The activated protein C (APC)–independent anticoagulant activity of protein S on tissue factor–induced thrombin generation was quantified in plasma. In absence of APC, protein S significantly decreased the endogenous thrombin potential (ETP) in a concentration-dependent manner. The APC-independent anticoagulant activity of protein S in plasma was not affected by phospholipid concentrations but strongly depended on tissue factor concentrations: protein S inhibited the ETP from 6% at 140 pM tissue factor to 74% at 1.4 pM tissue factor. Plasma with both 60% protein S and 140% prothrombin showed an ETP of 240% compared to normal plasma, suggesting an APC-independent protective role of protein S in the development of thrombosis as a result of protein S deficiency and the prothrombin-G20210A mutation. At high tissue-factor concentrations, protein S hardly expressed APC-independent anticoagulant activity but exerted potent APC-cofactor activity when thrombomodulin or APC were added to plasma. Neutralization of protein S under these conditions resulted in a 20-fold reduction of the anticoagulant activity of APC. The present study shows that protein S effectively regulates coagulation at 2 levels: at low procoagulant stimuli, protein S maintains the hemostatic balance by directly inhibiting thrombin formation, and at high procoagulant stimuli, protein S restores the hemostatic balance via its APC-cofactor activity.
Introduction
Human protein S was first described by DiScipio et al1,2 as a vitamin K–dependent glycoprotein that circulates in plasma. Protein S down-regulates blood coagulation via different mechanisms. Protein S is a nonenzymatic cofactor for activated protein C (APC) in the anticoagulant protein C pathway3,4 in which it accelerates the APC-mediated inactivation of factor Va5,6 and factor VIIIa.5,7 Protein S also expresses APC-independent anticoagulant activity by directly inhibiting prothrombin activation, a phenomenon that was observed both in purified systems8-10 and in plasma.9,11 Identification of interaction sites between protein S and factor Va12 and Xa13 supports a direct effect of protein S on prothrombin activation. Binding of protein S to negatively charged phospholipid surfaces is a prerequisite for protein S activity, as antibodies directed against the phospholipid-binding Gla-module of protein S completely abolish the anticoagulant activity of protein S both in the absence and in the presence of APC.9,14,15 On the basis of these experiments, it was proposed that competition for phospholipid binding sites, not a direct interaction with factor Va and factor Xa, is the main mechanism by which protein S inhibits prothrombin activation in the absence of APC.16,17 However, this hypothesis was based on experiments performed with purified protein S preparations that contain in vitro–generated multimeric forms of protein S.18 Due to high phospholipid-binding affinity (Kd < 1 nM), protein S multimers inhibit prothrombin activation at limiting phospholipid concentrations by occupying the phospholipid surface. In plasma, however, protein S multimers are absent, and native protein S is unlikely to effectively shield phospholipid surfaces due to its low affinity for phospholipids (Kd ∼ 250 nM).18
Protein S deficiency has been shown to be a risk factor of venous thrombosis.19,20 Since then, several reports confirmed the importance of protein S as a cofactor for APC in regulating thrombin formation. There are no reports yet that demonstrate the physiologic importance of an impaired activity of protein S in the absence of APC.
Protein S has been shown to increase the clotting time in plasma stimulated with both tissue factor and factor Xa in the absence of APC.9,21,22 This suggests that protein S inhibits the initiation of coagulation, but it is not clear to what extent protein S can control the propagation and/or termination phase. Thrombin generation measurements provide a useful tool to study coagulation as it allows quantification of the total amount of active thrombin generated during the coagulation processes (the endogenous thrombin potential: ETP).
In the present investigation we quantified the anticoagulant activity of native protein S in plasma. We examined the ability of protein S to inhibit thrombin formation, and we also examined how the inhibitory effect of protein S depends on the phospholipid concentration and procoagulant stimulus. In addition, we studied the effect of protein S on thrombin generation in a plasma system mimicking 2 conditions that affect the anticoagulant activity of protein S in the absence of APC: heterozygous protein S deficiency and the prothrombin G20210A polymorphism.
Materials and methods
Materials
HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer was obtained from Sigma (St Louis, MO), and bovine serum albumin (BSA) was obtained from ICN Pharmaceuticals (Aurora, OH). Fluorogenic substrate Z-Gly-Gly-Arg-AMC.HCl (I-1140) was from Bachem (Bubendorf, Switzerland). Recombinant tissue factor (thromboplastin) was from Dade Innovin (Dade Behring, Marburg, Germany). 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-Dioleoyl-sn-glycero-3-phosphoserine (DOPS), and 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) were obtained from Avanti Polar Lipids (Alabaster, AL). Phospholipid vesicles (20% DOPS, 20% DOPE, 60% DOPC) were prepared as described previously.23 Polyclonal anti–protein S (4.1 mg/mL, 27.3 μM) and anti–protein C (3.7 mg/mL, 24.7 μM) antibodies and normal rabbit serum (∼ 2 mg IgG per mL) were obtained from DAKO (Glostrup, Sweden). The monoclonal antibody CLB-PS13, which is directed against the Gla-domain of protein S, was a kind gift from Dr Jan van Mourik, Sanquin Research, Central Laboratory of the Blood Transfusion Service (CLB), Amsterdam, The Netherlands. Protein S–depleted plasma was obtained from Affinity Biologicals (Hamilton, ON, Canada). Human prothrombin was purified as described,24 and human factor Xa and APC were obtained from Enzyme Research Laboratories (South Bend, IN). Recombinant human thrombomodulin (TM) was a kind gift of Synapse BV (Maastricht, The Netherlands).
