Abstract
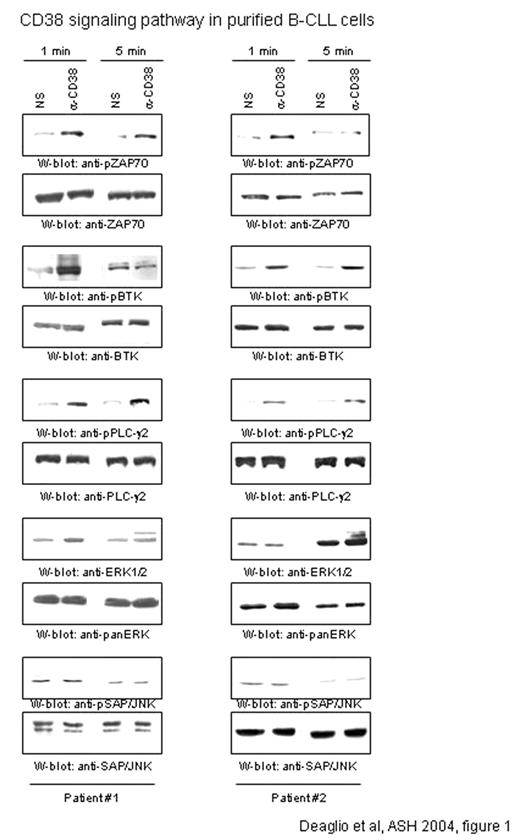
CD38 is a negative prognostic marker in B-chronic lymphocytic leukemia (B-CLL). In a previous study we observed that CD38 and IL-2 cooperate in inducing proliferation and survival of B-CLL cells. Now attention is given to the signaling pathway ruled by CD38 in B-CLL cells. We show that CD38 ligation triggers a prominent tyrosine phosphorylation of the ZAP70 kinase in 6 out of 9 patients studied (2 representative shown in figure 1). ZAP70 activation is evident 1 minute after receptor cross-linking and tends to diminish within 5 minutes. Tyrosine phosphorylation of syk is undetectable in these conditions. A functional relationship between CD38 and ZAP70 was confirmed by the analysis of a panel of B cell lines. Indeed, CD38 ligation in Nalm-6, a pre-B cell line identified as ZAP70+, wais followed by ZAP70 tyrosine phosphorylation, with a kinetics overlapping the one observed with the B-CLL samples.
Downstream events include the sequential activation of BTK and PLC-γ2, the latter likely responsible for the cytoplasmic Ca2+ mobilization, observed both in B-CLL and in the Nalm-6 cells. The signaling events culminate with the activation of ERK1/2, but not of SAP/JNK and p38 proteins. The results obtained with purified B-CLL cells from 2 representative patients are shown in figure 1.
Together, these data i) highlight for the first time a functional relationship between CD38 and ZAP70 in B cells and ii) identify crucial cytoplasmic steps in the signaling cascade promoted by CD38, thus providing a mechanistic base to explain why B-CLL patients with a CD38+/ZAP70+ clone are characterized by the most unfavourable clinical course. Selected bibliography
Figure.
Author notes
Corresponding author