Abstract
The bone marrow is the primary site for neutrophil production and release into the circulation. Because the CXC chemokine receptor-4/stromal derived factor-1 (CXCR4/SDF-1) axis plays a central role in the interactions of hematopoietic stem cells, lymphocytes, and developing neutrophils in the marrow, we investigated whether reciprocal CXCR4-dependent mechanisms might be involved in neutrophil release and subsequent return to the marrow following circulation. Neutralizing antibody to CXCR4 reduced marrow retention of infused neutrophils (45.7% ± 0.5% to 6.9% ± 0.5%) and was found to mobilize neutrophils from marrow (34.4% ± 4.4%). Neutrophil CXCR4 expression and SDF-1-induced calcium flux decreased with maturation and activation of the cells, corresponding to the decreased marrow homing associated with these characteristics in vivo. Infusion of the inflammatory mediator and CXCR2 ligand KC led to mobilization of neutrophils from marrow by itself and was augmented 3-fold by low doses of CXCR4-blocking antibody that otherwise had no mobilizing effect. Examination of KC and SDF-1 calcium signaling demonstrated that the effect of KC may, in part, be due to heterologous desensitization to SDF-1. These results suggest that the CXCR4/SDF-1 axis is critical in circulating neutrophil homeostasis and that it may participate in the rapid release of neutrophils from the marrow during inflammation through a novel interaction with inflammatory CXC chemokines. (Blood. 2004;104:565-571)
Introduction
The regulation of circulating neutrophil number and localization is a critical homeostatic mechanism, central to the development and resolution of systemic inflammatory states, yet it remains poorly understood. The bone marrow plays a central role in the regulation of neutrophil release under at least 2 circumstances: the homeostatic release of neutrophils that have reached maturity and the accelerated release of less mature cells as a response to an acute inflammatory response (the “left shift”). The mechanisms of release remain unclear, particularly as regards the release of mature neutrophils, but a variety of CXC chemokines such as interleukin 8 (IL-8) in humans or KC and macrophage inflammatory protein-2 (MIP-2) in mice have been implicated in the accelerated release in response to inflammation.1
Work has suggested that, in addition to the controlled release of neutrophils into the circulation, the bone marrow plays an important role in the selective retention of these cells once in circulation.2,3 Such retention appears in part to depend on intrinsic characteristics of the circulating neutrophil, so that less-mature and unstimulated cells are predominantly retained in the marrow following circulation, whereas senescent, activated, or damaged cells are cleared primarily by the liver and, to a lesser degree, the spleen.2,4,5 Circulating neutrophil sequestration in the marrow appears to lead either to recirculation (particularly in response to inflammatory stimuli) or to permanent clearance from circulation, perhaps depending on cell age and other factors.2 Because the marrow is thus involved in both release and retention of neutrophils, there may be circumstances in which neutrophil recirculation between marrow and peripheral blood occurs. We propose that reciprocal mechanisms are involved in the release of neutrophils from marrow into circulation and in the subsequent retention of neutrophils in marrow following circulation.
We have investigated the potential role of the CXC chemokine stromal derived factor-1 (SDF-1; CXCL12) and its receptor CXCR4 (CD184) in the marrow retention of circulating neutrophils as well as the release of neutrophils from marrow. This chemokine axis has previously been implicated in the marrow recirculation of B cells and hematopoietic stem cells6-9 and appears to play a critical role in the retention of myeloid cells within the marrow during their development.10,11 Although published reports are inconsistent, there is growing evidence that both marrow-derived and peripheral neutrophils express cell surface CXCR4 and respond to SDF-1 as measured by calcium flux and chemotaxis.12-16 Furthermore, animals in which either SDF-1 or CXCR4 have been disrupted show a dispersal of the developing myeloid progenitors into the peripheral circulation,17 and drugs targeting CXCR4 lead to the development of peripheral neutrophilia in both mice and humans.18 In this context, we posited that neutrophil release from and recirculation to the marrow might depend on the CXCR4/SDF-1 chemokine axis.
Materials and methods
Mice
Four- to 8-week-old C57Bl/6 mice were obtained from Harlan (Indianapolis, IN) and housed in the animal facilities of National Jewish Medical Research Center and the University of Vermont College of Medicine.
