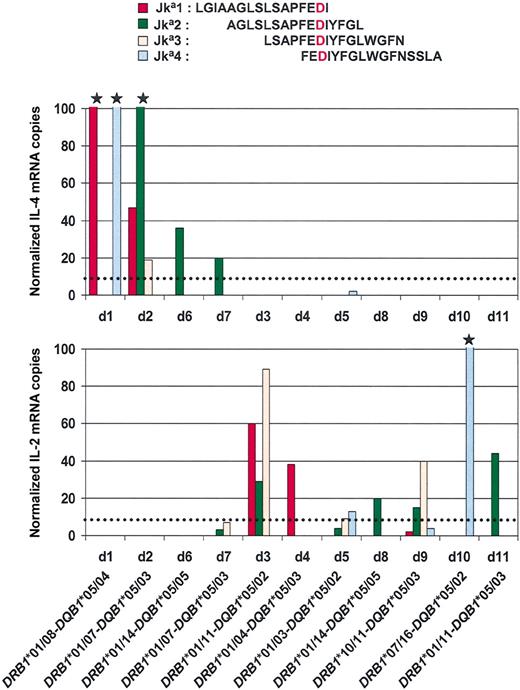
Although antibodies implicated in red blood cell (RBC) alloimmunization have been studied for many years, little is known about helper T-cell responses that drive their production.1-3 The aim of this work was to determine T-cell antigenic determinants involved in T-cell responses against RBC antigens, and the subsequent patterns of stimulatory cytokine production. The Kidd blood group antigen, Jka, was selected as a model. T lymphocytes from 11 donors whose anti-Jka alloimmunization was the result of previous pregnancies were stimulated during 6 hours by 4 overlapping peptides mimicking the Jka sequence 266 to 293 (Jka1, Jka2, Jka3, Jka4). The real-time reverse-transcriptase-polymerase chain reaction was chosen to quantify T helper 1 (Th1)- and Th2-type cytokines (respectively, interleukin-2 [IL-2] and IL-4). The results showed a clear Th1/Th2 dichotomy in cytokine responses induced by 2 immunodominant peptides: Jka1 and Jka2 (Figure 1). There were 2 populations of anti-Jka alloimmunized donors individualized: 4 donors with a Th1 (IL-2+/IL-4-) response, and 7 donors with a Th2 (IL-4+/IL-2-) response. In response to the same peptide, 2 different donors may produce different cytokines (IL-2 or IL-4). As a control of peptide-specific response, the nonalloimmunized population (7 donors) did not produce significant levels of cytokines (data not shown).
IL-2 and IL-4 mRNA quantifications among T cells stimulated by Jka synthetic peptides. The sequences of the 4 overlapping peptides tested are indicated at the top of the figure. The Asp280 (D) is indicated in red. The value of cytokine expression after stimulation was calculated after removing the background value of cytokine production by unstimulated peripheral blood mononuclear cells. The star indicates that the value obtained is more than 100. The dotted lines indicate the threshold for specific positive responses due to alloimmunization. Anti-Jka alloimmunized donors (d) are in abscissa. The donor DRB1*/DQB1* low-resolution typing is indicated at the bottom of the figure.
IL-2 and IL-4 mRNA quantifications among T cells stimulated by Jka synthetic peptides. The sequences of the 4 overlapping peptides tested are indicated at the top of the figure. The Asp280 (D) is indicated in red. The value of cytokine expression after stimulation was calculated after removing the background value of cytokine production by unstimulated peripheral blood mononuclear cells. The star indicates that the value obtained is more than 100. The dotted lines indicate the threshold for specific positive responses due to alloimmunization. Anti-Jka alloimmunized donors (d) are in abscissa. The donor DRB1*/DQB1* low-resolution typing is indicated at the bottom of the figure.
This Th1/Th2 dichotomy was not due to delays in kinetics of IL-2 and IL-4 productions, whatever the poststimulation time tested (3, 6, 20 hours; data not shown). Nor was it related to particular donor DRB1* or DQB1* molecules. On the contrary, the frequencies of DRB1*01 (82%) and DQB1*05 (100%) phenotypes observed in these alloimmunized donors producing anti-Jka, without other alloantibodies, were higher compared with the expected phenotypic frequencies in the white population, 18.1% and 20.8%, respectively.4 This observation raises the question as to whether these molecules are associated with genetic susceptibility to Jka alloimmunization, as previously demonstrated for DRB3*0101 and alloimmunization against platelet-specific antigen HPA-1a.5 The Jka1/Jka2 immunodominance in this population may be explained by the high affinity of both peptides for DRB1*01 as indicated by the HLA-peptide binding motif predictions (data not shown).6 Because DQB1*05 binding prediction data are not available, we cannot exclude that DQB1*05 increased frequency is the consequence of the known linkage disequilibrium with DRB1*01.7 Jka1 and Jka2 peptide presentation by HLA-class II molecules are currently being investigated.
In conclusion, our data suggest that (1) a clear dichotomy (IL-2/IL-4) exists among anti-Jka alloimmunized donors in response to specific Jka peptide stimulations; (2) the response induced by one peptide may vary (IL-2/IL-4) depending on the donor tested; and (3) as expected from peptide binding motif predictions, the Jka protein contains limited dominant T-helper epitopes, and the DRB1*01 molecule could be implicated in the Jka peptide presentation.
A better knowledge of cellular alloimmunization against Jka antigen could be extended to other RBC antigens. Identification of such immunodominant peptides, the cytokine patterns induced, and the HLA class II molecules implicated in their presentation would facilitate the design of new therapeutic strategies including the specific control of alloimmunization with peptide antigen tolerogens or the ex vivo induction of regulatory T cells.