFI; control IgG for T-bet: IC (T-bet) (▪); anti-T-bet: T-bet (▴); control IgG for PPAR-γ: IC (PPAR-γ) (▾); and anti-PPAR-γ: PPAR-γ (♦) are represented by in each group. Horizontal bars represent the mean of each set.
PPAR-γ expression in freshly isolated human NK cells. (A) RT-PCR detection of PPAR-γ mRNA and T-bet. Freshly isolated human NK cells were treated with IL-2, IL-4, IL-12, and IL-2 plus IL-12 for 24 hours. RT-PCR was performed to detect PPAR-γ, T-bet, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Densitometry analysis was performed to quantitate the expression level of PPAR-γ as indicated. (B) Identification of PPAR-γ protein by Western blotting. Immunodetection of PPAR-γ in cell lysates of freshly isolated human NK cells, NK92 cells, NKL cells, THP-1 cells, and U937 cells. NKL cells were treated with IL-4 for 24 hours. Both THP-1 and U937 cells were treated with phorbol-12-myristate-13-acetate (PMA) for 24 hours. (C) Detection of PPAR-γ and T-bet by flow cytometry in human NK cells. CD56+ cells were gated on human NK cell population, and expression of PPAR-γ and T-bet was detected by flow cytometry through intracellular staining. Shaded histograms are the Ab isotype control; open histograms are anti-T-bet and anti-PPAR-γ, respectively. Brackets represent the percentage of positive cells relative to the control. Numbers above the peaks refer to the mean fluorescence intensity (MFI). (D) Relative expression levels of PPAR-γ and T-bet in NK cells from 7 donors. MFI; control IgG for T-bet: IC (T-bet) (▪); anti-T-bet: T-bet (▴); control IgG for PPAR-γ: IC (PPAR-γ) (▾); and anti-PPAR-γ: PPAR-γ (♦) are represented by in each group. Horizontal bars represent the mean of each set.
Biologic functions of NK cells can be repressed by 15d-PGJ2, a natural ligand for PPAR-γ
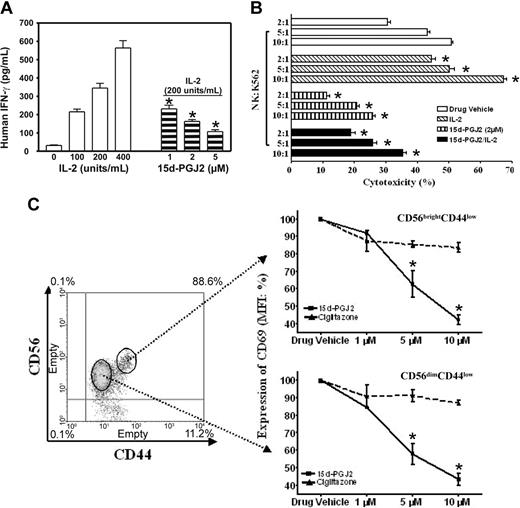
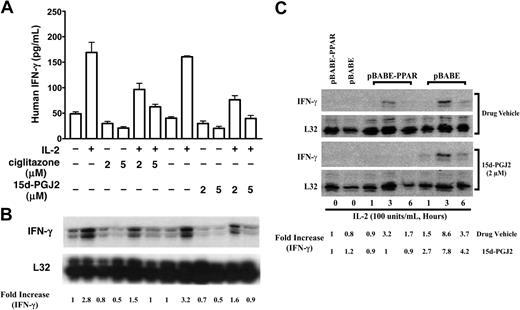
There are 2 major roles for NK cells: they function as regulators in innate resistance, antigen-specific adaptive immunity, and hematopoiesis through their production of cytokines, particularly IFN-γ; and they function as effectors in innate resistance through their cytotoxic activity.1,2,13 These functions are regulated by various stimuli such as IL-2. IL-2 augments mRNA expression and synthesis of IFN-γ in NK cells.11 Resting NK cells respond directly to IL-2 with enhanced cytotoxic activity in the absence of other stimuli. Long-term culture of NK cells with high-dose IL-2 leads to the generation of cells with a broad range of tumor target cell cytolytic capabilities.1,2,13 15d-PGJ2 is one of the most widely investigated natural ligands for PPAR-γ.29,41 We assayed IFN-γ production in freshly isolated human NK cells treated with 15d-PGJ2. Figure 2A shows that NK cells produced IFN-γ in response to IL-2 in a dose-dependent fashion, but IFN-γ expression was strongly inhibited by 15d-PGJ2 at concentrations as low as 1 μM, with optimal concentrations between 2 and 5 μM.
Regulation of NK function and activation markers by PPAR-γ natural ligand, 15d-PGJ2. (A) Inhibition of IFN-γ production in human NK cells. Human NK cells were treated with or without different dosages of 15d-PGJ2 for 30 minutes and then treated with IL-2 for 8 hours. The supernatant was collected to measure IFN-γ concentration. (B) Inhibition of cytolytic activity of human NK cells. Purified human NK cells were pretreated with or without 15d-PGJ2 (2 μM) for 1 hour and incubated with or without IL-2 (200 U/mL) for an additional 1 hour. The cells were then washed with RPMI 1640 without serum 3 times and incubated with 51Cr-loaded K564 cells for 4 hours as described in “Materials and methods.” (C) Repression of CD69 expression in activated human NK cells. Freshly isolated human NK cells were treated with different dosages of 15d-PGJ2 or ciglitazone for 30 minutes and then treated with IL-2 (200 U/mL) for 8 hours. The cells were then stained with fluorochrome-conjugated anti-CDs antibodies (APC-anti-CD56, FITC-anti-CD44, and PE-anti-CD69, respectively). Relative expression level of CD69 (mean fluorescence intensity percentage) is presented. Data represent the average from 3 different donors (mean ± SE). *Statistically significant changes compared with control samples (P < .05).