Reagents
Rabbit polyclonal antibodies (727/268b), previously demonstrated to block the murine CXCR4 receptor in vivo,15 were the kind gift of Dr J. C. Gutierrez-Ramos (Millennium Pharmaceuticals, Cambridge, MA). Rabbit immunoglobulin fraction (X0903) was obtained from Dako (Carpinteria, CA) for use as a control antibody in the blocking experiments. MEL14 (antimouse-l-selectin antibody; rat IgG2a) was obtained from Biosource (Camarillo, CA). Biotinylated rat antimouse CXCR4 monoclonal antibody (2B11/CXCR4) and control (A95-1; rat immunoglobulin G2b [IgG2b]) were obtained from PharMingen (San Diego, CA) for use in flow cytometry. Rat antimouse CD16/CD32 and fluorescein isothiocyanate (FITC)-conjugated rat antimouse Ly-6G (Gr-1) monoclonal antibodies were obtained from PharMingen as well. The CXC chemokines SDF-1α and KC were obtained from R&D Systems (Minneapolis, MN).
Preparation of murine peripheral blood neutrophils
Mouse peripheral blood neutrophils were isolated by methods previously reported.2 Briefly, mice were volume expanded and exsanguinated into a 3.8% citrate solution followed by centrifugation at 300g for 20 minutes. The cell pellet was resuspended in 6% dextran and 0.9% NaCl solution (in a ratio of 1:5.25, dextran-saline) to a final volume of 150% the original blood volume and sedimented at unity gravity for 30 minutes. The leukocyte-rich supernatant was aspirated, washed once in Hanks balanced salt solution (HBSS), layered over a Percoll gradient (78%, 66%, and 54%), and centrifuged at 1060g for 30 minutes. Cytospin samples of the 78%:66% interface revealed more than 90% neutrophils. Following red blood cell lysis with hypotonic saline, typical yields were approximately 2 to 4 × 105 peripheral blood neutrophils per mouse. Trypan blue dye exclusion showed the cells to be more than 97% viable following purification.
Preparation of morphologically mature murine bone marrow neutrophils
Femurs and tibias of killed mice were dissected, the marrow flushed with HBSS and layered on a 3-step Percoll (Pharmacia, Uppsala, Sweden) gradient (72%, 64%, and 52%) which was centrifuged at 1060g for 30 minutes.2 Cytospin samples of the 72%:64% interface revealed more than 95% morphologically mature-appearing neutrophils. This method exploits the previously noted correlation between marrow neutrophil density and maturity.19,20 Typical yields were approximately 1 to 2 × 107 neutrophils per mouse, which were more than 98% viable.
Preparation of murine peritoneal neutrophils
Mouse peritoneal exudate neutrophils were isolated following the methods of Savige et al21 4 hours after the intraperitoneal injection of 400 μL thioglycollate solution. Cytospin samples revealed nearly pure neutrophils (> 90%) with occasional macrophages. Typical yields were approximately 2 × 107 peritoneal neutrophils/mouse.
Labeling and circulation of infused neutrophils
Neutrophils isolated from marrow, peripheral blood, and sterile peritonitis were radiolabeled with 111In-tropolonate by using methods modified from Haslett et al.22 This method yielded 3 to 20 × 106 cell-associated cpm per 5 × 106 neutrophils after washing. A Beckman 7000 gamma counter (Beckman Coulter, Fullerton, CA) set to count both 173 KeV and 247 KeV peaks of 111In, was used to count radioactivity in all samples.
Aliquots of 5 × 106 labeled neutrophils in 200 μL phosphate-buffered saline with 0.1% bovine serum albumin (PBS/BSA) were infused intravenously through the left tail vein of each recipient mouse, 30 minutes after treatment (40-80 μg, by way of tail vein) with the CXCR4-blocking antibody, 727/268b15 or control immunoglobulin. A second control experiment was performed using MEL14, an anti-l-selectin antibody known to bind neutrophils, to assay for nonspecific effects on localization mediated through the bound antibody.
