Abstract
SHP-2 is a protein tyrosine phosphatase functioning as signal transducer downstream to growth factor and cytokine receptors. SHP-2 is required during development, and germline mutations in PTPN11, the gene encoding SHP-2, cause Noonan syndrome. SHP-2 plays a crucial role in hematopoietic cell development. We recently demonstrated that somatic PTPN11 mutations are the most frequent lesion in juvenile myelomonocytic leukemia and are observed in a smaller percentage of children with other myeloid malignancies. Here, we report that PTPN11 lesions occur in childhood acute lymphoblastic leukemia (ALL). Mutations were observed in 23 of 317 B-cell precursor ALL cases, but not among 44 children with T-lineage ALL. In the former, lesions prevalently occurred in TEL-AML1- cases with CD19+/CD10+/cyIgM- immunophenotype. PTPN11, NRAS, and KRAS2 mutations were largely mutually exclusive and accounted for one third of common ALL cases. We also show that, among 69 children with acute myeloid leukemia, PTPN11 mutations occurred in 4 of 12 cases with acute monocytic leukemia (FAB-M5). Leukemia-associated PTPN11 mutations were missense and were predicted to result in SHP-2 gain-of-function. Our findings provide evidence for a wider role of PTPN11 lesions in leukemogenesis, but also suggest a lineage-related and differentiation stage-related contribution of these lesions to clonal expansion. (Blood. 2004;104:307-313)
Introduction
SHP-2 is a cytoplasmic Src homology-2 (SH2) domain containing protein tyrosine phosphatase that plays a key role in intracellular signaling elicited by a number of growth factors, hormones, and cytokines.1,2 The accumulated data provide evidence that SHP-2 positively modulates the signal flow in most circumstances, even though it can also function as negative regulator depending on its binding partner and interactions with downstream signaling networks. Specifically, SHP-2 positively controls RAS function and is required for sustained activation of the mitogen-activated protein kinase (MAPK) cascade induced by several growth factors and cytokines.3-6 SHP-2 is widely expressed in both embryonic and adult tissues and is required in several developmental processes, including gastrulation and mesodermal patterning,7,8 development of terminal and skeletal structures,9,10 semilunar valvulogenesis,11 and hematopoiesis.9,12-14
Recently, we identified germline mutations in PTPN11, the gene encoding SHP-2, as major causative events in Noonan syndrome (NS),15,16 a disorder characterized by short stature, dysmorphic face, congenital heart disease, and skeletal anomalies.17 Children with NS are also predisposed to a spectrum of hematologic abnormalities and malignancies, including juvenile myelomonocytic leukemia (JMML),18-20 acute lymphoblastic leukemia (ALL),21-23 and acute myeloid leukemia (AML).23 Consistent with the crucial role of SHP-2 in RAS signaling and the higher prevalence of myeloproliferative disorders in infants and children with NS, we provided evidence that somatic PTPN11 mutations represent the most frequent molecular lesion in JMML.24 Interestingly, PTPN11 missense defects were also identified in a smaller percentage of children with myelodysplastic syndromes (MDSs) and de novo AML. A similar incidence and distribution of PTPN11 mutations in JMML and other myeloid malignancies was recently reported.25 Molecular modeling and functional data support a gain-of-function role of these mutations on SHP-2 catalytic activity.24
Because of the higher prevalence of ALL in children with NS and the key role of SHP-2 in lymphoid progenitor cell commitment and differentiation, we hypothesized a wider role of SHP-2 gain-of-function in leukemogenesis and considered PTPN11 as an excellent candidate gene that might be mutated in ALL. Here, we show that acquired missense mutations in PTPN11 represent a recurrent event in B-cell precursor ALL, are prevalently observed in children with the CD19+/CD10+/cyIgM- immunophenotype, frequently occur among patients with hyperdiploid DNA content, and are negatively associated with both TEL-AML1 gene rearrangements and oncogenic RAS mutations. We also report that, among children with acute myeloid leukemia, PTPN11 mutations are frequently found in children with acute monocytic leukemia. Our findings provide evidence for a wider role of PTPN11 lesions in leukemogenesis and suggest that the contribution of these mutations to expansion of the leukemic clone depends on stage of differentiation and lineage of the precursor cell.