Regulation of NK function and activation markers by PPAR-γ natural ligand, 15d-PGJ2. (A) Inhibition of IFN-γ production in human NK cells. Human NK cells were treated with or without different dosages of 15d-PGJ2 for 30 minutes and then treated with IL-2 for 8 hours. The supernatant was collected to measure IFN-γ concentration. (B) Inhibition of cytolytic activity of human NK cells. Purified human NK cells were pretreated with or without 15d-PGJ2 (2 μM) for 1 hour and incubated with or without IL-2 (200 U/mL) for an additional 1 hour. The cells were then washed with RPMI 1640 without serum 3 times and incubated with 51Cr-loaded K564 cells for 4 hours as described in “Materials and methods.” (C) Repression of CD69 expression in activated human NK cells. Freshly isolated human NK cells were treated with different dosages of 15d-PGJ2 or ciglitazone for 30 minutes and then treated with IL-2 (200 U/mL) for 8 hours. The cells were then stained with fluorochrome-conjugated anti-CDs antibodies (APC-anti-CD56, FITC-anti-CD44, and PE-anti-CD69, respectively). Relative expression level of CD69 (mean fluorescence intensity percentage) is presented. Data represent the average from 3 different donors (mean ± SE). *Statistically significant changes compared with control samples (P < .05).
To assess whether treatment of NK cells with 15d-PGJ2 could also affect their cytolytic activity, we measured 51Cr release of K562 target cells after a 4-hour incubation with NK cells at different E/T ratios (Figure 2B). With increasing numbers of NK cells, there were dramatic increases in the specific cytolytic activity as reflected by 51Cr release. However, after NK cells were treated with 15d-PGJ2, the IL-2-induced specific cytolytic activity of NK cells against target cells was significantly reduced. Notably, the intrinsic cytolytic activity of NK cells (without IL-2 treatment) was also reduced (Figure 2B). Herein, our data demonstrate that 15d-PGJ2 can inhibit 2 major biologic functions of NK cells.
Next, we examined whether the phenotype of NK cell activation was affected. As shown in Figure 2C, cells were gated into 2 subpopulations with CD56brightCD44high and CD56dimCD44low, respectively. 15d-PGJ2 reduced the expression of CD69 in both populations in a dose-dependent fashion. However, the same amount of ciglitazone had no effect. In addition, neither 15d-PGJ2 nor ciglitazone affected the expression of CD44 (data not shown). The negative effect of 15d-PGJ2 in the current study was not due to toxicity of the ligands, because we did not find changes of apoptosis in the NK cells treated with 15d-PGJ2 or ciglitazone in the range of 1 μM to 10 μM (data not shown). As reported, CD36 expression is regulated by PPAR-γ and its ligands in macrophages.40 We neither found significant expression of CD36 nor observed ligand-induced changes of its expression on the surface of CD56+ NK cells (data not shown).
PPAR-γ is expressed by NKL cells but not NK92 cells
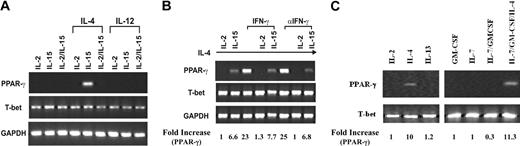
Because PPAR-γ expression in freshly isolated NK cells varied among donors, we speculated that the inhibitory effect of 15d-PGJ2 on NK function might not be solely attributed to the presence of PPAR-γ. Because the cultured cell lines are homogenous in population and convenient for molecular mechanism studies, we utilized 2 human NK cell lines, NKL and NK92 as model systems. IL-2 and IL-15 are 2 cytokines essential for the survival of NK cells. In our studies, both NKL and NK92 cell lines were maintained either in the presence of IL-2 alone, IL-15 alone, or IL-2 in combination with IL-15. As shown in Figure 3, IL-4 alone led to PPAR-γ expression in NKL cells that was decreased when treated with IL-15 simultaneously. Furthermore, any combination of IL-4 with IL-2 diminished the expression of PPAR-γ (Figure 3B). Although IFN-γ could reduce the expression of PPAR-γ in adipocytes,42 we found that it did not affect the expression of PPAR-γ in NKL cells (Figure 3B). Furthermore, IL-7, whose receptor shares the common γ chain, and IL-13, whose receptor uses IL-4Rα, did not influence PPAR-γ expression; nor did granulocyte-macrophage colony-stimulating factor (GM-CSF), whose receptor shares the common β chain (Figure 3C). In contrast to the findings of NKL cells, NK92 cells did not express PPAR-γ as determined by both RT-PCR (data not shown) and Western blot (Figure 1B). Consistent with the data from freshly isolated human NK cells, the expression of T-bet in both NK cell lines was not affected. Thus, PPAR-γ-positive NKL and PPAR-γ-negative NK92 cells are suitable models for us to study the molecular mechanisms of PPAR-γ in human NK biologic functions. The difference between NKL and NK92 with respect to the mechanism of IL-4 induction of PPAR-γ expression and differential effect of IL-2 and IL-15 awaits further investigation.
