Abstract
Introduction: FISH is able to recognize chromosomal deletions and translocations with a greater sensitivity than conventional cytogenetics. Specific abnormalities have been associated with prognosis. Initial observations suggest a poor outcome for patients with -17p13.1, chromosome 13 abnormalities (Δ13) and t(4;14)(p16.3;q32). In contrast a good outcome has been shown in some series for patients with t(11;14)(q13;q32). We analyzed the value of FISH in patients receiving high dose therapy .
Patients and Methods: We studied by cIg-FISH 226 patients undergoing high dose therapy at Mayo Clinic between 1/1990 and 9/2001. All patients had a pretransplant cIg-FISH done on cytospin slides from marrow aspirates for t(11;14)(q13;q32), t(4;14)(p16.3;q32), and -17p13.1(p53). Information was available regarding Δ13 for all patients (+ in 52%).
Results:
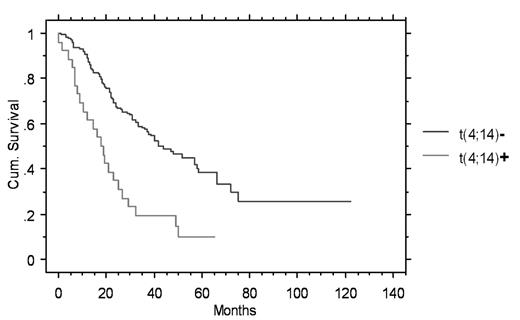
The prevalence of the abnormalities were: t(11;14)(q13;q32) 17% (n=197), t(4;14)(p16.3;q32) 13% (n=153), and -17p13.1 11% (n=168). The overall survival (OS)was significantly shortened in patients with t(4;14)(p16.3;q32) (18.2 vs. 43.3 mo, p=0.001) (figure) and patients with -17p13.1 (14.7 vs. 38.6 mo, p=.01). OS was not different for patients with the t(11;14)(q13;q32) (36.2 vs. 34.8 mo, p=ns). Likewise time to progression (TTP) was shortened in patients with t(4;14)(p16.3;q32) (8.5 vs 17.7 mo, p=.001) and -17p13.1 (8.3 vs. 16.2 mo, p=0.005). TTP was also not affected significantly by the t(11;14)(q13;q32) (20.7 vs. 14.9 mo, p=NS). To dissect the specific contribution of t(4;14)(p16.3;q32) we did a subset analysis of patients who also had Δ13, since 85% of patients with t(4;14)(p16.3;q32) are expected to have Δ13. Of 84 studied for both abnormalities 22 had both Δ13 and t(4;14)(p16.3;q32). The OS was significantly shorter in patients with both abnormalities versus those with Δ13 alone (26.6 vs. 18.2 months, p=0.001). When a multivariable analysis of the impact of Δ13 and t(4;14)(p16.3;q32) were placed into a Cox model the hazard function for t(4;14)(p16.3;q32) was greater than Δ13 (2.6 versus 1.6). Δ13 had only borderline significance in this model (p=0.06).
Conclusion: We have been unable to corroborate the improved outcome after transplant for patients with t(11;14)(q13;q32). As has been reported in patients with conventional and high dose therapy -17p13(p53) and t(4;14)(p16.3;q32) have clinical importance for estimation of OS and TTP. In this patient group the t(4;14)(p16.3;q32) carried a greater adverse impact than did Δ13, and identifies a subset of patients whose time to progression is 8.5 months. These patients likely do not benefit from autologous transplant and are candidates for novel therapeutic approaches.
Outcome
| . | Successful determination . | Patients with translocation or deletion . | Survival (months) with/without abnormality . | Time to Progression (months) with/without abnormality . |
|---|---|---|---|---|
| * p<0.01;**p<0.001 | ||||
| t(11;14)(q13;q32) | 197 | 34(17%) | 36.2/34.8 | 20.7/14.9 |
| p53 | 168 | 18(11%) | 14.7/38.6 * | 8.3/16.2 * |
| t(4;14)(p16.3;q32) | 153 | 26(17%) | 18.2/43.3 **figure | 8.5/17.7 ** |
| t(4;14)(p16.3;q32)/Δ13+ patients | 84 | 22(26%) | 18.2/26.6 | 8.2/12.8 * |
| . | Successful determination . | Patients with translocation or deletion . | Survival (months) with/without abnormality . | Time to Progression (months) with/without abnormality . |
|---|---|---|---|---|
| * p<0.01;**p<0.001 | ||||
| t(11;14)(q13;q32) | 197 | 34(17%) | 36.2/34.8 | 20.7/14.9 |
| p53 | 168 | 18(11%) | 14.7/38.6 * | 8.3/16.2 * |
| t(4;14)(p16.3;q32) | 153 | 26(17%) | 18.2/43.3 **figure | 8.5/17.7 ** |
| t(4;14)(p16.3;q32)/Δ13+ patients | 84 | 22(26%) | 18.2/26.6 | 8.2/12.8 * |
Author notes
Corresponding author