Three to 7 recipient mice were injected for each condition, and neutrophil localization was assayed 4 hours later, a time point at which marrow localization has reached a stable plateau following infusion.2 Mice were bled (10 μL) by way of the right tail vein and killed by cervical dislocation. Each mouse was dissected, and the lungs, spleen, kidneys, gut, liver, and right femur were removed and washed with 0.9% saline. The remaining mouse carcass was divided into head, tail, thorax, and hindquarters. 111Indium content in all tissues was expressed as a percentage of the total counts present in all tissues (the entire mouse). Values for the estimated total blood cpm were based on a predicted total blood volume of approximately 1.5 mL for the 4- to 8-week-old C57Bl/6 mice.23 The marrow content of C57Bl/6 mice femurs was estimated to represent 6% of the total marrow content on the basis of the work of Boggs.24
It is important to note that, although circulating labeled neutrophils in this system have previously been shown to have a blood half-life comparable to other species,2 most of the cells are found to initially marginate in the lungs following infusion with a slow washout similar to that seen in a variety of mammalian systems.25-27 That the majority of cells initially appear to marginate in this system likely reflects the extremely low circulating pool of neutrophils in the homeostatic mouse (5%-10% of circulating leukocytes), as there is no evidence of substantial neutrophil activation (in marrow or peripheral populations) or damage (in all 3 populations) prior to infusion.2
Mobilization of labeled neutrophils from marrow
The effects of injected antibody or chemokine on neutrophils previously localized to the marrow were examined by using methods previously used to monitor the release of labeled cells under homeostatic and inflammatory conditions.2 Briefly, indium-labeled morphologically mature marrow neutrophils (prepared as described in “Labeling and circulation of infused neutrophils”) were infused into otherwise untreated animals and allowed to localize to marrow for 4 hours, a time point at which such sequestration has reached a plateau (typically 60%-70% of infused cells2 ). Antibody or chemokine was then administered to the recipient animals, and the animals were subsequently bled, dissected, and assayed (as described in “Labeling and circulation of infused neutrophils”) to determine the change in neutrophil localization compared with control-treated animals.
To examine the effect of CXCR4 blockade on neutrophil release from the marrow, recipient mice were treated with CXCR4-blocking antibody (727/268b) or control immunoglobulin 2 μg intravenously at 4 hours after labeled neutrophil infusion. Mice were bled, killed, and dissected for assay as described earlier 2 hours or 16 hours after antibody infusion. The effect of the CXC chemokine KC (2 μg intravenously in 100 μL PBS/BSA) or low-dose CXCR4-blocking antibody (0.02 μg intravenously) on neutrophil release from the marrow was similarly examined. Mice were bled and dissected for assay at 30, 60, 90, and 120 minutes after KC administration. Finally, in one set of experiments both KC (2 μg intravenously) and low-dose CXCR4 blocking antibody (0.02 μg intravenously) were administered together, and the mice were bled and dissected for assay 60 minutes later.
Effects of blocking antibody, 727/268b, on neutrophil activation
Neutrophil F-actin polymerization and oxidant production were assayed to determine whether CXCR4 blocking antibody (727/268b) might cause activation of bound neutrophils. Isolated morphologically mature marrow neutrophils were incubated with buffer, 26.7 μg/mL blocking antibody (the equivalent dosing to 40 μg intravenously in a circulating blood volume of 1.5 mL23 ), control immunoglobulin (26.7 μg/mL), or 5 × 10-8M N-formylmethionine-leucine-phenylalanine (FMLP) for 5 minutes, and resulting F-actin polymerization was assayed as described.28 Superoxide production was measured by using the Amplex Red hydrogen peroxide assay (Molecular Probes, Eugene, OR) following exposure to buffer, blocking antibody (26.7 μg/mL), control antibody (26.7 μg/mL), or phorbol 12-myristate 13-acetate (PMA; 20 ng/mL) as described.29
Calcium flux assays
Calcium flux in response to varying concentrations of recombinant mouse SDF-1α (1, 0.1, 0.01 nM) was measured by the methods of Gonzalo et al.15 Cells were labeled with the fluorochrome Indo-1/am (Molecular Probes), and calcium mobilization in response to SDF-1 was assayed by using an LSR flow cytometer (Becton Dickinson, San Jose, CA). Results were expressed as the ratio of FL5/FL4 against time. CXCR4 blockade by 727/268b was assayed by incubating Indo-1/am-labeled marrow neutrophils for 10 minutes with antibody at 26.7 μg/mL. Blocked cells were then exposed to 1, 0.1, and 0.01 nM SDF-1α, as described above. In some experiments, murine neutrophils were exposed to 10 nM KC for 6 minutes at 37°C prior to treatment with 10 nM SDF-1α to test heterologous desensitization. In other experiments, cells were pretreated with 0.5 nM SDF-1α for 30 minutes at 37°C before exposure to KC or SDF-1 as indicated earlier.