PPAR-γ expression in the human NKL cell line. (A) IL-4-induced PPAR-γ expression in NKL cells. NKL cells were cultured with IL-2, IL-15, or IL-2 plus IL-15 with or without IL-4 or IL-12 for 72 hours. RT-PCR was performed to detect transcripts for PPAR-γ, T-bet, and GAPDH. (B) Effect of IFN-γ on PPAR-γ expression. IFN-γ or antibody against IFN-γ (αIFN-γ) was added to IL-4-treated NKL cells cultured with either IL-2 or IL-15 for 72 hours. Transcripts for PPAR-γ, T-bet, and GAPDH were analyzed by RT-PCR. (C) IL-2, IL-4, IL-7, and IL-13 as well as GM-CSF were incubated with NKL cells for 24 hours. Expression of PPAR-γ, T-bet, and GAPDH were measured by RT-PCR. The PPAR-γ mRNA levels were presented as arbitrary units that were derived from average normalization densitometry values of each PPAR-γ band by corresponding GAPDH band or T-bet band.
PPAR-γ expression in the human NKL cell line. (A) IL-4-induced PPAR-γ expression in NKL cells. NKL cells were cultured with IL-2, IL-15, or IL-2 plus IL-15 with or without IL-4 or IL-12 for 72 hours. RT-PCR was performed to detect transcripts for PPAR-γ, T-bet, and GAPDH. (B) Effect of IFN-γ on PPAR-γ expression. IFN-γ or antibody against IFN-γ (αIFN-γ) was added to IL-4-treated NKL cells cultured with either IL-2 or IL-15 for 72 hours. Transcripts for PPAR-γ, T-bet, and GAPDH were analyzed by RT-PCR. (C) IL-2, IL-4, IL-7, and IL-13 as well as GM-CSF were incubated with NKL cells for 24 hours. Expression of PPAR-γ, T-bet, and GAPDH were measured by RT-PCR. The PPAR-γ mRNA levels were presented as arbitrary units that were derived from average normalization densitometry values of each PPAR-γ band by corresponding GAPDH band or T-bet band.
Inhibition of IFN-γ production by 15d-PGJ2 from NK cells devoid of PPAR-γ
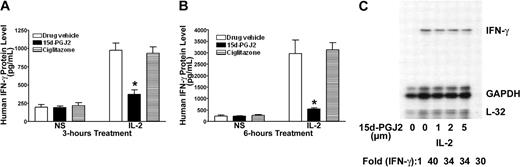
Although 15d-PGJ2 is the natural ligand for PPAR-γ, many of its effects can also be independent of the receptor.24,29 PPAR-γ-deficient NK92 cells provide a unique model to test its effect in NK cells. As shown in Figure 4, 15d-PGJ2 repressed IFN-γ production as it did on freshly isolated human NK cells. This inhibitory effect of 15d-PGJ2 was even more evident when treatment lasted for 6 hours (Figure 4B). In contrast, ciglitazone, the synthetic ligand for PPAR-γ, had no effect on IFN-γ production by NK92 cells. To determine whether the effect of 15d-PGJ2 on IFN-γ was due to changes in the IFN-γ mRNA level, we analyzed IFN-γ mRNA levels by RNAase protection assay (RPA). Surprisingly, 15d-PGJ2 did not affect IFN-γ mRNA in NK92 cells stimulated with IL-2 (Figure 4C). These data indicate that the effect of 15d-PGJ2 on IFN-γ expression in NK92 cells may be at posttranscriptional levels.
Inhibitory effect of 15d-PGJ2 on IFN-γ production in NK92 cells. (A-B) Inhibition of IL-2-induced IFN-γ production by 15d-PGJ2 but not ciglitazone in NK92 cells. NK92 cells were pretreated with either 15d-PGJ2 (2 μM) or ciglitazone (2 μM) or drug vehicle for 30 minutes and then further incubated with IL-2 (200 U/mL) for 3 hours (A) or 6 hours (B). Supernatant was collected at each time point or treatment for measurement of IFN-γ by ELISA. Data represent the average from 3 different experiments (mean ± SE). Statistically significant changes compared with control samples are indicated with an asterisk (P < .05). (C) Effect of 15d-PGJ2 on IL-2-induced IFN-γ mRNA in NK92 cells. NK92 cells were pretreated with different doses of 15d-PGJ2 for 1 hour and then with IL-2 (200 U/mL) for an additional 2 hours. Total RNA was extracted and subjected to RPA analysis as described in “Materials and methods.” The IFN-γ mRNA levels were presented as arbitrary units that were derived from average normalization densitometry values of each IFN-γ band by corresponding GAPDH band.