Normal pooled plasma
Blood was drawn from 74 healthy volunteers (45 men, 29 nonpregnant women not using oral contraceptives; mean age, 40.0; SD 8.9) on 3.8% citrate. The first few milliliters of blood were discarded. The residual volume was centrifuged for 15 minutes at 3000g, resulting in platelet-rich plasma, which was centrifuged again for 10 minutes at 11 000g. The platelet-poor plasmas were pooled and snap frozen.
Measurement of thrombin generation
Tissue factor and phospholipid vesicles were incubated at 6.25 times the final concentration for at least 1 hour at 37°C in HEPES buffered saline (HBS: 25 mM HEPES, 175 mM NaCl, pH 7.7) containing 5 mg/mL BSA (HBS-BSA) and 2 mM CaCl2. One mL of this tissue factor/phospholipid/CaCl2 mixture was mixed with 0.25 mL HBS-BSA containing 400 mM CaCl2, right before use. Plasma was incubated with antibodies directed against (activated) protein C (1.23 μM IgG) and with or without antibodies directed against protein S (2.73 μM IgG) for 15 minutes at 37°C. Subsequently, 80 μL plasma samples were transferred into a microtiterplate at 37°C, 20 μL of a prewarmed fluorogenic substrate solution (1875 μM I-1140 in HBS-BSA) was added, and coagulation was started by addition of 25 μL of a prewarmed phospholipid/tissue factor/CaCl2 mixture. Thrombin generation was followed continuously in time using a microtiterplate SPECTRAmax GEMINI XS fluorometer (Molecular Devices, Sunnyvale, CA) with excitation and emission wavelengths set at 368 and 460 nm, respectively.
To study the contribution of the APC-cofactor activity of protein S to the inhibition of thrombin generation, APC was included in the phospholipid/tissue factor/CaCl2 mixture (see previous paragraph) to yield a final APC concentration in plasma of 7.5 nM. In an additional experiment, purified thrombomodulin was added to plasma before coagulation was initiated. In these experiments thrombin generation was followed continuously in time by measuring fluorogenic substrate conversion in a microtiterplate Fluorskan Ascent fluorometer (Thermo Labsystems, Helsinki, Finland).
A calibrator was used to allow calculation of the amount of thrombin formed and correction of the obtained relative fluorescence units for inner-filter effects and fluorogenic substrate consumption.25 In addition, the contribution to substrate conversion by thrombin–α2-macroglobulin complexes was subtracted. Finally, the first derivative of the data was taken that yielded the thrombin generation curve, allowing calculation of the total area under the curve, the endogenous thrombin potential (ETP). In experiments (Figure 1 inset, 4, 6, and 7) in which the Fluorskan Ascent fluorometer was used, these corrections were performed automatically by means of the calibrated automated thrombogram (CAT) computer software25 provided by Synapse BV (Maastricht, the Netherlands). In all other experiments thrombin generation curves and ETP were obtained after manual correction.
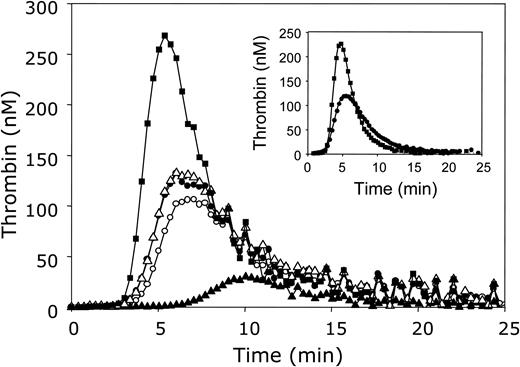
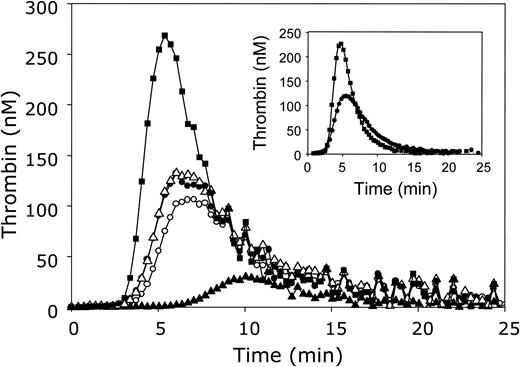
The influence of APC and protein S on thrombin generation in plasma. Thrombin generation was initiated with 3.5 pM tissue factor, 10 μM phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE), and 16 mM CaCl2 (final concentrations) in normal pooled plasma in the absence of APC and antibodies against protein C and protein S (○) or in the presence of antibodies against protein C (•), antibodies against both protein C and protein S (▪), 5 nM APC (▴), or 5 nM APC and antibodies against protein C (▵). Thrombin generation was quantified with the fluorogenic substrate I-1140 as described in “Materials and methods.” Inset: Thrombin generation was initiated under the same conditions in plasma in the presence of antibodies against protein C without (•) or with (▪) monoclonal antibodies CLB-PS13 against the Gla domain of protein S.
The influence of APC and protein S on thrombin generation in plasma. Thrombin generation was initiated with 3.5 pM tissue factor, 10 μM phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE), and 16 mM CaCl2 (final concentrations) in normal pooled plasma in the absence of APC and antibodies against protein C and protein S (○) or in the presence of antibodies against protein C (•), antibodies against both protein C and protein S (▪), 5 nM APC (▴), or 5 nM APC and antibodies against protein C (▵). Thrombin generation was quantified with the fluorogenic substrate I-1140 as described in “Materials and methods.” Inset: Thrombin generation was initiated under the same conditions in plasma in the presence of antibodies against protein C without (•) or with (▪) monoclonal antibodies CLB-PS13 against the Gla domain of protein S.
