Abstract
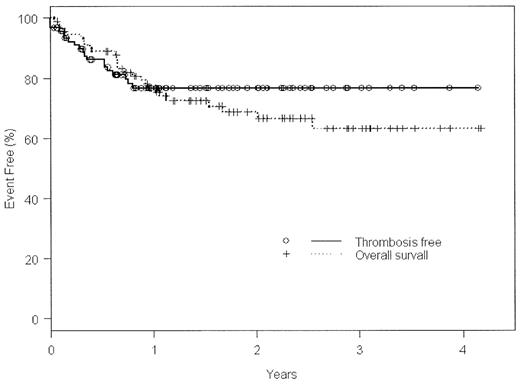
A strong link between asparaginase-containing (ase +) chemotherapy regimens and venous thrombotic events (VTE) has been established for pediatric acute lymphoblastic leukemia (ALL), but this association has not been studied in adult ALL. We recently adopted as our first-line therapy for adult ALL a treatment protocol (DFCI 00–01) that employs weekly high-dose ase throughout a 27 week intensification phase. We reviewed the records of 92 adult patients consecutively accrued at Princess Margaret Hospital (from Jan 2000 to Dec 2003) for venous thrombotic events (VTE). Median age at diagnosis of ALL was 41±17 y, or 19±3 y for those who presented after a history of pediatric ALL (n=6), and 42±16 y for adults who presented with de novo ALL (n=86). Thirty-five (37%) were female, 59 (63%) male. Twenty-four (26%) were Philadelphia-chromosome positive; 71% of these received regimens containing imatinib. At least 1 symptomatic, radiologically confirmed VTE was seen in 19 patients (21%), with 3 (3%) diagnosed prior to any antileukemic therapy. Overall survival (OS) versus thrombosis-free survival (TFS) is illustrated in the Kaplan-Meier curve below. Median time to VTE was 94±92 d [95% CI]. Average age of patients with VTE was 44 ±15y; no events were observed in patients with a prior pediatric history of ALL. The sites of VTE were: 10/22 (46%) in the lower extremities, 5/22 (23%) propagating from central venous access catheters (CVAC), 4/22 (18%) as pulmonary emboli (2 in isolation), and 3/22 (14%) CNS (2 cerebral venous sinus and 1 retinal venous thrombus). The first antileukemic regimen administered was DFCI 00–01 ± imatinib in 79/92 (86%), HyperCVAD ± imatinib in 7 (8%), an unclassified ase+ regimen in 1 (1%), or an unclassified asparaginase-excluding (ase−) regimen in 5 (5%). A significant difference in the rate of ase-dependent VTE was observed when all regimens (1° or subsequent) were counted, with 18 VTE during 103 ase+ regimens (17.5%), versus only 1 VTE in 32 ase− regimens (3.1%), [p=0.04]. Median time to VTE in DFCI 00-01was 109±98d, most often occurring during the intensification phase. The odds ratio for VTE with any T-cell subtype of ALL compared to any B-cell subtype was 4.6 [p=0.0043].
Conclusions: A high, significant VTE rate (~18%) was observed in adults with ALL undergoing ase+ antileukemic therapy, nearly 6X the rate observed either prior to treatment or during ase− regimens (~3%). Ase is thus strongly associated with VTE, warranting improved surveillance and antithrombotic prophylaxis. Risk factors (underlying thrombophilias, leukemic subtypes) similarly deserve further study.
Author notes
Corresponding author