Patients, materials, and methods
Patients
Children and adolescents with ALL (n = 362) and de novo AML (n = 69) were included in the study. Informed consent was obtained for each patient of the 2 cohorts. Approval for this study was obtained from the Istituto Superiore di Sanità, Fondazione Tettamanti and Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) review boards.
Between January 2001 and September 2002, 568 patients, aged 1 to 18 years, with ALL had been consecutively enrolled in the ongoing AIEOPBFM ALL 2000 study. Diagnosis was established according to standard morphologic, cytochemical, and immunologic criteria26 and centrally reviewed. According to surface/cytoplasmic antigen expression, B-cell precursor ALL was classified as pro-B ALL (CD19+, CD10-, CD20-, cyIgM-), common ALL (CD19+, CD10+, cyIgM-), pre-B (CD19+, CD10+/-, cyIgM+), or pre-B/B (CD19+, CD10+/-, cyIgM+, smIgM+, IgMκλ-). One case exhibited a bilineage leukemic condition with a mixed population expressing CD10+/CD33- (65% of cells) or CD10-/CD33+ (35% of cells). DNA samples were available in the diagnostic reference laboratory for 362 patients (64%). No statistically significant differences in clinical and laboratory features were observed between patients included or not in the study (data not shown).
Frozen material from 69 (52.7%) of 135 de novo AML cases, diagnosed in a single AIEOP institution since 1981, was available for the study. Diagnosis was established by standard morphologic, cytochemical, and immunologic criteria. According to the French-American-British (FAB) classification, patients were classified as M0 (n = 1, 1.4%), M1 (n = 15, 21.7%), M2 (n = 18, 26.1%), M3 (n = 11, 16.0%), M4 (n = 8, 11.6%), M5 (n = 12, 17.4%), M6 (n = 1, 1.4%), and M7 (n = 2, 2.9%); in 1 case the FAB subtype was unknown. Karyotype information was available for 60 patients (87%). Chromosomal aberrations characteristic for de novo AML, that is, t(8;21), t(15;17), inv(16), as well as other complex abnormalities were documented. Median age was 6.3 years (range, 0.2-17.6 years), 45 were boys (65.2%) and 24 were girls (34.8%). Median white blood cell (WBC) count was 26 × 109/L (range, 0.8-296 × 109/L).
Molecular analyses
DNA sample acquisition. Bone marrow aspirates were obtained at diagnosis, prior to therapy, as well as during the follow-up. Mononuclear cells were separated from aspirated bone marrow samples using a Ficoll gradient, and gDNA was isolated from lysates of these cells using a standard protocol.
Mutation analysis. The entire PTPN11 coding region (exons 1-15 and flanking intronic stretches) was screened for mutations. Polymerase chain reactions (PCRs) to amplify exons 2 to 15 were carried out as previously described16 ; exon 1 was amplified in 25 μL reaction volume containing 50 ng gDNA, 1 U AmpliTaq Gold (Applied Biosystems, Foster City, CA), 20 pmol each primer (MWG-Biotech, Ebersberg, Germany), 1.5 mM MgCl2, 10% dimethyl sulfoxide (DMSO), and 75 μM each dNTP and 1 × PCR Buffer II (Applied Biosystems), using primer pairs PTPN11-1sF, 5′-CGGAGCCTGAGCAAGGAGCG-3′; PTPN11-1sR, 5′-CGAGGGGACGAGGAGGGAACC-3′, and the following cycling parameters: 94°C, 8 minutes (first denaturing step); 94°C, 45 seconds; 60°C, 30 seconds; 72°C, 45 seconds; 33 cycles; 72°C, 15 minutes (last extension step). Mutational analysis was also performed on exons 1 and 2 of the NRAS and KRAS2 genes (primer sequences and PCR conditions are available on request). Unpurified PCR products were analyzed by denaturing high-performance liquid chromatography (DHPLC), using the Wave DNA Fragment Analysis System (Transgenomics, Omaha, NE) at column temperatures recommended by the WaveMaker version 4.1.31 software (Transgenomics). Heterozygous templates with previously identified mutations or synthetic templates containing heterozygous exonic single nucleotide changes were used as positive controls for all exons. Amplimers having abnormal denaturing profiles were purified (Microcon PCR; Millipore, Bedford, MA) and sequenced bidirectionally using the ABI BigDye Terminator Sequencing Kit version 3.1 (Applied Biosystems) and an ABI Prism 310 Genetic Analyzer (Applied Biosystems). Sequencing results were analyzed using the Sequencing Analysis version 3.6.1 and AutoAssembler version 2.1 software packages (both from Applied Biosystems).