Results
APC-independent anticoagulant activity of protein S
In order to investigate the APC-independent anticoagulant activity of protein S on thrombin generation in plasma, it had to be excluded that protein S contributes to the down-regulation of thrombin formation via its ability to act as a cofactor of APC. Because plasma may contain trace amounts of APC26 and since the high concentrations of thrombin that are formed during the clotting of plasma may activate protein C, APC-enzymatic activity was blocked by inhibitory antibodies against (activated) protein C.
Normal pooled plasma was incubated with polyclonal antibodies against protein C for 15 minutes at 37°C. As a reference, an identical incubation was performed in which the antiserum was replaced by HBS-BSA. Subsequently, the thrombin-specific fluorogenic substrate I-1140 was added to plasma, and coagulation was started by addition of a mixture of tissue factor, phospholipids, and CaCl2, resulting in final concentrations of 3.5 pM tissue factor and 10 μM phospholipid vesicles. Figure 1 demonstrates that the addition of antibodies against protein C caused a moderate increase in thrombin generation compared to control plasma in which buffer was added instead of antibody, illustrating the presence of APC during the thrombin generation process. However, from this experiment it could not be concluded that APC was fully inhibited by the antibodies. Therefore, 5 nM APC was added to normal pooled plasma in the presence or absence of antibodies against protein C. In the absence of antibodies against protein C, APC drastically reduced thrombin generation, whereas in the presence of antibodies the inhibitory effect of APC was completely abolished (Figure 1). The fact that thrombin generation curves measured in normal pooled plasma with or without added APC were identical when antibodies against protein C were present (Figure 1, open triangles and filled circles, respectively) and that the antibodies did not affect thrombin generation in protein C–deficient plasma (data not shown) demonstrate that the protein C antibodies specifically and fully inhibited APC in plasma. Although the presence of APC and/or in situ activation of protein C in plasma had a moderate effect on thrombin generation, it was decided to add antibodies against protein C to plasma in all further experiments.
Subsequently, in the absence of APC, inhibition of protein S in plasma by addition of polyclonal antibodies against protein S resulted in a large increase in thrombin generation (Figure 1). Both the lag time and the time required to reach the thrombin peak were shorter, and the ETP was increased to 156% when compared to plasma in which protein S was not inhibited by antibodies. In order to exclude nonspecific effects of the protein S and protein C antibodies on thrombin generation, an experiment was performed in which the antibodies were replaced by an equal amount of rabbit IgG. The fact that rabbit IgG had no effect on thrombin generation in normal pooled plasma and that the addition of the polyclonal antibodies against protein S and protein C did not affect thrombin generation in protein S– and protein C–depleted plasma, respectively, indicates that the increase in thrombin generation upon addition of antibodies to plasma is due to specific depletion of the protein of interest.
The use of the monoclonal antibody CLB-PS13 confirmed that protein S directly inhibits thrombin generation in plasma. The epitope recognized by CLB-PS13 is located in the Gla domain of protein S. Binding of this monoclonal antibody to protein S prevents interaction of protein S with negatively charged phospholipid surfaces and abolishes both the APC-cofactor and APC-independent activity of protein S.15,17 The inset in Figure 1 shows that, like the polyclonal antibody, CLB-PS13 significantly increased thrombin generation.
Since many vitamin K–dependent proteins undergo conformational changes upon ligating calcium ions27,28 and thrombin generation in our experiments was initiated by the addition of calcium ions, we tested whether rate-limiting Ca2+-dependent conformational changes might affect the direct regulation of thrombin generation by protein S. Therefore, plasma was incubated with CaCl2 (16 mM final concentration) for 15 minutes at which, under the conditions, no clotting occurred yet. Subsequently, tissue factor and phospholipids were added. It appeared that, except for a slight decrease in lag time, preincubation of plasma with calcium ions had no effect on thrombin generation and the ETP, either in the absence or presence of antibodies against protein S (data not shown).
Influence of protein S concentration on thrombin generation
In the previous experiment it was shown that protein S suppresses thrombin generation in plasma in the absence of APC. However, the amount of antibodies against protein S added to plasma resulted in complete neutralization of protein S in the plasma studied. Because homozygous protein S deficiencies are rare in nature and heterozygous protein S deficiencies are more common in populations presenting with thrombotic events, the dose-dependent effect of protein S on thrombin generation was studied. The protein S concentration in plasma was varied in 2 ways: (1) by adding increasing amounts of polyclonal antibodies against protein S to normal pooled plasma, or (2) by mixing protein S–depleted plasma with normal pooled plasma.
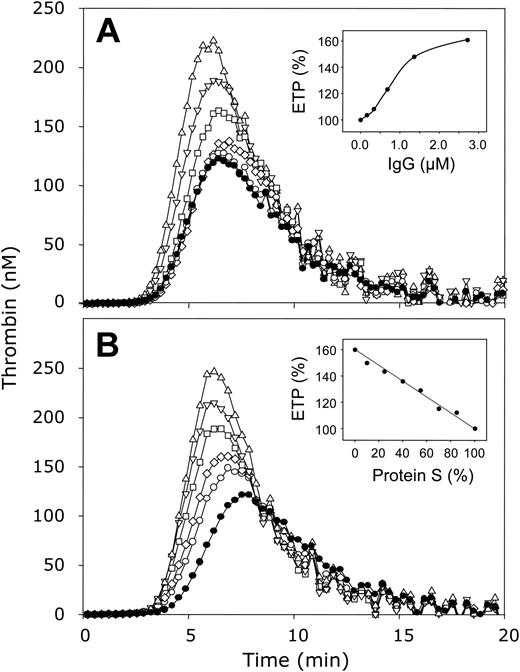
Polyclonal antibodies against protein S were added to normal pooled plasma resulting in final IgG concentrations ranging from 0 μM to 2.73 μM, and the plasma/antibody mixtures were incubated at 37°C for 15 minutes. Coagulation was initiated as described, resulting in final concentrations of 3.5 pM tissue factor and 10 μM phospholipids. A clear increase in thrombin generation was observed at increasing concentrations of antibodies against protein S (Figure 2A). Compared to thrombin generation in normal plasma that did not contain antibodies (ETP = 100%), neutralization of the APC-independent protein S activity in plasma by increasing the antibody concentration gradually increased the ETP to 161% at 2.73 μM IgG (inset, Figure 2A). Concentrations of more than 2.73 μM did not further increase the ETP (data not shown). In addition, the time required to the onset of thrombin generation, which coincides with the clotting of plasma, increased with increasing protein S concentration (Figure 2A).
The effect of varying protein S levels on thrombin generation in plasma in the absence of APC. (A) Normal pooled plasma was incubated with antibodies against protein C and 2.73 μM (▵), 1.37 μM (▿), 0.68 μM (□), 0.34 μM (⋄), 0.17 μM (○), or no (•) antibodies directed against protein S. (A, inset) ETP as function of the concentration antibodies against protein S. (B) Protein S–depleted plasma was mixed with varying amounts of normal pooled plasma resulting in protein S levels of 0% (▵), 40% (▿), 55% (□), 70% (⋄), 85% (○), and 100% (•). (B, inset) ETP as function of the protein S concentration. All plasma mixtures contained antibodies against protein C. Thrombin generation was initiated with 3.5 pM tissue factor, 10 μM phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE), and 16 mM CaCl2 (final concentrations) and quantified with the fluorogenic substrate I-1140 as described in “Materials and methods.”
The effect of varying protein S levels on thrombin generation in plasma in the absence of APC. (A) Normal pooled plasma was incubated with antibodies against protein C and 2.73 μM (▵), 1.37 μM (▿), 0.68 μM (□), 0.34 μM (⋄), 0.17 μM (○), or no (•) antibodies directed against protein S. (A, inset) ETP as function of the concentration antibodies against protein S. (B) Protein S–depleted plasma was mixed with varying amounts of normal pooled plasma resulting in protein S levels of 0% (▵), 40% (▿), 55% (□), 70% (⋄), 85% (○), and 100% (•). (B, inset) ETP as function of the protein S concentration. All plasma mixtures contained antibodies against protein C. Thrombin generation was initiated with 3.5 pM tissue factor, 10 μM phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE), and 16 mM CaCl2 (final concentrations) and quantified with the fluorogenic substrate I-1140 as described in “Materials and methods.”
In a separate experiment, the amount of protein S present in plasma was varied by mixing normal pooled plasma with protein S–depleted plasma. Varying the plasma protein S concentration from 100% to 0% resulted in a protein S–dependent effect on thrombin generation in which the lag time decreased (Figure 2B) and the ETP gradually increased from 100% in normal plasma (100% protein S) to 160% in protein S–depleted plasma (0% protein S) (inset, Figure 2B).
APC-independent activity of protein S depends on the concentration of the procoagulant stimulus
In the previous experiments, coagulation was started with 3.5 pM tissue factor, at which the anticoagulant effect of protein S was clearly visible. To investigate the effect of the strength of the procoagulant stimulus on the inhibition of thrombin generation by protein S, coagulation was initiated with varying concentrations of tissue factor (1.4 pM to 140 pM) at a constant phospholipid concentration (10 μM). In the absence of antibody, that is, in the presence of protein S, a clear dose-dependent effect of the tissue factor concentration on thrombin generation was observed (Figure 3, open symbols). At 140 pM tissue factor, after a short lag time, a large amount of thrombin was formed. Lowering the tissue factor concentration resulted in a dose-dependent increase of the lag time and decrease of both the height of the thrombin generation peak and the ETP.
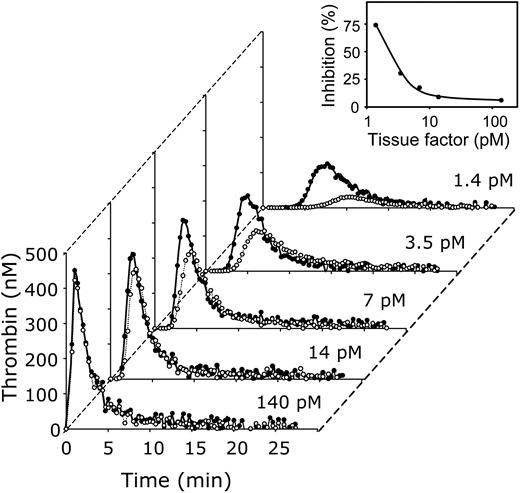
The effect of varying concentrations of tissue factor on thrombin generation in plasma in the absence of APC and presence or absence of protein S. Thrombin generation was initiated in normal pooled plasma with indicated concentrations of tissue factor, 10 μM phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE), and 16 mM CaCl2 (final concentrations) in the presence (•) or absence (○)of antibodies against protein S. Inset: Inhibition of the ETP by protein S as function of the tissue factor concentration. All plasma mixtures contained antibodies against protein C. Thrombin generation was quantified with the fluorogenic substrate I-1140 as described in “Materials and methods.”
The effect of varying concentrations of tissue factor on thrombin generation in plasma in the absence of APC and presence or absence of protein S. Thrombin generation was initiated in normal pooled plasma with indicated concentrations of tissue factor, 10 μM phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE), and 16 mM CaCl2 (final concentrations) in the presence (•) or absence (○)of antibodies against protein S. Inset: Inhibition of the ETP by protein S as function of the tissue factor concentration. All plasma mixtures contained antibodies against protein C. Thrombin generation was quantified with the fluorogenic substrate I-1140 as described in “Materials and methods.”
The effect of protein S on thrombin generation varied greatly with the tissue factor concentration. Neutralization of protein S with antibodies (Figure 3, filled symbols) had virtually no effect on the lag time and the ETP at high tissue factor concentrations (140 pM and 14 pM). At these tissue-factor concentrations protein S inhibited thrombin generation approximately 6%. The effect of protein S became more pronounced when the tissue factor concentration was further decreased. At 7 pM and 3.5 pM tissue factor, protein S neutralization resulted in an increase of the ETP to 121% and 143% of the ETP determined in the absence of antibody, respectively (inset, Figure 3). At the lowest tissue factor concentration (1.4 pM), the presence of protein S antibodies increased the ETP to a vast 380% (that is, protein S inhibits thrombin generation ∼ 74%) and considerably reduced the lag time compared to plasma that did not contain protein S antibodies.
The effect of phospholipid concentration on the APC-independent anticoagulant activity of protein S
Binding of protein S to an anionic lipid surface is essential for the expression of its anticoagulant activity.9,29 Moreover, it has been speculated that the mechanism underlying the APC-independent anticoagulant activity of protein S is competition for phospholipid binding sites.16,17 According to this hypothesis, the inhibitory effect of protein S on thrombin generation should be maximal at low phospholipid concentration and decrease at increasing concentrations of phospholipids.
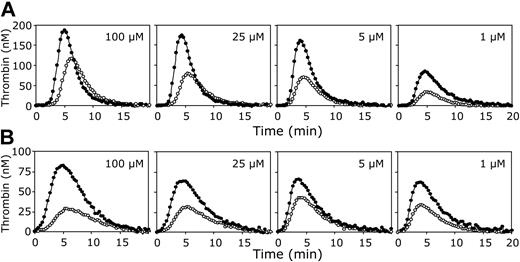
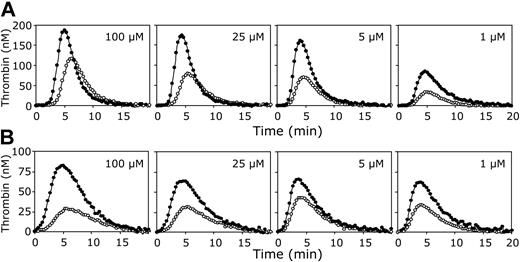
To investigate the effect of the phospholipid concentration on the APC-independent anticoagulant activity of protein S, thrombin generation was measured in normal pooled plasma with and without antibodies against protein S, in which clotting was initiated with a fixed amount of tissue factor (3.5 pM) at varying phospholipid concentrations (1-100 μM). Figure 4A demonstrates that the anticoagulant effect of protein S in the absence of APC was virtually independent of the phospholipid concentration. Calculation of the ETP of plasmas with and without protein S showed no substantial variation in the ability of protein S to inhibit thrombin generation at varying amounts of phospholipids.
The effect of varying phospholipid concentrations on thrombin generation in plasma. Thrombin generation was initiated in normal pooled plasma with 3.5 pM tissue factor (A) or 250 pM factor Xa (B), 16 mM CaCl2, and indicated concentrations of phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE; 100, 25, 5 or 1 μM final concentrations) in the presence (•) or absence (○) of antibodies against protein S. All plasma mixtures contained antibodies against protein C. Thrombin generation was quantified with the fluorogenic substrate I-1140 as described in “Materials and methods.”
The effect of varying phospholipid concentrations on thrombin generation in plasma. Thrombin generation was initiated in normal pooled plasma with 3.5 pM tissue factor (A) or 250 pM factor Xa (B), 16 mM CaCl2, and indicated concentrations of phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE; 100, 25, 5 or 1 μM final concentrations) in the presence (•) or absence (○) of antibodies against protein S. All plasma mixtures contained antibodies against protein C. Thrombin generation was quantified with the fluorogenic substrate I-1140 as described in “Materials and methods.”
The same results were obtained when a lower tissue factor concentration (1.4 pM) was used as procoagulant stimulus. Although the overall thrombin generation was lower and the inhibitory activity of protein S was more pronounced, there was also no influence of the amount of phospholipids on the APC-independent anticoagulant activity of protein S at a low tissue factor concentration (data not shown).
To address the question whether the APC-independent anticoagulant effect of protein S depends on the nature of the procoagulant trigger, tissue factor was replaced by factor Xa to initiate thrombin generation. When 250 pM factor Xa was added to plasma, thrombin formation started immediately, while the amount of thrombin formed was lower compared to the tissue factor–initiated thrombin generation (Figure 4B). Nevertheless, as for tissue factor–initiated coagulation, the ability of protein S to inhibit thrombin formation initiated with factor Xa did not decrease at higher phospholipid concentrations.
Significance of the APC-independent anticoagulant activity of protein S
The experiments presented above demonstrate that in the absence of APC, protein S has a pronounced inhibitory effect on the thrombin generation in plasma at low procoagulant stimuli. However, the relevance of the APC-independent anticoagulant activity of protein S in vivo remains unclear.