Inhibitory effect of 15d-PGJ2 on IFN-γ production in NK92 cells. (A-B) Inhibition of IL-2-induced IFN-γ production by 15d-PGJ2 but not ciglitazone in NK92 cells. NK92 cells were pretreated with either 15d-PGJ2 (2 μM) or ciglitazone (2 μM) or drug vehicle for 30 minutes and then further incubated with IL-2 (200 U/mL) for 3 hours (A) or 6 hours (B). Supernatant was collected at each time point or treatment for measurement of IFN-γ by ELISA. Data represent the average from 3 different experiments (mean ± SE). Statistically significant changes compared with control samples are indicated with an asterisk (P < .05). (C) Effect of 15d-PGJ2 on IL-2-induced IFN-γ mRNA in NK92 cells. NK92 cells were pretreated with different doses of 15d-PGJ2 for 1 hour and then with IL-2 (200 U/mL) for an additional 2 hours. Total RNA was extracted and subjected to RPA analysis as described in “Materials and methods.” The IFN-γ mRNA levels were presented as arbitrary units that were derived from average normalization densitometry values of each IFN-γ band by corresponding GAPDH band.
15d-PGJ2 facilitates IFN-γ protein degradation independent of PPAR-γ
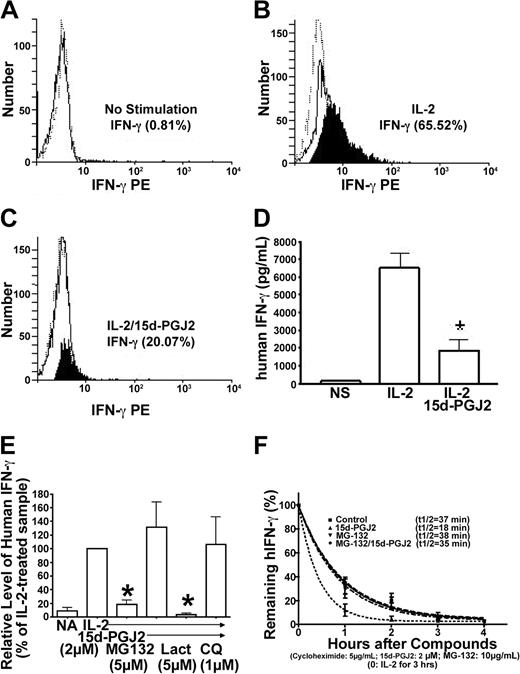
Intracellular processing of IFN-γ protein in NK cells can be monitored through intracellular staining by flow cytometry. By combining flow cytometry with measuring IFN-γ in the supernatant of matched samples, changes at the secretion level can be distinguished from changes in both synthesis and degradation. As shown in Figure 5A-C, intracellular IFN-γ increased in NK92 cells treated with IL-2 but decreased dramatically after cells were treated with 15d-PGJ2. A similar change in secreted IFN-γ from the matched samples was observed as assayed by ELISA (Figure 5D). If 15d-PGJ2 mainly affected secretion, the intracellular IFN-γ should not have changed significantly. Therefore, the inhibitory effect of 15d-PGJ2 on IFN-γ protein processing is mainly at the intracellular level.
Reduction of IFN-γ protein by 15d-PGJ2 is mediated through MG132-sensitive and chloroquine-sensitive pathways. (A-C) Detection of intracellular IFN-γ protein by flow cytometry in permeabilized NK92 cells. NK92 cells were pretreated with 15d-PGJ2 (3 μM) or drug vehicle (no stimulation). IL-2 (200 U/mL) was then added for 4 hours. Detailed procedures are described in “Materials and methods.” The histogram plots are defined as follows: PE-anti-IFN-γ (solid line), PE-conjugated control antibody (dashed line), and the net difference between PE-anti-IFN-γ and PE-conjugated control antibody (filled). (D) Detection of extracellular IFN-γ protein in the supernatant by ELISA. Supernatant was collected from the same cells that were analyzed for detection of intracellular IFN-γ protein by flow cytometry and assayed for IFN-γ protein as described in “Materials and methods.” (E) Effect of MG132 or chloroquine on IFN-γ inhibition by 15d-PGJ2. NK92 cells were preincubated with 15d-PGJ2 (2 μM) for 1 hour, and then IL-2 (200 U/mL), MG132 (5 μM), lactacystin (5 μM), or chloroquine (1 μM) was added for 8 hours. The supernatant was collected at each point, and IFN-γ protein was measured by ELISA. Relative level of IFN-γ was presented as changes of percentage compared with IL-2-treated sample (100%). (F) Kinetic analysis of IFN-γ protein degradation. NK92 cells were treated with IL-2 (200 U/mL) for 3 hours, and then cycloheximide (5 μg/mL) was added to inhibit protein synthesis. The mixtures were divided into 4 aliquots: control, 15d-PGJ2 (2 μM), MG132 (5 μM), and MG132/15d-PGJ2. The time of cycloheximide addition was designated as the zero-hour point, and the amount of IFN-γ measured by ELISA at this time point was arbitrarily set as 100%. Remaining IFN-γ at each time point (%) = (amount of IFN-γ at this time point - amount of IFN-γ at the previous time point)/amount of IFN-γ at the previous time point × 100%. The equation for the curve is Y = span × e (K × X) + plateau, where span and plateau are fixed at 100% and 0%, respectively, and half-life = 0.69/K. Data represent the average from 3 different experiments (mean ± SE). *Statistically significant changes compared with control samples (P < .05).