Determination of neutrophil surface CXCR4 expression
Isolated neutrophils (1 × 105) were resuspended in staining buffer (PBS with 5% fetal calf serum [FCS]), incubated with anti-CD16/CD32 monoclonal antibodies for 30 minutes at 4°C. They were next stained sequentially with anti-Gr-1-FITC monoclonal antibody, biotinylated anti-CXCR4, or isotype control monoclonal antibodies and then streptavidin-PE (phycoerythrin), each for 30 minutes at 4°C. Cells were collected using a FACScan flow cytometer (Becton Dickinson) and analyzed by WinMDI software. Results were expressed as a ratio of the mean fluorescence intensity of cells stained with CXCR4 antibody versus isotype control antibody. Three to 5 separate experiments were performed for each analyzed population of neutrophils.
Results
CXCR4 is critically involved in interaction of circulating neutrophils with marrow
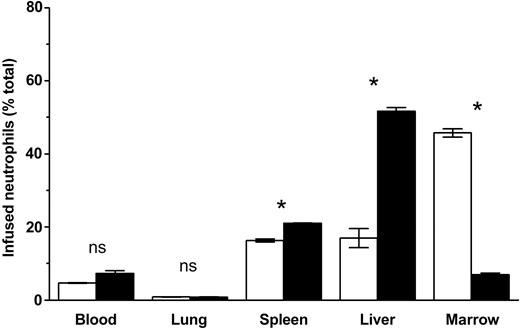
Because SDF-1, signaling through CXCR4, has been suggested to be involved in granulopoiesis,10,11,17 and because inhibitors of CXCR4 lead to leukocytosis,18 we questioned whether CXCR4 contributed to the homing of neutrophils to marrow. Infusion of CXCR4 blocking antibody resulted in a marked inhibition of marrow homing. The approximately 80% inhibition of peripheral neutrophil homing to marrow that is associated with antibody blocking of CXCR4 (45.7% ± 0.5% versus 6.9% ± 0.5%) is demonstrated in Figure 1. No such effect was seen with control immunoglobulin (Figure 1) or l-selectin antibody treatment (data not shown). Inhibition of marrow homing was associated with a reciprocal increase in hepatic and splenic sequestration, as well as a trend toward higher levels of circulating labeled cells. Although sequestration to the liver and spleen is associated with activated neutrophils, the blocking antibody did not cause significant evidence of neutrophil activation as assayed by actin polymerization, superoxide production, or calcium flux compared with control whole immunoglobulin fraction (data not shown). Furthermore, antibody directed against l-selectin did not affect marrow uptake of labeled cells, suggesting that the observed anti-CXCR4 effect is not due to opsonization, or a general antiadhesive effect.
CXCR4 blockade decreases localization of infused labeled peripheral blood neutrophils. To examine the effects of CXCR4 blockade on circulating neutrophil retention, mice were treated with either 40 μg/mouse blocking antibody (▪) or control immunoglobulin (□) intravenously 30 minutes before indium-labeled isolated peripheral neutrophils were infused. The mice were then bled and dissected 4 hours after cell infusion, and these tissues were assayed by gamma counting. Data points are the means of 3 to 7 separate mice (± SEM). *Significantly different when compared with control-treated animals, P < .01. ns indicates not significant.
