Abstract
BACKGROUND
Surprisingly, anti-tumor responses can occur in patients who reject donor grafts following nonmyeloablative hematopoietic cell transplantation (Dey et al., Biol Blood Marrow Transplant 7:604). In murine mixed chimeras prepared with nonmyeloablative conditioning, we previously showed that recipient leukocyte infusions (RLI) induced anti-tumor responses against host-type tumors (Rubio et al. Blood 102:2300).
To further investigate the clinical relevance of this RLI model, we:
Evaluated the effect of RLI from tumor-bearing mice
Compared RLI with allogeneic lymphocyte infusion in untreated mice
METHODS
Mixed chimerism was achieved in BALB/c (H-2d) mice conditioned with depleting anti-CD4 and CD8 mAbs on Day-5, cyclophosphamide 200 mg/kg on Day -1 and 7 Gy thymic irradiation on Day 0 prior to transplantation of 25x106 B10.BR (H-2k) bone marrow cells. Some groups received RLI (3x107 BALB/c spleen cells) seven weeks post-BMT. Some RLI donor mice received BALB/c A20 B cell lymphoma cells (1x105) two weeks before RLI. Some groups received RLI depleted of B cells using B220 negative selection by MACS column. A20 cells (5x105) were given i.v. one week after RLI in chimeras or after allogeneic lymphocyte infusion (3x107 B10.BR spleen cells) to untreated BALB/c mice.
RESULTS
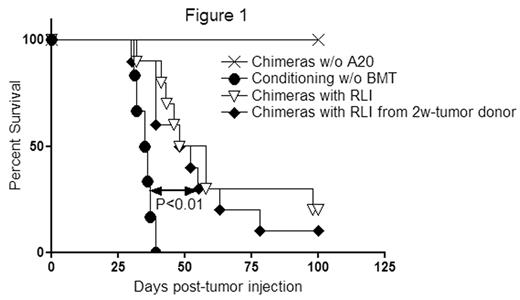
In the clinical setting, RLI would be obtained from tumor-bearing hosts. We therefore examined whether RLI is still effective when the lymphocytes are obtained from the tumor-bearing mice. Contamination of B220+ cells in the RLI was less than 0.03%. RLI recipients from the tumor-bearing mice (n=10) showed similar tumor survival compared to recipients of RLI from naïve donors (n=10) (median survival time [MST] 53 versus 50 days respectively, p=0.48) (Figure 1). Thus, with a purging procedure, the same anti-tumor effect was achieved with RLI from tumor-bearing hosts as from non-tumor-bearing hosts.
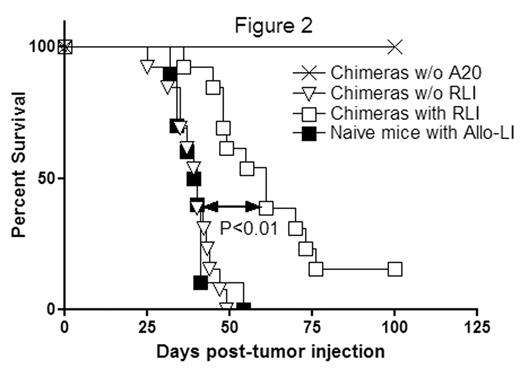
Allogeneic lymphocyte injection is a potentially feasible anti-tumor therapy. We therefore compared anti-tumor effects of allogeneic lymphocyte infusion into naïve mice with that of RLI given to mixed chimeras. Chimeric RLI recipients mice (n=13) had longer survival (MST 61 days) than naïve mice receiving allogeneic lymphocytes (n=10) (MST 39.5 days, P<0.01) (Figure 2). This result suggests not only that the anti-tumor effect of RLI therapy is stronger than allogeneic lymphocyte infusion therapy but also suggests that rejection of allogeneic cells is insufficient and mixed chimerism is required prior to the induced rejection to achieve maximum anti-tumor effects.
CONCLUSION
Together, these data reinforce the potential of RLI therapy to be a new HSCT strategy which does not have the risk of graft-versus-host disease.
Author notes
Corresponding author