Reduction of IFN-γ protein by 15d-PGJ2 is mediated through MG132-sensitive and chloroquine-sensitive pathways. (A-C) Detection of intracellular IFN-γ protein by flow cytometry in permeabilized NK92 cells. NK92 cells were pretreated with 15d-PGJ2 (3 μM) or drug vehicle (no stimulation). IL-2 (200 U/mL) was then added for 4 hours. Detailed procedures are described in “Materials and methods.” The histogram plots are defined as follows: PE-anti-IFN-γ (solid line), PE-conjugated control antibody (dashed line), and the net difference between PE-anti-IFN-γ and PE-conjugated control antibody (filled). (D) Detection of extracellular IFN-γ protein in the supernatant by ELISA. Supernatant was collected from the same cells that were analyzed for detection of intracellular IFN-γ protein by flow cytometry and assayed for IFN-γ protein as described in “Materials and methods.” (E) Effect of MG132 or chloroquine on IFN-γ inhibition by 15d-PGJ2. NK92 cells were preincubated with 15d-PGJ2 (2 μM) for 1 hour, and then IL-2 (200 U/mL), MG132 (5 μM), lactacystin (5 μM), or chloroquine (1 μM) was added for 8 hours. The supernatant was collected at each point, and IFN-γ protein was measured by ELISA. Relative level of IFN-γ was presented as changes of percentage compared with IL-2-treated sample (100%). (F) Kinetic analysis of IFN-γ protein degradation. NK92 cells were treated with IL-2 (200 U/mL) for 3 hours, and then cycloheximide (5 μg/mL) was added to inhibit protein synthesis. The mixtures were divided into 4 aliquots: control, 15d-PGJ2 (2 μM), MG132 (5 μM), and MG132/15d-PGJ2. The time of cycloheximide addition was designated as the zero-hour point, and the amount of IFN-γ measured by ELISA at this time point was arbitrarily set as 100%. Remaining IFN-γ at each time point (%) = (amount of IFN-γ at this time point - amount of IFN-γ at the previous time point)/amount of IFN-γ at the previous time point × 100%. The equation for the curve is Y = span × e (K × X) + plateau, where span and plateau are fixed at 100% and 0%, respectively, and half-life = 0.69/K. Data represent the average from 3 different experiments (mean ± SE). *Statistically significant changes compared with control samples (P < .05).
Dynamic changes of intracellular IFN-γ protein can be controlled by both protein synthesis and degradation. A recent study suggests that ligands for PPAR-γ can function through regulation of the proteasome pathway independent of receptors.43 We investigated the effects of proteasome inhibitors on 15d-PGJ2-mediated down-regulation of IFN-γ in PPAR-γ-negative NK92 cells. We found that the reduction of IFN-γ by 15d-PGJ2 was reversed by addition of MG132 (Figure 5E). However, we found that the effect of 15d-PGJ2 on IFN-γ production from NK92 cells was unaffected by lactacystin, suggesting that degradation mechanisms triggered by 15d-PGJ2 might not be solely through the proteasome pathway. Based on this finding, we examined whether the lysosomal degradation pathway was involved in the action of 15d-PGJ2. As shown in Figure 5E, chloroquine, an inhibitor of lysosomal function, could partially reverse the effect of 15d-PGJ2 on IFN-γ, thus indicating the involvement of lysosomal degradation of intracellular IFN-γ. We next evaluated the effects of 15d-PGJ2 on the decay rate of IFN-γ by utilizing cycloheximide to inhibit protein synthesis. We observed that the data best fitted a “top to zero 1-phase exponential decay model”(Figure 5F). The half-life of IFN-γ in the presence of 15d-PGJ2 was significantly shortened, which could be reduced in the presence of the inhibitor. Taken together, the 15d-PGJ2-induced reduction of IFN-γ protein can be mediated at an intracellular posttranscriptional level through proteasome and/or lysosome pathways.
PPAR-γ activation represses IFN-γ expression in NK cells
Because 15d-PGJ2 is a natural ligand for PPAR-γ, we next investigated whether PPAR-γ activation had any effect on IFN-γ gene expression. The reduction of IFN-γ expression in IL-4-treated NKL cells by 15d-PGJ2 or ciglitazone was observed at both the mRNA and protein levels (Figure 6A,B). This result is different from our observation with PPAR-γ-deficient NK92 cells, in which 15d-PGJ2 facilitated only IFN-γ protein reduction but not its transcription, and ciglitazone had no effect on both mRNA and protein levels of IFN-γ (Figure 4). To further evaluate the role of PPAR-γ in NK cells, we introduced PPAR-γ in PPAR-γ-deficient NK92 cells via retrovirus infection. As shown in Figure 6C, NK92 cells with PPAR-γ expression had reduced IL-2-induced IFN-γ expression. In addition, 15d-PGJ2 further inhibited IFN-γ expression in NK92 cells with PPAR-γ expression (Figure 6C). Taken together, our data indicate that PPAR-γ activation in NK cells represses IFN-γ expression not only at the protein level but also at the mRNA expression level.
Effect of PPAR-γ activation on IFN-γ expression in NK cells. (A-B) Inhibition of IL-2-induced IFN-γ response by 15d-PGJ2 or ciglitazone in PPAR-γ-positive NKL cells. PPAR-γ-positive NKL cells were preincubated with either 15d-PGJ2, ciglitazone, or drug vehicle for 1 hour, and IL-2 (200 U/mL) was added to the cells for 6 hours. IFN-γ production in the supernatant was measured by ELISA (A). Results are shown as a representative experiment conducted in duplicate (mean ± SD). Total RNA was isolated from the corresponding samples that were treated for 2 hours, and IFN-γ mRNA was analyzed by RPA (B). (C) Reduction of IL-2-induced IFN-γ mRNA in NK92 cells upon ectopic expression of PPAR-γ. PPAR-γ-null NK92 cells were retrovirally infected with or without PPAR-γ (pBABE-PPAR or pBABE) as described in “Materials and methods” and then treated with IL-2 (200 U/mL) and 15d-PGJ2 (2 μM), respectively, for a period of time as indicated in the figure. The total RNA was isolated and subjected to RPA analysis. The PPAR-γ mRNA levels were presented as arbitrary units that were derived from normalization densitometry values of each PPAR-γ band by corresponding L32 band.