CXCR4 blockade decreases localization of infused labeled peripheral blood neutrophils. To examine the effects of CXCR4 blockade on circulating neutrophil retention, mice were treated with either 40 μg/mouse blocking antibody (▪) or control immunoglobulin (□) intravenously 30 minutes before indium-labeled isolated peripheral neutrophils were infused. The mice were then bled and dissected 4 hours after cell infusion, and these tissues were assayed by gamma counting. Data points are the means of 3 to 7 separate mice (± SEM). *Significantly different when compared with control-treated animals, P < .01. ns indicates not significant.
Maturation/activation-related changes in marrow homing in circulation are governed by a CXCR4-dependent mechanism
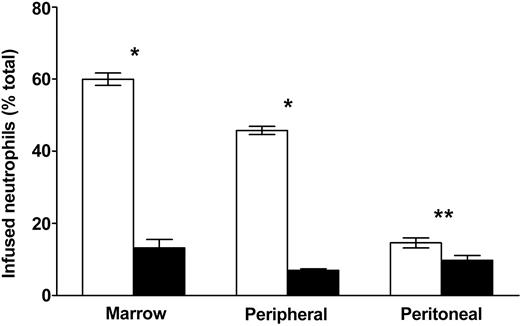
We have previously suggested that more mature (peripheral blood) and postmature (inflammatory exudate) neutrophils demonstrate diminished homing to marrow compared with unstimulated, less immature marrow neutrophils.2 To determine whether maturation/activation state-linked changes in homing might be related to CXCR4 responses, we compared the marrow retention of marrow-derived, peripheral, and peritoneal exudate cells in the presence or absence of antibody with CXCR4. As seen in Figure 2, in the control animals infused peripheral neutrophils were retained in marrow less than neutrophils isolated from marrow, whereas peritoneal exudate neutrophils were retained far less than either of these cell populations. Following antibody blockade, all 3 cell populations exhibited a significant decrease in homing to the marrow. Titration of the blocking antibody demonstrated that maximal effect was achieved at 40 μg/mouse with peripheral and exudate neutrophils, whereas 80 μg/mouse was required with marrow neutrophils. Furthermore, optimal blockade of marrow, peripheral, and exudate neutrophil populations resulted in similar residual levels of marrow homing (13.2% ± 2.3% versus 6.9% ± 0.5% versus 9.5% ± 1.3%, respectively), suggesting that the difference in homing between these populations in the control animals is largely due to a CXCR4-dependent mechanism.
Marrow localization of neutrophils following circulation decreases with maturation and activation of the cells and is diminished by CXCR4 blockade. The effects of CXCR4 blockade on marrow localization of relatively immature, mature, and activated neutrophils were examined by treating mice with 40 to 80 μg/mouse blocking antibody (▪) or whole immunoglobulin (control; □) intravenously 30 minutes before infusing indium-labeled cells. Localization was then assayed 4 hours after cell infusion. Source of neutrophil population is on the abscissa. Data points are the means of 3 to 7 separate experiments (± SEM). Significantly different when compared with control-treated animals, *P < .001, **P < .05.
Marrow localization of neutrophils following circulation decreases with maturation and activation of the cells and is diminished by CXCR4 blockade. The effects of CXCR4 blockade on marrow localization of relatively immature, mature, and activated neutrophils were examined by treating mice with 40 to 80 μg/mouse blocking antibody (▪) or whole immunoglobulin (control; □) intravenously 30 minutes before infusing indium-labeled cells. Localization was then assayed 4 hours after cell infusion. Source of neutrophil population is on the abscissa. Data points are the means of 3 to 7 separate experiments (± SEM). Significantly different when compared with control-treated animals, *P < .001, **P < .05.