Heterozygous (type I) protein S deficiency and the prothrombin G20210A mutation are 2 pathophysiologic conditions that are associated with an increased risk of venous thrombosis. To analyze the effect of these conditions on the APC-independent anticoagulant activity of protein S, their phenotypes were modeled in plasma. Protein S deficiency was modeled by mixing normal pooled plasma with protein S–depleted plasma, resulting in 60% total protein S levels. The increase in prothrombin levels associated with the prothrombin G20210A mutation was mimicked by the addition of purified prothrombin to normal pooled plasma, resulting in a prothrombin level of 140%. Final concentrations of 3.5 pM tissue factor and 10 μM phospholipid vesicles were used to initiate coagulation, and thrombin generation was followed with the fluorogenic substrate I-1140.
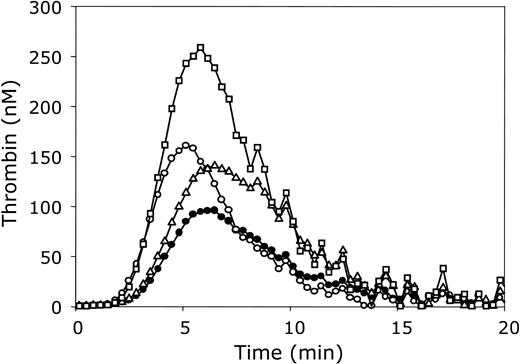
An increase in plasma prothrombin concentration to a level representative for carriers of the prothrombin G20210A mutation increased the ETP to 160% of that of normal plasma (Figure 5). There was no effect on the lag time. Reduction of the protein S concentration to 60% of normal plasma resulted in an increase of the ETP to 130% and in a slight decrease of the lag time (Figure 5). Thrombin generation also was quantified in plasma containing 140% prothrombin and 60% protein S. In this plasma (Figure 5) the ETP was substantially higher (ETP = 240%) than in normal plasma (ETP = 100%) and than in plasma containing 60% protein S (ETP = 130%) or 140% prothrombin (ETP = 160%).
Significance of the APC-independent anticoagulant activity of protein S in plasma. Thrombin generation was initiated with 3.5 pM tissue factor, 10 μM phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE), and 16 mM CaCl2 (final concentrations) in normal pooled plasma (•) and in plasma containing 140% prothrombin (▵), 60% protein S (○), or 140% prothrombin and 60% protein S (□). All plasma mixtures contained antibodies against protein C. Thrombin generation was quantified with the fluorogenic substrate I-1140 as described in “Materials and methods.”
Significance of the APC-independent anticoagulant activity of protein S in plasma. Thrombin generation was initiated with 3.5 pM tissue factor, 10 μM phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE), and 16 mM CaCl2 (final concentrations) in normal pooled plasma (•) and in plasma containing 140% prothrombin (▵), 60% protein S (○), or 140% prothrombin and 60% protein S (□). All plasma mixtures contained antibodies against protein C. Thrombin generation was quantified with the fluorogenic substrate I-1140 as described in “Materials and methods.”
APC-independent versus APC-cofactor activity of protein S
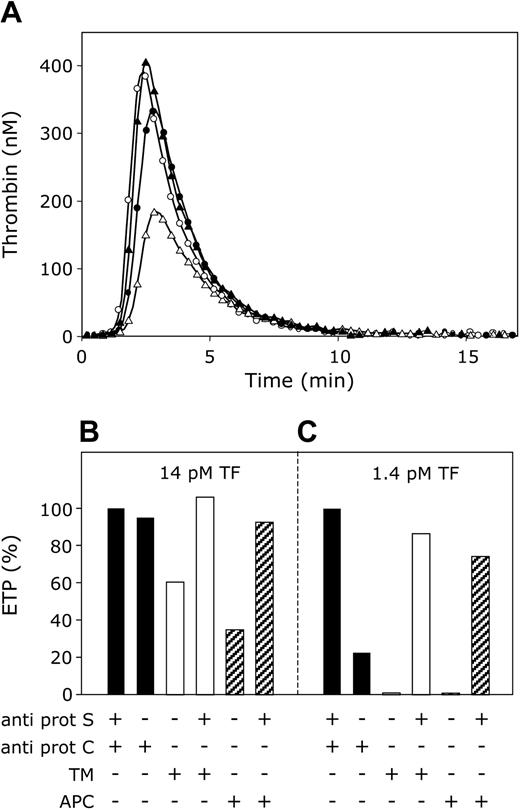
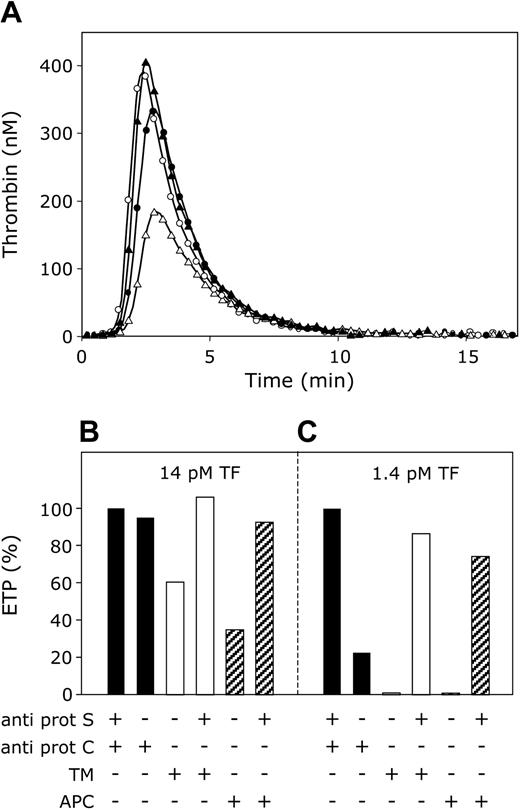
In the absence of APC, protein S inhibited thrombin generation most effectively when the procoagulant stimulus was low ([tissue factor] = 1.4 pM), whereas at high tissue factor concentrations (14 pM) the ability of protein S to inhibit the ETP in the absence of APC became minimal. These findings prompted us to compare the APC-independent and APC-cofactor activities of protein S by measuring the effect of protein S on thrombin generation initiated in plasma with 1.4 pM and 14 pM tissue factor in the absence and presence of APC or thrombomodulin. When thrombin formation was initiated with 14 pM tissue factor in plasma in which both protein S and protein C were neutralized with antibodies, a maximal ETP was obtained (Figure 6A, open circles). Omission of antibodies against protein S resulted in a small decrease in the ETP (Figure 6A, filled circles), representing the low anticoagulant activity of protein S in the absence of APC under these conditions. Subsequently, antibodies against protein C also were omitted, and activation of plasma protein C during thrombin generation was promoted by the addition of thrombomodulin to plasma (40 nM final concentration). As a result, the ETP was significantly reduced to 60% of its original value (Figure 6A, open triangles). This reduction of the ETP could almost exclusively be attributed to the APC-cofactor activity of protein S, as the inhibitory effect of in situ–activated protein C on the ETP was completely abolished by the addition of anti–protein S antibodies (Figure 6A, filled triangles). The quantitative aspects of the effects of protein S and thrombomodulin (APC) on the ETP are summarized in Figure 6B. This bar diagram also depicts the effect of APC added to plasma. Like thrombomodulin, APC considerably inhibits thrombin generation in a protein S–dependent manner.
APC-cofactor versus APC-independent anticoagulant activity of protein S. (A) Thrombin generation was initiated with 14 pM tissue factor, 10 μM phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE), and 16 mM CaCl2 (final concentrations) in normal pooled plasma with anti–protein S and anti–protein C antibodies (○), anti–protein C antibodies (•), 40 nM thrombomodulin (▵), 40 nM thrombomodulin, and anti–protein S antibodies (▴). (B,C) Endogenous thrombin potentials (ETP) calculated from time courses of thrombin generation initiated with either 14 pM tissue factor (B) or 1.4 pM tissue factor (C). ETP were determined in plasma without added APC or thrombomodulin (▪), addition of 40 nM thrombomodulin (□) or 7.5 nM APC (▨), and presented in percentage of ETP in plasma with antibodies against protein S and protein C.
APC-cofactor versus APC-independent anticoagulant activity of protein S. (A) Thrombin generation was initiated with 14 pM tissue factor, 10 μM phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE), and 16 mM CaCl2 (final concentrations) in normal pooled plasma with anti–protein S and anti–protein C antibodies (○), anti–protein C antibodies (•), 40 nM thrombomodulin (▵), 40 nM thrombomodulin, and anti–protein S antibodies (▴). (B,C) Endogenous thrombin potentials (ETP) calculated from time courses of thrombin generation initiated with either 14 pM tissue factor (B) or 1.4 pM tissue factor (C). ETP were determined in plasma without added APC or thrombomodulin (▪), addition of 40 nM thrombomodulin (□) or 7.5 nM APC (▨), and presented in percentage of ETP in plasma with antibodies against protein S and protein C.
This experiment also was performed at 1.4 pM tissue factor (Figure 6C). At this tissue factor concentration the APC-independent anticoagulant activity of protein S was very pronounced, and in the absence of APC (that is, in the presence of protein C antibodies), protein S inhibited thrombin generation by 80%. Addition of thrombomodulin or APC resulted in an extra inhibition of thrombin generation to higher than 95%. Under these conditions, anti–protein S antibodies abrogated the inhibition of the ETP by the APC-independent as well as the APC-cofactor activity of protein S (Figure 6C).
APC-cofactor activity of protein S in plasma
Interestingly, the presence of anti–protein S antibodies almost completely abolished the anticoagulant activity of APC. An experiment was performed to quantify the contribution of protein S in the anticoagulant protein C pathway in plasma. The effect of APC on thrombin generation was measured in plasma in the presence or absence of protein S (Figure 7). APC dose dependently inhibited the ETP in the presence of protein S but became a poor inhibitor in the absence of protein S. Linear fits through the initial parts of the curves indicated that protein S enhances the anticoagulant activity of APC in plasma 21.5-fold.
APC-cofactor activity of protein S. ETP calculated from thrombin generation curves initiated with 14 pM tissue factor, 10 μM phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE), 16 mM CaCl2, and indicated concentrations of APC (final concentrations), in normal pooled plasma with (○) or without (•) anti–protein S antibodies. ETP are presented as percentages of the ETP determined in plasma without added APC.
APC-cofactor activity of protein S. ETP calculated from thrombin generation curves initiated with 14 pM tissue factor, 10 μM phospholipid vesicles (20/60/20 DOPS/DOPC/DOPE), 16 mM CaCl2, and indicated concentrations of APC (final concentrations), in normal pooled plasma with (○) or without (•) anti–protein S antibodies. ETP are presented as percentages of the ETP determined in plasma without added APC.