Effect of PPAR-γ activation on IFN-γ expression in NK cells. (A-B) Inhibition of IL-2-induced IFN-γ response by 15d-PGJ2 or ciglitazone in PPAR-γ-positive NKL cells. PPAR-γ-positive NKL cells were preincubated with either 15d-PGJ2, ciglitazone, or drug vehicle for 1 hour, and IL-2 (200 U/mL) was added to the cells for 6 hours. IFN-γ production in the supernatant was measured by ELISA (A). Results are shown as a representative experiment conducted in duplicate (mean ± SD). Total RNA was isolated from the corresponding samples that were treated for 2 hours, and IFN-γ mRNA was analyzed by RPA (B). (C) Reduction of IL-2-induced IFN-γ mRNA in NK92 cells upon ectopic expression of PPAR-γ. PPAR-γ-null NK92 cells were retrovirally infected with or without PPAR-γ (pBABE-PPAR or pBABE) as described in “Materials and methods” and then treated with IL-2 (200 U/mL) and 15d-PGJ2 (2 μM), respectively, for a period of time as indicated in the figure. The total RNA was isolated and subjected to RPA analysis. The PPAR-γ mRNA levels were presented as arbitrary units that were derived from normalization densitometry values of each PPAR-γ band by corresponding L32 band.
Repression of NK cell cytolytic function is independent of PPAR-γ
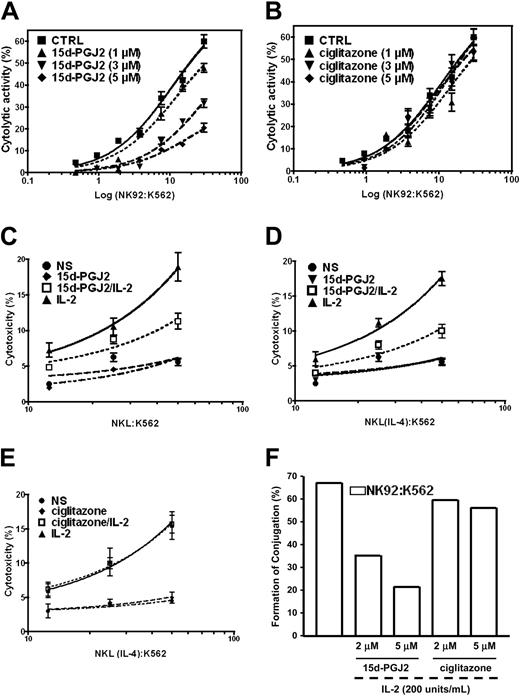
Cytolytic activity is another important biologic function of NK cells. Because the natural ligand of PPAR-γ can repress this function of freshly isolated human NK cells, we wondered whether the inhibitory effect of 15d-PGJ2 on the cytolytic activity of human NK cells is mediated through PPAR-γ. Similar to the results obtained with freshly isolated human NK cells, we observed that 15d-PGJ2 significantly reduced the cytolytic activity of IL-2-stimulated NK92 cells that are devoid of PPAR-γ (Figure 7A). Interestingly, the synthetic PPAR-γ ligand, ciglitazone, did not have any significant inhibitory effect (Figure 6B). We further examined the effects of both natural and synthetic PPAR-γ ligands in NKL cells treated with IL-4. The cytolytic activity of NKL cells before or after PPAR-γ expression induced by IL-4 displayed a similar response to 15d-PGJ2 (Figure 7C,D), whereas ciglitazone had no effect regardless of PPAR-γ expression (Figure 7E). These data indicate that PPAR-γ is not necessary for the regulation of NK cytolytic activity.
Effect of PPAR-γ activation on NK cytolytic activity. (A) PPAR-γ-null NK92 cells were preincubated with different concentrations of 15d-PGJ2 for 1 hour, and the cells were washed 3 times with RPMI 1640 without serum. The washed cells were incubated with 51Cr-loaded K562 cells for 4 hours. The detailed procedures are described in “Materials and methods.” (B) Effect of ciglitazone, a synthetic ligand for PPAR-γ, on cytolytic activity of NK92 cells. PPAR-γ-null NK92 cells were preincubated with different concentrations of ciglitazone for 30 minutes. The remaining procedures were identical to those described in panel A. Inhibition of cytolytic activity of PPAR-γ-positive NKL cells by 15d-PGJ2 but not ciglitazone. NKL cells without IL-4 priming (C) or NKL cells with IL-4 priming (D,E) were pretreated with either 15d-PGJ2 (3 μM) (C,D) or ciglitazone (3 μM) (E) for 1 hour and then were incubated with or without IL-2 (200 U/mL) for another 1 hour. The cells were washed with RPMI 1640 without serum 3 times and incubated with 51Cr-loaded K564 cells for 4 hours. The detailed procedures are described in “Materials and methods.” All values are means ± SEM from 3 independent experiments. (F) Effect of 15d-PGJ2 on conjugation formation. NK92 cells were incubated with 400 nM Ca-AM for green labeling, and K562 cells were labeled red with 250 μM HE. The percentage of conjugated effector or target cells was determined by gating the green and dual-labeled events or the red and dual-labeled events, respectively. Data are shown as a bar presentation of the data obtained from flow cytometry analysis of conjugation formation. Data represent the average from 3 different experiments (mean ± SE).