RT-PCR assay. RNA was purified from bone marrow mononuclear cells by standard phenol-chloroform extraction method. Reverse transcription-PCR (RT-PCR) was performed as previously described.27 All samples were analyzed by single-step PCR for the presence of the MLL-AF4, BCR-ABL and TEL-AML1 fusion transcripts; in addition, PTPN11 mutated cases were also analyzed for the presence of the E2A-PBX1 fusion gene product. Amplification of the housekeeping ABL gene transcript was performed in all samples to guarantee for good quality cDNA synthesis. After amplification, 10 μL PCR products were run on a 2.5% agarose gel stained with ethidium bromide and visualized under a UV lamp.
Cytogenetic, FISH, and DNA index analyses. Cytogenetic analysis was performed on leukemic bone marrow mononuclear cells methanolacetic acid fixed chromosome preparations by standard QFQ-banding. Rearrangement of the MLL gene was investigated by fluorescence in situ hybridization (FISH) analysis from methanol-acetic acid-fixed interphase nuclei, by using the “Dual color, break apart MLL probe” (Vysis, Downers Grove, IL), covering the MLL locus on chromosome 11q23. DNA index was calculated according to the guidelines provided by the Committee on Nomenclature of the Society for Analytical Cytology.28
Statistical analyses
Descriptive analyses were conducted to examine the distribution of variables of interests (age at diagnosis, gender, immunophenotype, presence/absence of gene mutations or rearrangements, DNA index). The Pearson χ2 test was used to evaluate statistical significance (at 95% level) of differences in proportions among groups; the Fisher exact test (2-tailed P values) was alternatively adopted when an expected cell value in a contingency table was less than 5. Exact confidence intervals of proportions (at 95% level) were calculated based on binomial distribution. Mean values of continuous variables were compared among groups by means of the nonparametric Kruskal-Wallis rank test. Unconditional logistic regression models were adopted to investigate the relationship between frequency of PTPN11 mutations and covariates of interest. All analyses were carried out with the STATA statistical package release 7.0 (Stata, College Station, TX).
Results
PTPN11 mutations in childhood ALL
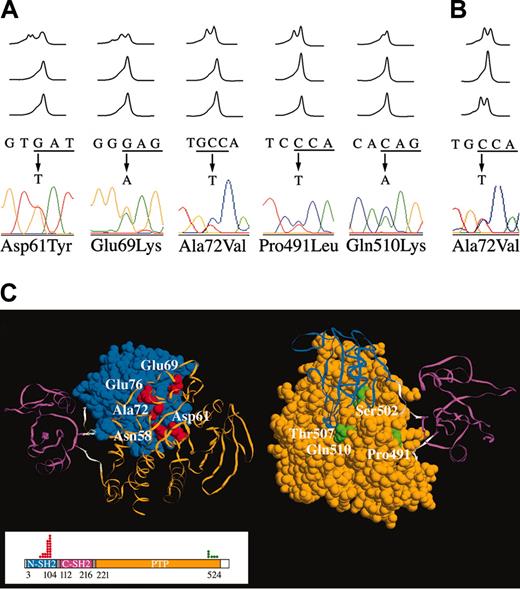
We investigated the prevalence of PTPN11 mutations in bone marrow mononuclear cells from a cohort of 362 children and adolescents with ALL using DHPLC. PTPN11 defects were identified in 23 (7.3%) of 317 children with B-cell precursor ALL (Table 1), whereas no mutation was observed in the T-lineage ALL cohort (44 cases). Mutation analysis of DNAs from bone marrow samples obtained during disease remission demonstrated absence of the mutated allele in all cases (Figure 1A), providing evidence that all mutations were somatic events acquired in the leukemic cells. Consistently, none of these defects was observed among more than 200 control individuals.15,16 All mutations were missense changes, 18 affecting exon 3 and 5 residing in exon 13. Among them, the 181G>T (Asp61Tyr), 182A>T (Asp61Val), 205G>A (Glu69Lys), 214G>A (Ala72Thr), 215C>T (Ala72Val), 226G>A (Glu76Lys), and 227A>G (Glu76Gly) changes had been previously reported in children with JMML.24,25 Several lesions were found recurrently, and mutations affecting residues Asp61, Glu69, Ala72, Glu76, and Pro491 accounted for 87% of all defects. As previously observed in JMML, codon 76 represented a mutational hot spot (35% of total mutations), with 3 different amino acid substitutions predicted among 8 individuals. All mutations affected amino acid residues located in the N-SH2 and PTP functional domains. Specifically, all mutated residues except Pro491 clustered in regions of these domains that are involved in the N-SH2/PTP intramolecular interaction that normally stabilizes SHP-2 in its catalytically inactive conformation (Figure 1C). According to the protein crystallographic structure,29 Pro491 is spatially far from the N-SH2/PTP interaction surfaces, residing on the PTP surface exposed toward the linker stretch connecting the C-SH2 domain to the PTP domain (residues 217-223).
Somatic PTPN11 mutations in childhood acute leukemia. (A) Representative DHPLC profiles showing the occurrence of missense mutations in 5 children with B-cell precursor ALL; in all cases, mutations were observed at diagnosis (top), but were undetectable during remission, after 33 (middle) and 78 (bottom) days of follow-up. Corresponding nucleotide changes and electropherograms are also shown. (B) DHPLC profiles, and corresponding nucleotide change and electropherogram, showing the 215C>T substitution in one case with FAB-M5a identified at diagnosis (top) and relapse (bottom), but not during remission (middle). (C) Location of SHP-2 mutated residues in childhood acute leukemia. Exposed surface or Cα trace of N-SH2 (blue), C-SH2 (magenta), and PTP (orange) domains, and N-SH2/CSH2 and C-SH2/PTP linkers (gray) of the catalytically inactive conformation of SHP-2.29 The views are rotated to show the interdomain interacting surfaces and the exposed residues of the N-SH2 (left) and PTP (right) surfaces. Mutated N-SH2 residues (Asn58, Asp61, Glu69, Ala72, and Glu76) are indicated in red; mutated PTP residues (Pro491, Ser502, Thr507, and Gln510) are indicated in green. SHP-2 domain organization is shown in the boxed area. The numbers below the domain structure indicate the amino acid boundaries of those domains. Dots above the domain structure refer to number of cases with mutations documented in this study.
Somatic PTPN11 mutations in childhood acute leukemia. (A) Representative DHPLC profiles showing the occurrence of missense mutations in 5 children with B-cell precursor ALL; in all cases, mutations were observed at diagnosis (top), but were undetectable during remission, after 33 (middle) and 78 (bottom) days of follow-up. Corresponding nucleotide changes and electropherograms are also shown. (B) DHPLC profiles, and corresponding nucleotide change and electropherogram, showing the 215C>T substitution in one case with FAB-M5a identified at diagnosis (top) and relapse (bottom), but not during remission (middle). (C) Location of SHP-2 mutated residues in childhood acute leukemia. Exposed surface or Cα trace of N-SH2 (blue), C-SH2 (magenta), and PTP (orange) domains, and N-SH2/CSH2 and C-SH2/PTP linkers (gray) of the catalytically inactive conformation of SHP-2.29 The views are rotated to show the interdomain interacting surfaces and the exposed residues of the N-SH2 (left) and PTP (right) surfaces. Mutated N-SH2 residues (Asn58, Asp61, Glu69, Ala72, and Glu76) are indicated in red; mutated PTP residues (Pro491, Ser502, Thr507, and Gln510) are indicated in green. SHP-2 domain organization is shown in the boxed area. The numbers below the domain structure indicate the amino acid boundaries of those domains. Dots above the domain structure refer to number of cases with mutations documented in this study.
