Abstract
Procoagulant activity on tumor cells can enhance their ability to spread via the circulation to colonize distant organs. Toward defining the relative importance of the main host responses to coagulation for hematogenous metastasis, we examined lung metastases after intravenous injection of melanoma cells in Nf-E2-/- mice, which have virtually no circulating platelets; Par4-/- mice, which have platelets that fail to respond to thrombin; Par1 and Par2-/- mice, which have markedly attenuated endothelial responses to coagulation proteases; and Fib-/- mice, which lack fibrinogen. In a severe combined immunodeficiency (SCID) background, median lung tumor count in Nf-E2-/-, Par4-/-, and Fib-/- mice was 6%, 14%, and 24% of wild type, respectively; total tumor burden was only 4%, 9%, and 3% of wild type, respectively. Similar results were seen in a syngeneic C57BL6 background. By contrast, deficiencies of protease-activated receptor 1 (PAR1) or PAR2 did not provide protection. These results provide strong genetic evidence that platelets play a key role in hematogenous metastasis and contribute to this process by both thrombin-dependent and -independent mechanisms. Importantly, PAR4 heterozygosity conferred some protection against metastasis in this model. Thus even partial attenuation of platelet function may be sufficient to provide benefit. (Blood. 2004;104:397-401)
Introduction
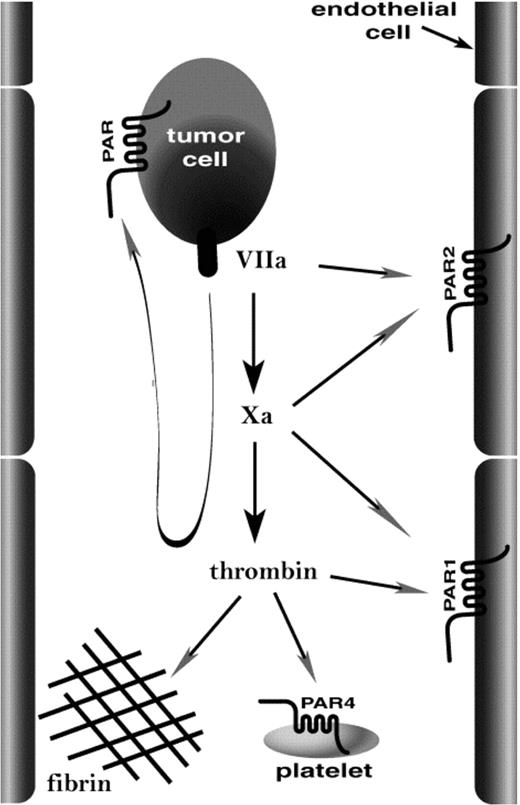
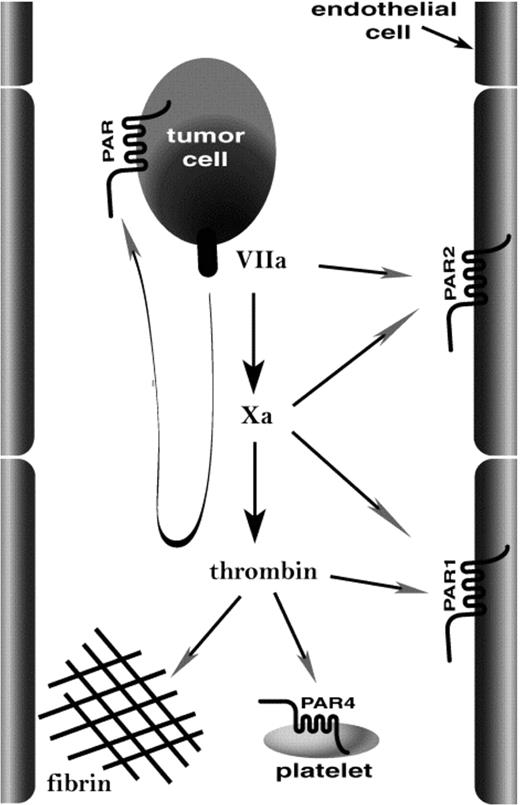
Several critical steps must be fulfilled to enable tumor cells to metastasize. The success of one such step, tissue colonization by tumor cells moving through the vascular compartment (hematogenous metastasis), is more likely if the tumor cell can activate the coagulation cascade. Such tumor cell procoagulant activity has been correlated with malignant progression of several types of human cancer.1-6 In mouse models, introduction of tissue factor, the main trigger for coagulation, into nonmetastatic cell lines can confer efficient hematogenous metastasis,7,8 and inhibitors of coagulation can inhibit hematogenous metastasis.8-12 It seems likely that procoagulant activity promotes hematogenous metastasis at least in part by helping tumor cells to lodge in the microvasculature9,10 so as to allow diapedesis into tissue13 or intravascular growth of secondary tumors.14 This might be accomplished by a number of host responses to coagulation proteases (Figure 1). Platelets can act as bridges between endothelial cells and lymphocytes15 or monocytes,16 and thrombin activation of platelets can enhance this function.16 Thus, coagulation proteases might activate platelets such that they bind tumor cells to the vessel wall. Platelet activation and fibrin formation in response to coagulation proteases formed on the tumor cell surface might also promote its incorporation into microthrombi that lodge in the microvasculature.13 Alternatively, endothelial cells respond to coagulation proteases by displaying a variety of adhesion molecules,17 and this activity might tether tumor cells or tumor cell-platelet complexes to the vessel wall (Figure 1). We used knock-out mice to examine the relative importance of platelets, platelet and endothelial cell activation by coagulation proteases, and fibrinogen in a model of hematogenous metastasis.