Effect of PPAR-γ activation on NK cytolytic activity. (A) PPAR-γ-null NK92 cells were preincubated with different concentrations of 15d-PGJ2 for 1 hour, and the cells were washed 3 times with RPMI 1640 without serum. The washed cells were incubated with 51Cr-loaded K562 cells for 4 hours. The detailed procedures are described in “Materials and methods.” (B) Effect of ciglitazone, a synthetic ligand for PPAR-γ, on cytolytic activity of NK92 cells. PPAR-γ-null NK92 cells were preincubated with different concentrations of ciglitazone for 30 minutes. The remaining procedures were identical to those described in panel A. Inhibition of cytolytic activity of PPAR-γ-positive NKL cells by 15d-PGJ2 but not ciglitazone. NKL cells without IL-4 priming (C) or NKL cells with IL-4 priming (D,E) were pretreated with either 15d-PGJ2 (3 μM) (C,D) or ciglitazone (3 μM) (E) for 1 hour and then were incubated with or without IL-2 (200 U/mL) for another 1 hour. The cells were washed with RPMI 1640 without serum 3 times and incubated with 51Cr-loaded K564 cells for 4 hours. The detailed procedures are described in “Materials and methods.” All values are means ± SEM from 3 independent experiments. (F) Effect of 15d-PGJ2 on conjugation formation. NK92 cells were incubated with 400 nM Ca-AM for green labeling, and K562 cells were labeled red with 250 μM HE. The percentage of conjugated effector or target cells was determined by gating the green and dual-labeled events or the red and dual-labeled events, respectively. Data are shown as a bar presentation of the data obtained from flow cytometry analysis of conjugation formation. Data represent the average from 3 different experiments (mean ± SE).
Effector cell recognition of and binding to target cells leading to the formation of conjugates is crucial for NK cytolytic activity.1,3,13 We next investigated whether the effect of 15d-PGJ2 on NK cytolytic activity was due to the disruption of effector-target conjugate formation as monitored by flow cytometry. NK92 cells (effector) were labeled with calcein acetoxymethylester, which penetrates the cell membrane and stains the cell by green fluorescence after being enzymatically modified by esterase. Hydroethidine was used for labeling K562 cells (target). After penetration of the cell and being enzymatically oxidized, hydroethidine is intercalated into the DNA and stains the cell with a red fluorescence. Pretreatment of NK92 with 15d-PGJ2 for 45 minutes significantly reduced the frequency of conjugate formation. There was 50% inhibition at 3 μM of 15d-PGJ2 and further inhibition at 5 μM, whereas ciglitazone at the same concentrations failed to show any effect (Figure 6F). Our data indicate that the 15d-PGJ2-mediated inhibitory effect on the cytolytic function of human NK cells is associated with an interruption of effector-target conjugate formation and is not dependent on PPAR-γ.
Discussion
In addition to their important role both in the defense against infections and in the regulation of immune responses, NK cells may also function as a bridge between the innate and the adaptive immune system through the regulation of autoimmune responses.2,5 Defects in the biologic functions of NK cells have been reported in NOD mice.5 IFN-γ, a major NK cell product, has been shown to mediate the penetration of diabetogenic T cells into pancreatic cells.10 Furthermore, by depletion of IFN-γ, the onset of diabetes can be delayed.9 Removal of NK cells abrogates cyclophosphamide-induced diabetes in NOD mice and prevents streptozotocin-induced diabetes in CD-1 mice.7,8 Drugs that belong to the family of thiazolidinediones have been effective in diabetes both in patients and in animal models.44 Thiazolidinediones have also been identified as ligands for PPAR-γ and function as immune modulators through the PPAR-γ pathway.19,22,23,40,45,46 Herein, we demonstrate a regulatory role for PPAR-γ and its ligands in the biologic functions of human NK cells. PPAR-γ is expressed by human NK cells at variable levels among donors, suggesting that it is regulated by exogenous factors, and IL-4 was found to be the key cytokine for PPAR-γ induction in NK cells. 15d-PGJ2 reduces IFN-γ production and cytolytic activity, whereas ciglitazone activates PPAR-γ to block IFN-γ production without effects on cytolytic activity.