Neutrophils from marrow are more responsive to SDF-1 than are peripheral or exudate neutrophils
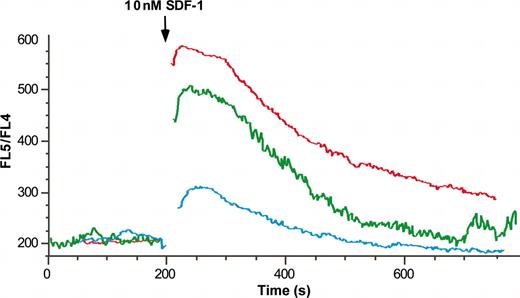
To determine whether the differential localization of immature, mature, and postinflammatory neutrophils could be due to differential sensitivity to SDF-1 through CXCR4, signaling in isolated marrow, peripheral and peritonitis-derived neutrophils was evaluated by using calcium flux in Indo-1-am-loaded cells. As seen in Figure 3, SDF-1 induced a calcium flux in marrow-derived, peripheral, and inflammatory exudate neutrophils. The response to 1 nM (Figure 3) and 0.5 nM SDF-1 (data not shown) was strikingly different between these cell populations, as the peritoneal cells exhibited a minimal response, whereas the marrow-derived cells demonstrated a robust response, and peripheral neutrophils were intermediate. A diminished response but with similar population differences was seen in response to 0.1 nM SDF-1 (data not shown). CXCR4-blocking antibody (26.7 μg/mL) was found to markedly diminish calcium flux in response to 1 nM SDF-1 and completely attenuate the response to concentrations of 0.1 to 0.5 nM SDF-1 (data not shown).
SDF-1α-induced calcium transient decreases with neutrophil maturation and activation. Isolated marrow (red line), peripheral (green line), and exudate (blue line) neutrophils were labeled with the fluorochrome Indo-1/am and calcium mobilization in response to 10 nM SDF-1 was assayed by flow cytometry. Results are expressed as the ratio of FL5/FL4 against time.
SDF-1α-induced calcium transient decreases with neutrophil maturation and activation. Isolated marrow (red line), peripheral (green line), and exudate (blue line) neutrophils were labeled with the fluorochrome Indo-1/am and calcium mobilization in response to 10 nM SDF-1 was assayed by flow cytometry. Results are expressed as the ratio of FL5/FL4 against time.
CXCR4 is expressed on the neutrophil surface and its level is altered on neutrophils of different stages of maturation and activation
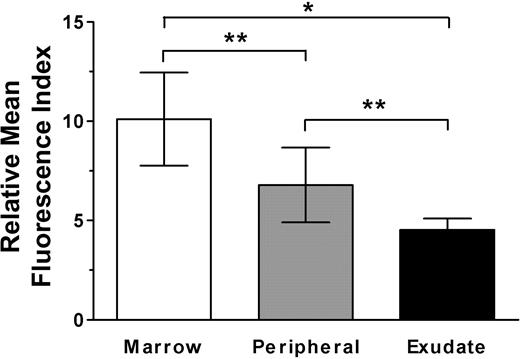
Because one mechanism for diminished response to SDF-1 is the level of expression of CXCR4 on the surface of the cell,30 we examined surface staining with anti-CXCR4 antibody on neutrophils of different stages of maturation and activation. As seen in Figure 4, surface expression of CXCR4 was diminished in peripheral neutrophils compared with marrow neutrophils and further reduced in peritoneal exudate neutrophils, corresponding to both the measured calcium flux responses to SDF-1, as well as the marrow homing behaviors of these populations of cells. This data may explain the higher dose of CXCR4-blocking antibody (80 μg/mouse) required to achieve maximal blockade of infused marrow neutrophils. It also suggests that a fall in CXCR4 expression accompanies late maturation (and activation) of the cells, an inverse of the increase in expression seen during early stages of neutrophil maturation.16 Such a decrease is in contrast to the findings of Nagase et al,31 who found that cultured peripheral neutrophils apparently increased CXCR4 expression over 48 hours.
Surface expression of CXCR4 decreases with neutrophil maturation and activation. Isolated bone marrow, peripheral, and exudate neutrophils were stained with biotinylated anti-CXCR4 or isotype control antibodies followed by streptavidin-phycoerythrin and were analyzed by flow cytometry. Results are expressed as a ratio of the mean fluorescence intensity of cells stained with CXCR4 antibody versus isotype control antibody. Data points are the means of 3 to 5 separate experiments performed for each analyzed population of neutrophils (± SEM). Expression significantly different when compared with each other, *P < .001, **P < .02.
