Abstract
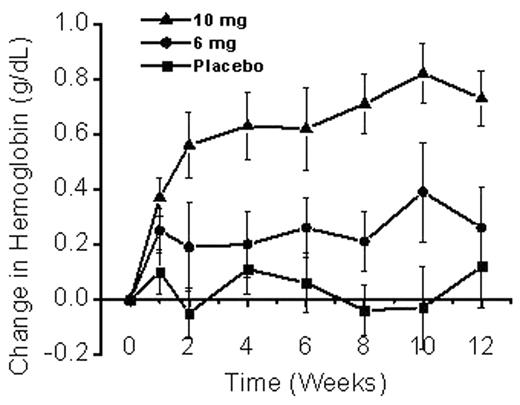
The rate and extent of hemoglobin S (HbS) polymer formation in the circulating sickle erythrocyte (RBC) is strongly influenced by the intracellular concentration of HbS. ICA-17043 (bis(4-fluorophenyl)phenyl acetamide) is a novel Gardos channel inhibitor that specifically blocks Ca++-dependent K+ efflux from the human RBC. By limiting the loss of K+, Cl−, and water, this drug preserves the hydration status of the RBC, prevents increases in HbS concentration and thereby inhibits HbS-polymerization. We hypothesized that ICA-17043 could decrease both the hemolytic rate and vaso-occlusive complications of sickle cell anemia (SCA). In a recently completed multicenter, parallel-group, randomized, double-blind, dose-range-finding study, we examined the efficacy and safety of ICA-17043 in patients with SCA. In this 12-week study, 90 patients were randomized into 3 arms: placebo; low dose ICA-17043 (6 mg); and high dose ICA-17043 (10 mg). The primary outcome variable was the change in hemoglobin (Hb) from baseline to the end of the study. A total of 90 subjects were enrolled in this study, 80 of whom completed the 12-week treatment period. Using an intent-to-treat analysis, we found a significant increase from baseline of the total Hb when the subjects in the high dose arm (0.68 ± 0.11 g/dL, S.E.) were compared to those in the placebo arm (0.01 ± 0.12 g/dL), p < 0.001. Although an increase in Hb was also seen in the subjects in the low dose arm (0.28 ± 0.15 g/dL), this smaller change failed to reach statistical significance.
In addition to the increase in Hb (as well as similar increases in hematocrit and RBC count), statistically significant decreases were noted after 12 weeks on 10 mg of ICA-17043 vs. placebo in the following variables: a) dense RBC (% with Hb >41 g/dL) [−2.41 vs. −0.08, p < 0.001]; b) reticulocyte count (%) [−4.12 vs. −0.46, p <0.001]; c) LDH (u/L) [−121 vs. −15, p = 0.002]; and d) indirect bilirubin (mg/dL) [−1.18 vs. 0.12, p < 0.001]. ICA-17043 was well tolerated by the subjects. The most common adverse experiences were diarrhea and nausea, both of which occurred more frequently in the subjects receiving active treatment than in those on placebo. No patients died during the study, and only 3 of the 10 patients who dropped out of the study did so because of adverse events. In summary, we found that 12 weeks of treatment with ICA-17043 at a dose of 10 mg/d produced a significant increase in Hb and significant decreases in the various markers of RBC destruction. Furthermore, we found this drug to be safe and well tolerated. Therefore, based on the beneficial, dose-dependent RBC effects, a Phase III study has now been designed that will examine the clinical effect of ICA-17043 on the frequency of vaso-occlusive events in patients with sickle cell disease.
Author notes
Corresponding author