PTPN11 defects were not randomly distributed in the ALL cohort (P = .019) because the lesions occurred prevalently among patients with the common (CD19+/CD10+/cyIgM-) immunophenotype, where they accounted for 10.6% of cases (95% CI, 6.6%-16.0%). Three mutations occurred in children with pre-B ALL (2.7% of cases; 95% CI, 0.6%-7.6%). One of the common ALL cases with mutated PTPN11 showed CD10 antigen expression only in a subset of cells and expression of the CD33 myeloid antigen in a different subset of cells, indicating a mixed population.
Because gene rearrangements and other chromosomal abnormalities account for a relatively large portion of childhood ALL cases, major chromosomal translocations were systematically investigated in all patients carrying a mutated PTPN11 gene. We did not observe the E2A-PBX1, BCR-ABL, and MLL-AF4 gene rearrangements among children carrying a mutated PTPN11 gene (Table S1; see the Supplemental Materials link at the top of the online article on the Blood website). Significantly, none of cases exhibited the TEL-AML1 fusion gene. That karyotypically cryptic gene rearrangement represents the most recurrent lesion in children with B-lineage ALL and was observed in 25% of common ALL cases in our series (Table 2). This mutually exclusive distribution was statistically significant (P = .007). Because the TEL-AML1 gene rearrangement has been documented to be strongly associated with nonhyperdiploid DNA content30 (present study; Figure S1), we compared the prevalence of PTPN11 mutations in patients with and without hyperdiploidy (Table 2). A higher prevalence of mutations in children and adolescents with hyperdiploid DNA content was observed. This association was statistically significant within both the B-cell precursor ALL (14 of 102 versus 8 of 206; P = .004) and common ALL (11 of 66 versus 8 of 114; P = .042) cohorts.
Analysis of distribution of PTPN11 mutations by age at diagnosis and gender did not reveal any statistically significant differences either among patients with B-cell precursor ALL or those with common ALL (Table S1). However, a statistically significant higher prevalence of PTPN11 mutations in patients with age at diagnosis older than 15 years was observed compared with younger patients, within the B-cell precursor ALL cohort (4 of 12 versus 19 of 205, P = .007, Fisher exact test; Figure S1). Moreover, results from unconditional logistic regression analysis highlighted that PTPN11 mutations were significantly associated with both hyperdiploid DNA content and age at diagnosis among common ALL cases. Specifically, the probability of a PTPN11 mutation was approximately 3-fold and 5-fold higher in patients, respectively, with hyperdiploid DNA content (odds ratio [OR] = 2.80; 95% CI, 1.04-7.53) and age at diagnosis older than 15 years (OR = 5.38; 95% CI, 1.17-24.78), taking age at diagnosis younger than 15 years and diploidy as reference categories.
Deregulated RAS signaling in childhood ALL
Increasing evidence supports the idea that SHP-2 positively controls cell proliferation and survival of hematopoietic cells by acting as positive modulator of RAS function.31-33 Consequently, we investigated the cumulative prevalence of up-regulated RAS signaling in 166 common ALL cases by performing mutational screening of exons 1 and 2 of the NRAS and KRAS2 genes (Table 1). Sixteen mutations affecting codons 12, 13, and 61 of NRAS were observed (Table S2), accounting for 9.6% of cases (95% CI, 5.6%-15.2%). Mutations affecting exon 1 of KRAS2 were identified in 24 cases (14.5%; 95% CI, 9.5%-20.7%). Among the 23 cases with mutated PTPN11 gene, only one showed concomitant lesions in the NRAS or KRAS2 genes (Table 2). In that case, leukemic cells were not available to evaluate if mutations were present in independent subclones. These results documented that, among children with common ALL, PTPN11, NRAS, and KRAS2 gene mutations account for approximately 45% of TEL-AML1- cases.