PPAR-γ ligands, including 15d-PGJ2 and thiazolidinediones, can act at transcriptional and posttranscriptional levels with or without PPAR-γ.33,39,41,47 Utilization of 2 human NK cell lines (NK92 and NKL) allowed us to demonstrate that 15d-PGJ2 inhibits IFN-γ expression through PPAR-γ-dependent and -independent pathways, whereas the inhibition of cytolytic activity is independent of PPAR-γ. In the absence of PPAR-γ, 15d-PGJ2 reduces IFN-γ protein without any effect on IFN-γ mRNA. Instead, 15d-PGJ2 facilitates the reduction of IFN-γ through MG132- and/or chloroquine-sensitive pathways. Lactacystin is a more specific proteasome inhibitor without any effects on serine or cysteine proteases. MG132 has more broad and nonspecific effects other than its effect on the proteasome pathway.48 The effect of 15d-PGJ2 on intracellular protein degradation has been reported as one of the mechanisms for down-regulation of cyclin D1.43 Protein degradation through proteasome and/or lysosme pathways has been suggested in the regulation of cytokine-mediated signaling pathways and nuclear receptor-mediated gene transcription.49-51 The detailed molecular mechanisms of how the lysosome plays a role in 15d-PGJ2-mediated reduction of IFN-γ warrant further investigation.
An essential step to initiate NK cytolytic activity is the conjugate formation between NK cells and target cells in which cell surface receptors such as CD2 and CD11a play an important role.52 Previous studies indicated that PPAR-γ agonists can repress CD11a independent of PPAR-γ.53 Thus, it may be possible that disruption of conjugate formation between NK cells and target cells by 15d-PGJ2 independent of PPAR-γ is due to changes of distribution of these molecules on the cell membrane of effector cells. CD69 has a functional role in NK cell cytolytic activity as demonstrated in both human beings and mice.54 Thus, the reduction of CD69 expression by 15d-PGJ2 on the surface of NK cells as shown in this study may also contribute to its negative effect on NK cell cytolytic activity.
PPAR-γ is a ligand-activated transcription factor that can be heterodimerized with RXR and bind to DNA sequences containing PPAR response element (PPRE) in the promoter region of target genes.18 PPAR-γ also has a negative effect on gene expression mediated by other transcription factors, including nuclear factor-κB (NF-κB), activator protein-1 (AP-1), and signal transducers and activators of transcription (STATs).19,41,47,55 Transcription factors including AP-1, NF-κB, and STATs as well as T-bet play very important roles in IFN-γ gene expression of T cells and NK cells.56-62 We demonstrated here that PPAR-γ activation inhibited expression of IFN-γ mRNA, because both natural and synthetic ligands could regulate IFN-γ mRNA expression in PPAR-γ-positive NK cells. In addition, expression of PPAR-γ in the PPAR-γ-null NK92 cells and subsequent activation by 15d-PGJ2 strongly repressed IFN-γ mRNA expression. Full activity of transcription factors cannot be achieved without their interaction with cofactors like cyclic adenosine monophosphate-response element binding protein-binding protein (CBP)/p300.63 The abundance of the coactivators like CBP/p300 is limited in most cells and any competition for these proteins will ultimately affect the expression patterns of most genes.64 Our preliminary studies indicate that sequestering CBP/p300 from the transcriptomes by PPAR-γ is one of the mechanisms for the regulatory role of this transcription factor on IFN-γ gene expression (data not shown).
The expression of PPAR-γ in NK cells may have a profound biologic effect under physiological conditions. The fact that some long-chain unsaturated fatty acids and their metabolites are potential endogenous ligands for PPAR-γ further highlights the importance of PPAR-γ as one of key molecular targets through which NK cell biologic function and differentiation can be modulated.41,65 Indeed, IL-4, a type 2 cytokine that can enhance PPAR-γ expression in many immune cells including NK cells (as shown by the current study), can also enhance arachidonic acid metabolism in macrophages.20 Furthermore, IL-4 can activate a cytosolic phospholipase A2 to produce endogenous PPAR-γ ligands via lipoxygenase or cyclo-oxygenase pathways.66,67 The formation and nuclear localization of 15d-PGJ2 in vivo further implicates the important role of PPAR-γ and its ligands as important regulatory factors.27,28,36 Although the reported in vivo concentration of 15d-PGJ2 may not be sufficient enough to achieve the biologic effects in the adipocytes that were observed in ex vivo or in vitro studies,28,29 one cannot rule out the possibility that the multiple endogenous PPAR-γ ligands formed in vivo synergistically regulate cellular functions. Furthermore, because 15d-PGJ2 has a very short half-life and a strong tendency to bind the intracellular proteins, it is possible that the reported in vivo concentration of this ligand may be underestimated.31
Thiazolidinediones have been very effective in patients with diabetes, but their effects on immune system function have only begun to be appreciated. The current findings, in which ciglitazone only targets the production of IFN-γ without effects on the NK cytolytic function, may be beneficial for patients with diabetes in helping them to maintain immune surveillance capability against invading microbes and to skew immune responses from “ill” (type 1) toward “healthy” (type 2). The current findings further expand our understanding of how the immune system may be regulated by fatty acids and their metabolites. The data presented here will also provide a useful and thoughtful framework for understanding NK function in the treatment of disease.
Prepublished online as Blood First Edition Paper, July 20, 2004; DOI 10.1182/blood-2004-02-0664.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Bill Bere, Stephanie Krebs, Anna Mason, Mike Sanford, and Jami Willette-Brown for their excellent technical assistance. We acknowledge Dr B. M. Spiegelman and his laboratory for providing the constructs pBABEpuro and pBABEpuro-PPAR-γ2 and Dr Michael J. Robertson (Indiana University, Indianapolis, IN) for providing NKL cells. We thank Dr Dan McVicar and Dr Frank Gonzalez for thoughtful suggestions and critical comments on the manuscript and Susan Charbonneau for editorial assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal