Abstract
PKCβ isoforms are important in B-cell signaling and proliferation. PKCβ overexpression in diffuse large B-cell lymphoma relates with poor prognosis but the clinical significance of PKCβ in MM is not yet established. Herein, we report antimyeloma activity of the PKCβ inhibitor enzastaurin HCl (LY317615) both as a single agent and in combination with bortezomib and dexamethasone.
Methods: Six MM cell lines (NCI-H929, RPMI8226, IM9, U266, ARH-77 and HS Sultan) with varying degree of PKC activity were treated with graded dose of enzastaurin and studied for induction of apoptosis by flow cytometric analysis of annexin and propidium iodide stainings, and inhibition of proliferation by MTT assay.
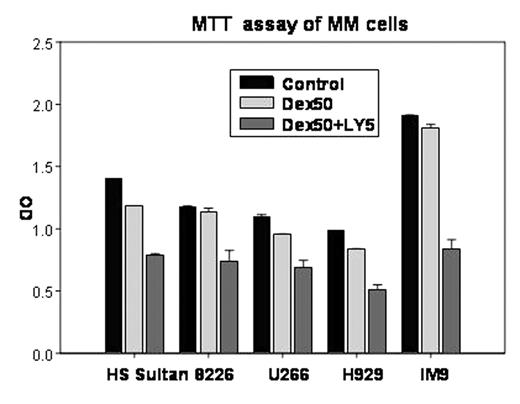
Results: IC50 doses of both effects were low at 1-5 μM in all cell lines and did not correlate with the baseline PKC activity. Interestingly, the combination of enzastaurin at non-toxic concentrations with varying doses of dexamethasone and bortezomib yielded synergistic enhancement of cell death and inhibition of proliferation in all cell lines. Multiparameter immunoblottings of cell lysate after enzastaurin treatment showed that, at these functional doses, PI3/AKT, Erk and JNK pathways were unaffected. Additional studies are ongoing to evaluate the effect of enzastaurin on activities of apoptotic proteins, and verify its effect in primary samples.
Conclusions: Our preliminary data indicate that the PKCβ inhibitor enzastaurin demonstrates single-agent antimyeloma activity and can sensitize MM cells to bortezomib and dexamethasone. Enzastaurin is promising for further studies as an approach to increase activity and decrease toxicity of MM therapy.
Author notes
Corresponding author