To investigate the distribution and prevalence of oncogenic NRAS and KRAS2 lesions in the other ALL subtypes included in the study, children with pro-B (n = 9), pre-B (n = 99), and T-cell (n = 34) ALL were also screened. Consistent with available molecular evidence,34-36 mutations were observed in all the cohorts, with a combined prevalence of 9% (95% CI, 1.9%-23.7%) among the T-ALL cases and 11% (95% CI, 0.3%-48.2%) and 23% (95% CI, 15.3%-32.8%) among the pro-B and pre-B cases, respectively (Table 1 and Table S2). The distributions of PTPN11 and RAS gene mutations differed significantly when compared among all the different ALL subtypes (P = .012) as well as among B-cell precursor ALL immunophenotypes (P = .026). These results support that the contribution of RAS mutations to leukemogenesis is not restricted to specific subtypes, a notable difference from what was observed for PTPN11 mutations.
Multiple lesions affecting codons 12 and 13 of the NRAS and KRAS2 genes were observed in 5 children (Table S2). Leukemic cells were not available to evaluate if lesions were present in distinct cell subpopulations. DHPLC profiles and electropherograms, however, indicated that these mutations might be present only in a fraction of leukemic cells, suggesting that these lesions do not represent primary events during leukemogenesis but are acquired during progression of disease.
NRAS mutations were found to be largely mutually exclusive with the TEL-AML1 gene rearrangement (χ2 = 5.95, P = .015), whereas no association was observed between KRAS2 and TEL-AML1 (χ2 = 0.003; P = .957). No association between RAS gene mutations and age at diagnosis was observed, whereas, as observed for PTPN11 mutations, RAS defects had significant higher prevalence among cases with hyperdiploid DNA content (Figure S1).
PTPN11 mutations in childhood AML
Sixty-nine children with de novo AML were included in the study to investigate prevalence, spectrum, and distribution of PTPN11 lesions in this heterogeneous group of myeloid malignancies. Somatic PTPN11 lesions were identified in 4 children (6% of cases; 95% CI, 1.6%-14.2%; Table 1), confirming the relatively low prevalence of this class of molecular lesions we previously documented.24 All mutations were missense (Table 3) and affected residues located in the interacting surfaces of the N-SH2 and PTP domains. Two amino acid substitutions (Ala72Val and Glu76Lys) have been observed previously in JMML and childhood MDSs,24,25 as well as in the present ALL cohort. A novel change (1520C>A), resulting in the substitution of residue Thr507 by lysine was identified in one case. Bone marrow specimens at remission were available in 3 cases and showed absence of the mutated allele. A subsequent relapse occurred in one child, and the presence of the mutated allele was also documented (Figure 1B).
Significantly, all cases carrying a mutated PTPN11 gene exhibited acute monocytic leukemia (FAB-M5 subtype). These results combined with those from our previous study24 indicate that PTPN11 is frequently mutated in children within this leukemic condition (5 of 20; eg, 25% of cases; 95% CI, 8.7%-49.1%). Cytogenetic data were available for 2 of the 4 cases. One patient exhibited the complex karyotype 46,XX, invdup(1)(q31), t(2;17)(q14;q24), t(2;19)(p22;q13); mosaicism for t(2;10)(q36;q22) was observed in the second case. FISH analysis carried out on 3 of the 4 cases showed rearrangement of the MLL gene (data not shown).
The distribution of NRAS and KRAS2 gene mutations in the AML cohort was also investigated. gDNA was available for 49 cases, and mutational screening identified NRAS lesions in 5 (10%; 95% CI, 3.4%-22.2%). Mutations occurred in children with different AML subtypes, including 2 with FAB-M1, 2 with FAB-M2, and 1 with FAB-M4 (Table S2). No NRAS mutation was identified among the 4 cases with mutated PTPN11.
Discussion
In the present study, we documented that somatic missense mutations in PTPN11 represent a recurrent molecular event in childhood acute leukemia. Specifically, we provided first evidence that PTPN11 is mutated in B-cell precursor ALL, particularly among children with common ALL, where these lesions accounted for 11% of cases. Our data also suggest that, as observed in myeloid malignancies, up-regulated RAS signaling, due to mutations in RAS genes or in genes coding for proteins controlling RAS function, represent a major pathway driving the aberrant growth of malignant B-cell precursors. Finally, we confirmed and further extended our previous observations on the relevance of PTPN11 lesions in pediatric AML by documenting that these defects frequently occur in children with acute monocytic leukemia, where they account for approximately one fourth of cases.