Discussion
In this study we quantified the APC-independent anticoagulant activity of protein S in its natural environment, in plasma. Although it was previously recognized that protein S prolongs the clotting of plasma in the absence of APC, clotting times do not offer information about the entire process of thrombin formation. We therefore studied the influence of protein S on thrombin generation in plasma, which provides insight on the effect of protein S on the initiation, propagation, and termination of thrombin formation. To investigate the anticoagulant activity of protein S in the absence of APC, we quantified thrombin generation in plasma with or without functional protein S by determining the ETP. Protein S present in plasma was functionally inhibited by addition of polyclonal antibodies against protein S. To rule out a contribution of APC, all experiments were performed in plasma to which a polyclonal antibody against (activated) protein C was added.
In the absence of APC, plasma protein S inhibited thrombin generation in a dose-dependent manner, independent of the phospholipid concentration. However, the extent of inhibition varied considerably with the amount of tissue factor used for initiation of coagulation. When tissue factor concentrations decreased, the inhibitory potential of protein S increased more than 10-fold. Apparently, in the absence of APC, protein S controls coagulation efficiently when thrombin generation is limited. When the procoagulant stimulus is higher, the action of protein S could be overruled by the procoagulant driving force, and without APC, protein S is unable anymore to down-regulate thrombin generation. A possible explanation for this phenomenon might be that at high tissue factor concentrations large amounts of factor Xa are formed that will result in high concentrations of prothrombinase complexes. As a consequence, prothrombin activation will become diffusion limited,30 a condition at which inhibitors work less efficiently than in the case of an enzyme-controlled reaction.
Although at high tissue factor concentrations virtually no APC-independent anticoagulant activity of protein S was observed, protein S still expressed APC-cofactor activity under these conditions, since in situ–generated or externally added APC effectively inhibited the endogenous thrombin potential in a protein S–dependent manner.
There is as yet no consensus about how protein S exerts its APC-independent anticoagulant activity. Several studies indicated that protein S may directly inhibit prothrombin activation via interactions with the protein components of the prothrombinase complex.8-10 However, it also was proposed that the APC-independent anticoagulant activity of protein S is due to occupation of phospholipid binding sites,16,17 thereby preventing the assembly of the prothrombinase complex and the interaction with its substrate, prothrombin. The latter model may explain why purified protein S is very active at low concentrations of phospholipids and has reduced ability to inhibit prothrombin activation at high concentrations of phospholipids. It should be emphasized, however, that the majority of the studies in which the APC-independent anticoagulant activity of protein S was investigated were performed with purified protein S preparations. In a previous report, we have shown that purified protein S preparations contain a small fraction of multimers that bind with a very high affinity (Kd < 1 nM) to anionic phospholipids and which effectively inhibit prothrombin activation at low phospholipid concentrations.18 Multimer-free protein S binds with much lower affinity (Kd = 250 nM) to phospholipids and inhibits prothrombin activation also at higher phospholipid concentrations.
As protein S multimers are absent in plasma,18 we studied the native form of protein S in our experiments. The observation that the APC-independent anticoagulant activity of protein S in plasma is not influenced by variation of the phospholipid concentration suggests that the inhibition of thrombin formation is not due to occupation of phospholipid binding sites but involves direct interactions of protein S with components of the prothrombinase complex. The observation that the monoclonal antibody CLB-PS13, which is directed against the Gla-domain of protein S, abrogates the direct anticoagulant activity of protein S in plasma indicates that protein S has to bind to phospholipid in order to interact with protein components of the prothrombinase complex and to inhibit prothrombin activation.
Currently, thrombosis is considered a typical multifactorial disease in that combinations of separate risk factors most often will lead to thrombotic complications. We have developed a plasma model in which we were able to study the effect of a combination of heterozygous protein S deficiency and carriership of the prothrombin G20210A mutation on thrombin generation. In the present study, its phenotype was modeled by supplementing plasma containing either 60% or 100% protein S with prothrombin to a final plasma level of 140%. In addition to the individual procoagulant effects of heterozygous protein S deficiency and the prothrombin G20210A mutation, which cause increases of the ETP by 30% and 60%, respectively, we demonstrated that a combination of these risk factors has a synergistic effect on thrombin formation. The large increase of the ETP (140%) in plasma containing the combined defects indicates that impairment of protein S function in the absence of activated protein C particularly comes to expression at high prothrombin levels.
During homeostasis, the hemostatic system is in balance. It has been reported that tissue factor circulates at low concentration in blood,31 providing a constant procoagulant stimulus. Intravascular clotting caused by these small amounts of tissue factor is an unwanted phenomenon that has to be controlled by the anticoagulant system. On the basis of our observations, we propose that protein S plays a major role in maintaining the hemostatic balance by inhibiting thrombin generation independent of APC at low tissue factor stimuli. When a more severe disturbance of the hemostatic balance occurs, the APC-cofactor activity of protein S becomes important in the down-regulation of coagulation. Strikingly, the present data show that the anticoagulant effect of APC is over 95% dependent on the presence of protein S. Hence, native multimer-free protein S effectively regulates thrombin formation at 2 levels: independent of APC at small disturbance of the hemostatic balance when insufficient amounts of APC are generated, and as essential cofactor of APC at stronger procoagulant forces. Failure to restore the hemostatic balance at low procoagulant stimuli might add to the development of thrombotic disease, and therefore, an impaired APC-independent anticoagulant activity of protein S may contribute to the increased thrombotic risk associated with heterozygous protein S deficiency.19,20
Prepublished online as Blood First Edition Paper, August 3, 2004; DOI 10.1182/blood-2004-03-1146.
Supported in part by a fellowship from The Royal Netherlands Academy of Arts and Sciences (KNAW) (T.M.H.) and by research grants from the Netherlands Organisation for Scientific Research (NWO) (VIDI 917.36.372 to T.M.H).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.