How might procoagulant activity promote localization of tumor cells in the microvasculature? The image illustrates candidate targets of coagulation proteases generated on the tumor cell. Tissue factor/factor VIIa and factor Xa might activate endothelial PAR2, and thrombin might activate endothelial PAR1 and platelet PAR4 and mediate fibrin formation. These same proteases might act at PARs on the tumor cell itself. Our data emphasize the importance of platelets and fibrinogen as important host effectors of hematogenous metastasis.
How might procoagulant activity promote localization of tumor cells in the microvasculature? The image illustrates candidate targets of coagulation proteases generated on the tumor cell. Tissue factor/factor VIIa and factor Xa might activate endothelial PAR2, and thrombin might activate endothelial PAR1 and platelet PAR4 and mediate fibrin formation. These same proteases might act at PARs on the tumor cell itself. Our data emphasize the importance of platelets and fibrinogen as important host effectors of hematogenous metastasis.
Materials and methods
Cells
The B16-F10 murine melanoma cell line9 was from American Type Culture Collection (Manassas, VA). To facilitate quantitation of tumor burden, B16-F10 cells were stably transfected with a pRK-luciferase construct kindly provided by Dr Marc Shuman (University of California, San Francisco).18 A clone with high constitutive luciferase expression (B16-F10-luc) was selected for further use. Cells were cultured in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, and 400 μg/mL G418.
Mice
Generation of mice deficient in protease-activated receptor 1 (PAR1), PAR2, and PAR4 has been described.19-22 Fibrinogen-deficient mice21 and NF-E2-deficient mice23 were generously provided by Drs Jay L. Degen (Children's Hospital, Cincinnati) and Stuart Orkin (Harvard Medical School), respectively. PAR1, PAR2, PAR4, and fibrinogen lines had been backcrossed 5 to 6 generations into the C57BL6/J strain. Littermate mice for experiments that used PAR1, PAR4, and fibrinogen null mice were generated from crosses between heterozygous (+/-) females and +/- or homozygous (-/-) males, except for studies that involved PAR2 deficiency. Intercrosses of Par2+/- mice yielded very low numbers of live-born knock outs in the C57BL6/J strain background, but crosses of those Par2-/- mice that survived yielded relatively normal litter sizes (G. R. Sambrano and S.R.C., unpublished observations, July 1999). Accordingly, PAR2 knock outs for experiments were generated from Par2-/- “survivor” intercrosses; age- and sex-matched wild-type mice in the same strain background from the same colony were used as controls. Nf-E2+/- intercrosses also yielded very low numbers of live-born knock outs in the C57BL6/J strain, but crosses of Nf-E2-/- survivors offered no advantage. To permit studies of NF-E2 knock outs and to study the effect of other knock outs in a different strain, the mutant alleles were bred into a CB-17/severe combined immunodeficient (SCID) strain background (Taconic Farms, Germantown, NY). These immunodeficient SCID mice permitted engraftment of the melanoma cells in a host strain where viable NF-E2 knock outs could be generated in sufficient numbers. Presence of the SCID mutation was screened for by the ability of the restriction enzyme AluI to cleave a 64-base pair polymerase chain reaction product generated using primers m6-DNA-PK(+)GGAAAAGAATTGGTATCCAC and m8-DNA-PK(-)GTTGGCCCCTGCTAACTTTC flanking the nonsense mutation as described by Araki et al.24
Metastasis model
Tumor cells harvested from subconfluent cultures were injected into the lateral tail veins of 6- to 9-week-old experimental mice in a single bolus of 2.5 × 105 cells in 200 μL Hanks balanced salt solution. Where indicated, 20 mg/kg recombinant hirudin (Refludan; kindly provided by Dr Joyce Chan, Berlex Biosciences, Richmond, CA) in 200 μL saline or saline control was injected subcutaneously 20 minutes prior to tumor cell injection. Then, 14 days later, the mice were killed and their lungs harvested. The right lung was fixed in 4% paraformaldehyde and the number of tumors visible at the lung surface was counted; the melanomas appear black against the pink lung surface. Surface tumors larger than 75 μm in diameter were counted under an Olympus SZH10 dissection microscope (Melville, NY). An arbitrary upper limit for counting was set at 150 surface tumors; higher burden was also expressed as 150 tumors. The left lung was frozen in liquid nitrogen for later determination of luciferase activity. Lungs were powdered with pestle and mortar in liquid nitrogen and then suspended in Cell Culture Lysis Reagent (Promega, Madison, WI). Luciferase activity was determined with a luminescent substrate (Promega) according to the manufacturer's instructions and correlated to a standard curve made with recombinant luciferase. Total lung protein in the same samples was determined by DC Protein Assay kit (Bio-Rad, Hercules, CA), and tumor burden was expressed as nanograms luciferase per gram lung protein. Fold or percent reduction in tumor burden was relative to wild-type controls and based on median values unless otherwise stated. Experiments were performed in 3- to 4-week intervals on all 6- to 9-week-old mouse littermates available on the scheduled date. Knock outs and wild-type controls were represented in each experiment, although not necessarily in equal numbers. The graphs shown represent the accumulated data from experiments of a given design. Statistical significance was determined using Mann-Whitney test, or a Kruskal-Wallis test with Dunn multiple comparison if more than 2 genotypes were compared.
Results
To probe the roles of various targets of coagulation proteases in hematogenous metastasis, we used a well-studied mouse model in which B16-F10 melanoma cells are injected by tail vein and lung metastases are measured by counting tumors. The melanoma cells used in this study also expressed luciferase, and lung luciferase content was used as an index of total tumor burden. Studies were performed in a C.B-17SCID background and, where possible, were repeated in a C57BL6 background.
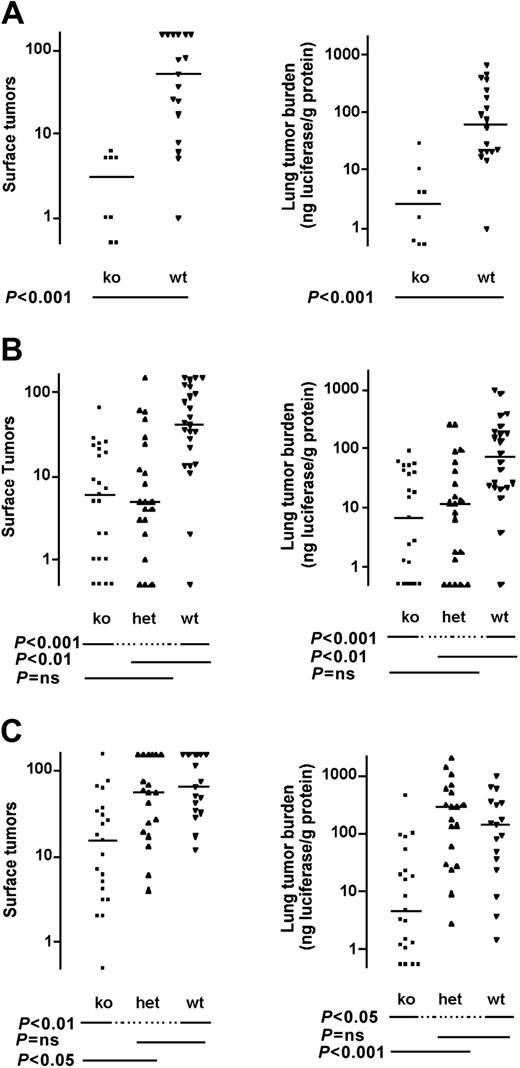
Electron microscopic studies reveal that B16 melanoma cells trapped in the lung vasculature are associated with platelets and fibrin,13 and available data suggest that both can contribute to hematogenous metastasis (see “Discussion”). To provide a genetic test of the relative importance of platelets and thrombin-induced platelet activation in this process, we examined Nf-E2-/- and Par4-/- mice. The former have virtually no circulating platelets,23 and the latter have platelets that fail to respond to thrombin.22 Nf-E2-/- mice showed marked protection against hematogenous metastasis; the median tumor count was only 6% of that seen in wild type, and tumor burden was only 4%. Heavy tumor burden was never observed in NF-E2 knock outs: the maximum number of surface tumors observed in an Nf-E2-/- mouse was 8 versus hundreds in control mice (Figures 2A, 3).
Effect of NF-E2 (platelet), PAR4, and fibrinogen deficiency on pulmonary metastasis in immunodeficient C.B-17 SCID mice. (A) Nf-E2-/-, (B) Par4-/-, and (C) fibrinogen (Fib-/-) mice, and appropriate controls were injected with B16-F10-luc cells via tail vein and killed 14 days later. The number of tumors visible on the lung surface (left panels) or total tumor burden as determined by lung luciferase activity (right panels) was determined. Horizontal lines indicate medians. P values were determined using Mann-Whitney test (A) or Kruskal-Wallis test with Dunn multiple comparison (B-C). ns indicates not significant; ko, knock out; het, heterozygote; and wt, wild type.
Effect of NF-E2 (platelet), PAR4, and fibrinogen deficiency on pulmonary metastasis in immunodeficient C.B-17 SCID mice. (A) Nf-E2-/-, (B) Par4-/-, and (C) fibrinogen (Fib-/-) mice, and appropriate controls were injected with B16-F10-luc cells via tail vein and killed 14 days later. The number of tumors visible on the lung surface (left panels) or total tumor burden as determined by lung luciferase activity (right panels) was determined. Horizontal lines indicate medians. P values were determined using Mann-Whitney test (A) or Kruskal-Wallis test with Dunn multiple comparison (B-C). ns indicates not significant; ko, knock out; het, heterozygote; and wt, wild type.
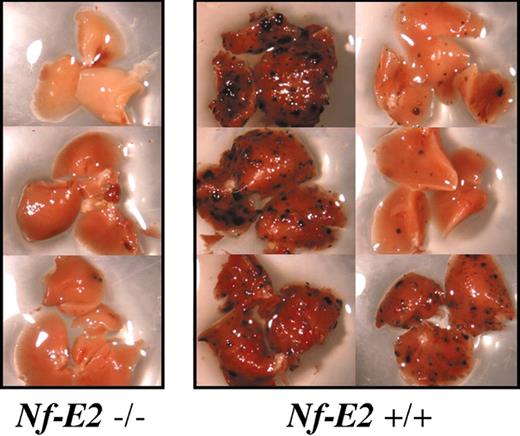
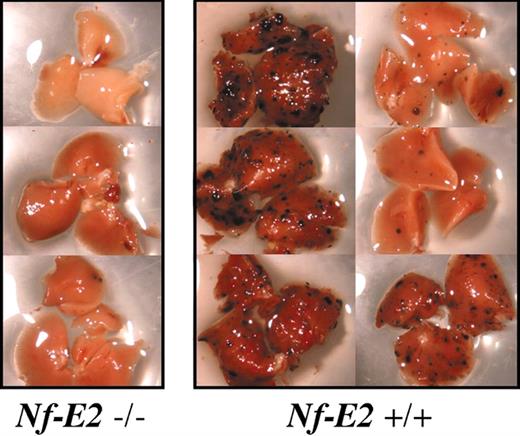
Pulmonary metastasis in platelet-deficient mice. Example of tumor burden in right lobes of lungs from Nf-E2-/- mice and wild-type littermates from a single experiment. Note the almost complete absence of surface tumors in lungs from platelet-deficient (Nf-E2-/-) animals.
Pulmonary metastasis in platelet-deficient mice. Example of tumor burden in right lobes of lungs from Nf-E2-/- mice and wild-type littermates from a single experiment. Note the almost complete absence of surface tumors in lungs from platelet-deficient (Nf-E2-/-) animals.
Hematogenous metastasis was also reduced in PAR4 knock outs. Median surface tumor count was 14% of wild type and total tumor burden was 9% (Figure 2B). Perhaps surprisingly, PAR4 heterozygosity also conferred protection against metastasis; tumor count in Par4+/- mice was 12% of wild type and total tumor burden was 16%.
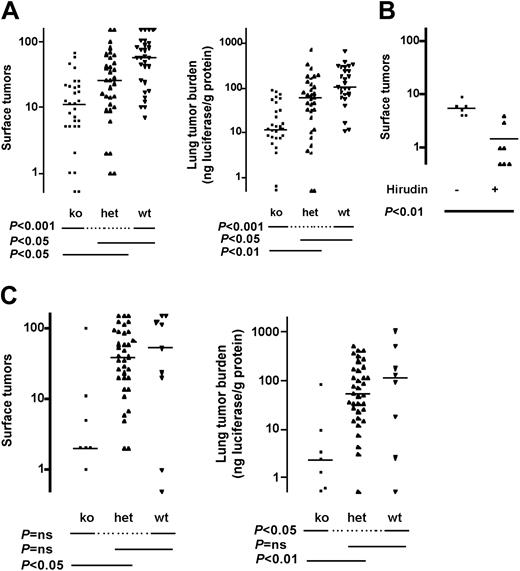
Palumbo et al have demonstrated that fibrinogen-deficient (Fib-/-) mice are protected against hematogenous metastasis of B16-F10 melanoma cells in a C57BL6 background.25 To compare the relative importance of fibrinogen versus platelets and thrombin-induced platelet activation, we examined Fib-/- mice in the CB17-SCID background. In this background, Fib-/- mice showed substantial protection against metastasis, with 24% of wild-type tumor counts and 3% tumor burden (Figure 2C).
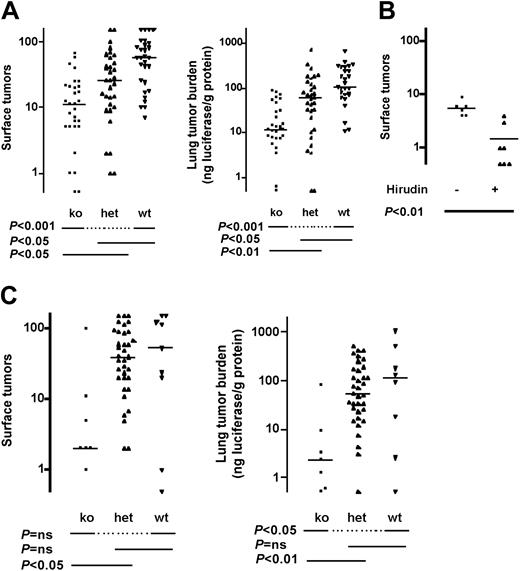
Similar results were obtained in the well-studied C57BL6 background, which is immunocompetent but syngeneic with the melanoma line used in these studies. Median tumor count in Par4-/- mice was 19% of wild type and tumor burden was 11% (Figure 4A). Par4+/- mice again showed protection; median tumor counts and tumor burden were 46% and 58% of wild type, respectively. Treatment of Par4-/- mice with hirudin provided additional protection against thrombosis, consistent with roles for thrombin beyond platelet activation in this model (Figure 4B). Indeed, in Fib-/- mice, median tumor counts and tumor burden were 3% and 2% of wild type in this background, respectively, in accord with Palumbo et al25 (Figure 4C).
Effect of fibrinogen and PAR4 deficiency on pulmonary metastasis in immunocompetent mice. Mice deficient in PAR4 (Par4-/-, A) or fibrinogen (Fib-/-, C) and littermate controls in a C57BL/6 strain background were injected with B16-F10-luc cells via tail vein and pulmonary metastasis was determined as in Figure 2. (B) Par4-/- mice were injected subcutaneously with 20 mg/kg recombinant hirudin in saline or with saline alone 15 minutes prior to tumor cell injection. Horizontal lines indicate medians. P values were determined using Kruskal-Wallis test with Dunn multiple comparison (A,C) or Mann-Whitney test (B). ko indicates knock out; het, heterozygous, wt, wild type; and ns, not significant.
Effect of fibrinogen and PAR4 deficiency on pulmonary metastasis in immunocompetent mice. Mice deficient in PAR4 (Par4-/-, A) or fibrinogen (Fib-/-, C) and littermate controls in a C57BL/6 strain background were injected with B16-F10-luc cells via tail vein and pulmonary metastasis was determined as in Figure 2. (B) Par4-/- mice were injected subcutaneously with 20 mg/kg recombinant hirudin in saline or with saline alone 15 minutes prior to tumor cell injection. Horizontal lines indicate medians. P values were determined using Kruskal-Wallis test with Dunn multiple comparison (A,C) or Mann-Whitney test (B). ko indicates knock out; het, heterozygous, wt, wild type; and ns, not significant.
The results described are consistent with the hypothesis that both platelet activation by thrombin and fibrin formation contribute to the ability of tissue factor and other procoagulant activities to promote hematogenous metastasis. Additional targets of coagulation proteases include PAR1 and PAR2, which in mice are the major endothelial cell receptors for thrombin and tissue factor/factor VIIa and Xa.26,27 Activation of these receptors can trigger expression of a variety of chemokines and adhesion molecules that might be involved in recruiting tumor cells to the microvasculature.17 However, no effect of PAR1 or PAR2 deficiency on tumor count or burden was seen in the same model in which effects of fibrinogen deficiency, PAR4 deficiency, and even PAR4 heterozygosity were easily detected (Figure 5).
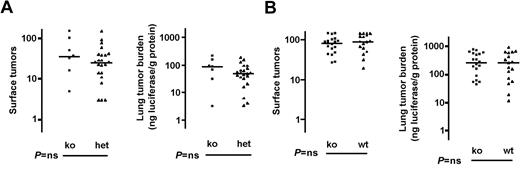
Effect of deficiencies in endothelial cell PARs on pulmonary metastasis. Mice deficient in PAR1 (Par1-/-, A) or PAR2 (Par2-/-, B) and littermate (PAR1) or matched (PAR2, see “Materials and methods”) controls in a C57BL/6 strain background were injected with B16-F10-luc cells via tail vein and pulmonary metastasis determined as in Figure 2. Horizontal lines indicate medians. Note that a second comparison in which Par1+/+ and -/- mice were compared (instead of Par1+/- and -/-) also showed no difference (not shown). P values were determined using Mann-Whitney test. ns indicates not significant; ko, knockout; and wt, wild type.
Effect of deficiencies in endothelial cell PARs on pulmonary metastasis. Mice deficient in PAR1 (Par1-/-, A) or PAR2 (Par2-/-, B) and littermate (PAR1) or matched (PAR2, see “Materials and methods”) controls in a C57BL/6 strain background were injected with B16-F10-luc cells via tail vein and pulmonary metastasis determined as in Figure 2. Horizontal lines indicate medians. Note that a second comparison in which Par1+/+ and -/- mice were compared (instead of Par1+/- and -/-) also showed no difference (not shown). P values were determined using Mann-Whitney test. ns indicates not significant; ko, knockout; and wt, wild type.
Discussion
This study provides strong genetic evidence that platelets are important for hematogenous metastasis of melanoma cells in a mouse model, and that thrombin activation of platelets makes an important contribution. Our data further suggest that platelet activation and fibrin formation are both important mechanisms by which tumor cell “procoagulant activity” promotes metastasis.
The protection seen in Nf-E2-/- and Par4-/- mice is almost certainly due to altered platelet number and function, respectively. Nf-E2-/- mice lack circulating platelets,23 and Par4-/- mice have platelets that cannot be activated by thrombin.22 High-level PAR4 expression is relatively restricted to platelets in the adult mouse,22,28 and thrombin signaling in Par4-/- mouse microvascular endothelial cells appears to be like wild type.27 Recent bone marrow reconstitution studies show that the impaired hemostasis and protection against thrombosis seen in Par4-/- mice are indeed accounted for by loss of PAR4 function in bone marrow-derived cells.29 Furthermore, results obtained with NF-E2- and PAR4-deficient mice were qualitatively similar and concordant with the results of previous studies32-34 that examined the role of platelets in hematogenous metastasis.
The nearly complete protection against hematogenous metastasis seen in Nf-E2-/- mice was striking and suggests that platelets are relatively important in this process. The greater protection seen in Nf-E2-/- versus Par4-/- mice suggests that platelets can contribute to hematogenous metastasis by both thrombin-dependent and thrombin-independent mechanisms. This is consistent with the recent demonstration that platelets can interact with tumor cells in a lectin-dependent manner that may be enhanced by thrombin signaling but is not dependent upon it.30
The ability of hirudin treatment to enhance protection against hematogenous metastasis in Par4-/- mice is consistent with a role for thrombin in this process that is independent of platelet activation. This complements the observation of Palumbo et al, who showed that hirudin enhanced protection seen in Fib-/- mice, suggesting a role for thrombin that is independent of fibrin formation.25 Thus together, our studies suggest that thrombin-induced platelet activation and fibrin formation both make important and at least partially independent contributions to hematogenous metastasis.
PAR1 deficiency does not affect thrombin signaling in mouse platelets but markedly attenuates thrombin signaling in mouse microvascular endothelial cells.27 PAR2 deficiency attenuates tissue factor/factor VIIa and Xa signaling in the latter but is partially redundant with PAR1.26 Our failure to detect protection against hematogenous metastasis in Par1-/- and Par2-/- mice in a model in which a defect even in PAR4 heterozygotes was readily detected supports the specificity of our results with the PAR4 nulls. Given the at least partially redundant roles of PAR1, PAR2, and PAR4 in mouse microvascular endothelial cells,26,27 we cannot exclude a role for endothelial activation by coagulation proteases in hematogenous metastasis based on our results. However, the effect of PAR4 heterozygosity on mouse platelet responsiveness to thrombin is subtle compared with the effect of PAR1 deficiency on mouse endothelial cell responsiveness to thrombin.22,27 Thus failure to see any trend toward protection in the PAR1 nulls militates against an important role for endothelial cell activation by thrombin in this process. This, of course, does not address the role of activation of PAR1 and/or PAR2 in the tumor cells themselves.31
Our results build on a substantial body of data that suggests a role for platelets and thrombin in hematogenous metastasis. For example, melanoma cells can bind and activate platelets and have been found in association with platelets in the mouse lung.13 Moreover, antibody-induced thrombocytopenia as well as antibodies to the platelet fibrinogen receptor can attenuate hematogenous metastasis32-34 as can the thrombin inhibitor hirudin.9 In addition to providing an important genetic confirmation of these pharmacologic studies, our observation that Nf-E2-/- and Par4-/- mice are protected against hematogenous metastasis extends them in interesting ways. The level of protection afforded NF-E2-deficient mice was somewhat greater than that seen in the antibody studies.32,33,35 This may be due to the fact that the lack of platelets and, hence, platelet function in Nf-E2-/- mice are sustained over time and profound in degree. The finding that PAR4 deficiency confers strong protection against metastasis is novel and provides both a strong link between thrombin-induced platelet activation and hematogenous metastasis and one explanation for the role of tumor cell tissue factor expression and/or procoagulant activity in this process. The difference between Nf-E2-/- and Par4-/- mice confirms a thrombin-independent contribution of platelets in hematogenous metastasis, and the ability of hirudin to enhance protection in Par4-/- mice confirms a role for thrombin that is distinct from platelet activation, consistent with known importance of fibrinogen in this process.25 Moreover, it suggests that in addition to functioning as a platelet ligand, fibrinogen conversion to fibrin is important.25
In summary, our results suggest a key role for platelets and an important role for thrombin-induced platelet activation in hematogenous metastasis. They also suggest that thrombin-induced platelet activation and fibrin formation are both important, make at least partially independent contributions in this process, and likely account, at least in part, for the ability of tumor cell tissue factor and other procoagulant activities to promote hematogenous metastasis. The surprising protection afforded even Par4+/- mice, which suffer no spontaneous bleeding and have only variably prolonged tail bleeding times,22 emphasizes the importance of platelet activation by thrombin and suggests that even partial attenuation of platelet activation is sufficient to confer protection in this model. Thus inhibition of platelet function might be explored as a possible strategy for prophylaxis against metastasis.
Prepublished online as Blood First Edition Paper, March 18, 2004; DOI 10.1182/blood-2004-02-0434.
Supported by National Institutes of Health HL65590 and HL65185 (S.R.C.). E.C. was supported by the Julius H. Comroe fellowship.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Fred Elfman and Marc Shuman for helpful discussions and Susanna Williams and Nathanael Horton for technical assistance.