Several lines of evidence support the notion that PTPN11 mutations represent events that contribute to leukemogenesis. First, molecular analysis of bone marrow specimens documented that mutations were observed at disease presentation, but were undetectable at remission, supporting the presence of the mutated gene in the leukemic clone. Consistently, in previous studies15,16 we did not observe any of these mutations in more than 200 control individuals. Second, the spectrum and distribution of mutations indicated that these lesions are not random, but affect specific domains of the protein and are predicted to promote SHP-2 gain-of-function. Third, among children with ALL, PTPN11 defects were negatively associated with major gene rearrangements (TEL-AML1, E2A-PBX1, BCR-ABL, and AF4-MLL), and other gene lesions (NRAS and KRAS2). Fourth, PTPN11 mutations appeared to be associated preferentially with certain acute leukemia subtypes, suggesting a specific role in disease pathogenesis. Finally, we documented the same mutation at initial diagnosis and relapse in the only relapsing case with a PTPN11 lesion and serial samples in our series.
SHP-2 contains 2 tandemly arranged amino-terminal SH2 domains (N-SH2 and C-SH2), a single catalytic (PTP) domain, and a carboxy-terminal tail (Figure 1C). Crystallographic data indicate that SHP-2 is basally inactive with an autoinhibited conformation because of the intramolecular interaction between the N-SH2 and PTP domains.29 Catalytic activation requires disruption of such interaction, which is promoted by the conformational change of the N-SH2 domain subsequent to its binding to phosphotyrosyl-containing motifs of signaling partners. Similarly to what we observed for most mutations causing NS,15,16 our previously published24 and present data document that the vast majority of leukemia-associated PTPN11 lesions affect residues located at or close to the N-SH2/PTP-interacting surfaces. Significantly, both the spectrum and location of mutations support a model in which PTPN11 lesions up-regulate SHP-2 physiologic activation by impairing the switching between the inactive and active conformations, favoring a shift in the equilibrium toward the latter. Consistent with this view, neither mutations affecting residues that are essential for phosphatase activity, nor nonsense, frameshift, or splicing defects have been observed among the numerous mutations (N > 200) identified thus far15,16,24,25 (present study; and M.T. and B.D.G., unpublished data, May 2004). Accordingly, in vitro functional studies on 2 JMML-associated SHP-2 mutants, Asp61Gly and Glu76Lys,24 the latter here documented to be recurrent in ALL and AML, as well as 3 mutants identified in NS (Ser72Ala, Ile282Val, and Asn308Asp)37 demonstrated increased basal PTPase activity. This supports the idea that these substitutions weaken the autoinhibitory interaction between the N-SH2 and PTP domains controlling SHP-2 activation. Functional studies are required to understand the significance of substitutions affecting Pro491.
Missense PTPN11 mutations occur as germline lesions in NS and related developmental disorders, and as somatic defects in hematologic malignancies. The present study confirms previous data from our group and others indicating that the identity of the affected residues or the type of substitution differ between developmental and hematologic disorders, even though the resulting molecular defects appear to be functionally similar.15,16,24,25,38 Such dramatic genotype-phenotype relationship defines a novel class of missense mutations in the PTPN11 gene with a role in leukemia. The prognostic significance of these mutations remains unknown, and prospective studies will be needed to clarify their clinical significance.
We documented that the vast majority of cases with B-cell precursor ALL do not harbor mutations in both PTPN11 and RAS genes. Because SHP-2 is a positive modulator of RAS function, this finding suggests that mutated SHP-2 and RAS proteins might elicit their effects through a common pathway, and that missense mutations in PTPN11 might represent a novel class of lesions that lead to hyperactive RAS. This view was originally introduced by the observation that PTPN11, NRAS, KRAS2, and NF1 mutations are largely mutually exclusive in JMML,24,25,39 and supported by functional analyses documenting ligand-dependent prolonged activation of ERK2 in cells expressing mutated SHP-2 proteins at least under selected experimental conditions.24 RAS activation is an essential component of proliferative and antiapoptotic responses to a number of hematopoietic growth factors and cytokines,40-42 and up-regulation of RAS signaling has a pivotal role in promoting proliferation and survival of malignant myeloid cells, as documented by RAS point mutations and a number of genetic lesions that deregulate RAS function.43,44 Here, we showed that the combined prevalence of PTPN11, NRAS, and KRAS2 mutations accounts for approximately one third of pediatric cases with common ALL. Significantly, whereas PTPN11 mutations appeared to be preferentially associated with the common immunophenotype, mutations in NRAS or KRAS2 were uniformly distributed among the B-cell precursor ALL subtypes with a cumulative prevalence ranging between 9% and 24%. Because there are some limitations to the accuracy of this estimate (no attempt was made to look for mutations located on other exons and known to activate RAS function); the true prevalence of NRAS and KRAS2 oncogenic lesions in our series could be slightly higher. Consequently, these data support a relevant role of deregulated RAS signaling in B-cell precursor ALL and suggest that additional genes coding for transducers involved in this signal transduction pathway might represent novel candidate genes in B-cell precursor ALL pathogenesis.
A major finding of the present study concerns the mutually exclusive occurrence of PTPN11 and TEL-AML1 lesions among patients with B-cell precursor ALL. The TEL-AML1 gene rearrangement is strongly associated with nonhyperdiploid DNA content and occurs frequently among children with that form of leukemia. In contrast, a significant association with hyperdiploidy and advanced age at diagnosis (> 15 years) was observed for PTPN11 mutations. These findings suggest that PTPN11 and TEL-AML1 lesions might contribute with different, and possibly alternative, mechanisms to ALL pathogenesis. Although additional studies will be required to address this issue, the high prevalence of PTPN11 mutations among adolescents and their exclusive occurrence among TEL-AML1- cases suggests that PTPN11 lesions might be relevant for the pathogenesis of ALL in adults.
In conclusion, the present findings provide the first genetic evidence of a mutated protein tyrosine phosphatase acting as oncoprotein in both lymphoid and myeloid malignancies. The existing data do not permit any firm conclusions regarding whether somatic PTPN11 mutations represent primary permissive events or second hits acquired during disease progression that might be important for emergence of fully developed leukemia. The finding, however, that they appear to be almost exclusively restricted to specific acute leukemia conditions, that is, common ALL and acute monocytic leukemia, strongly suggests that gain-of-function of SHP-2 contributes to expansion of the leukemic clone depending on stage of differentiation and lineage of the precursor cell.
Prepublished online as Blood First Edition Paper, February 24, 2004; DOI 10.1182/blood-2003-11-3876.
Supported in part by grants from Ricerca finalizzata 1% FSN-2003 (Stabilità del genoma: bersagli molecolari nella prevenzione e nel controllo delle neoplasie) and Ricerca corrente ISS-2003 (PTPN11 e tumori: epidemiologia molecolare e studi funzionali) (M.T.); Associazione Italiana per la Ricerca sul Cancro (AIRC), Fondo per gli Investimenti della Ricerca di Base (FIRB), Fondazione Cariplo and Fondazione Tettamanti (A.B., G.C.); Ministero dell'Instruzione, dell' Università a della Ricerca-Consiglio Nazionale delle Ricerche (MIUR-CNR) and Fondazione Città Della Speranza (G.B.); and the US Public Health Service (grants HL71207 and HD01294) (B.D.G.).
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to patients and their families who participated in the study and the clinicians of the AIEOP centers for providing bone marrow samples. We thank J. D. Licht (Mount Sinai School of Medicine, New York, NY) for critical comments on the manuscript. We are also grateful to L. Morelli and M. Conte (Istituto Superiore di Sanità, Rome, Italy) for their helpful assistance in DNA sequencing, and to G. Giudici and S. Bungaro (Centro Ricerca Tettamanti, Monza, Italy) for cytogenetic and FISH analyses.