Surface expression of CXCR4 decreases with neutrophil maturation and activation. Isolated bone marrow, peripheral, and exudate neutrophils were stained with biotinylated anti-CXCR4 or isotype control antibodies followed by streptavidin-phycoerythrin and were analyzed by flow cytometry. Results are expressed as a ratio of the mean fluorescence intensity of cells stained with CXCR4 antibody versus isotype control antibody. Data points are the means of 3 to 5 separate experiments performed for each analyzed population of neutrophils (± SEM). Expression significantly different when compared with each other, *P < .001, **P < .02.
Blocking CXCR4 results in mobilization of neutrophils from marrow
Because previous studies18 have suggested that strategies to inhibit CXCR4 resulted in release of neutrophils into circulation, we wanted to determine whether blocking CXCR4 by using antibody 727/268b would similarly mobilize neutrophils previously retained within the marrow. As seen in Figure 5, injection of CXCR4 blocking antibody resulted in the release of previously sequestered labeled neutrophils from the marrow (34.4% of those sequestered) with dispersal to the blood, liver, lung, and spleen. Such an effect was transient, however, as similar (high) levels of marrow-sequestered labeled cells were found in blocking and control antibody-treated animals 16 hours after antibody infusion (data not shown), suggesting that neutrophils mobilized by CXCR4 blocking antibody return to the marrow as the antibody is cleared.
Blocking CXCR4 results in mobilization of neutrophils from the marrow. To determine the effects of CXCR4 blockade on marrow-sequestered neutrophils, mice were treated with CXCR4-blocking antibody (▪) or control immunoglobulin (□), 2 μg intravenously 4 hours after labeled marrow neutrophils were sequestered to the marrow, as described. Subsequent changes in neutrophil localization were assayed 2 hours after antibody infusion. Data points are the means of 3 to 7 separate experiments (± SEM). *Significantly different when compared with control treated animals, P = .01. ns indicates not significant.
Blocking CXCR4 results in mobilization of neutrophils from the marrow. To determine the effects of CXCR4 blockade on marrow-sequestered neutrophils, mice were treated with CXCR4-blocking antibody (▪) or control immunoglobulin (□), 2 μg intravenously 4 hours after labeled marrow neutrophils were sequestered to the marrow, as described. Subsequent changes in neutrophil localization were assayed 2 hours after antibody infusion. Data points are the means of 3 to 7 separate experiments (± SEM). *Significantly different when compared with control treated animals, P = .01. ns indicates not significant.
CXCR2 chemokine KC induces neutrophil release from marrow
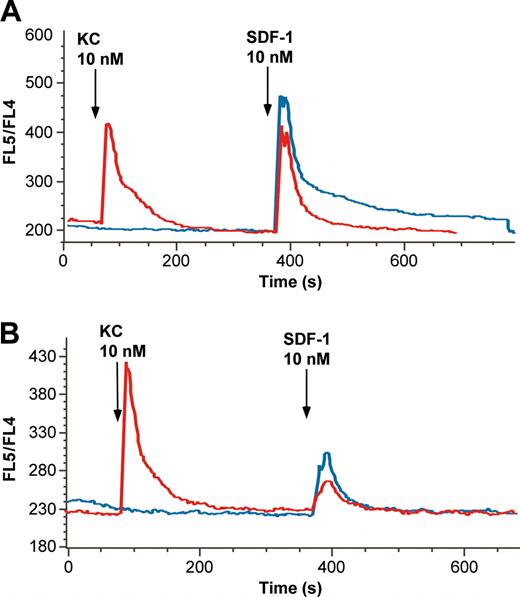
Chemoattractant ligands have been shown to induce release of immature neutrophils from marrow.1 To confirm that neutrophils experimentally localized to marrow would similarly respond to CXCR2 ligands, we infused the CXC chemokine KC into mice in which labeled neutrophils had been infused 4 hours earlier. As seen in Figure 6A, mobilization of labeled cells peaked at 30 minutes after KC and decreased thereafter, suggesting a reversible effect of KC on neutrophil release. Released neutrophils were found to disperse to blood, liver, spleen, and lung (data not shown). To determine whether the mobilizing effects of KC and CXCR4 blocking antibody might interact, we administered a low dose of antibody (that by itself had no mobilization effect) and KC simultaneously and examined a time point following infusion at which the effect of KC had previously been shown to wane (60 minutes). Under these circumstances, labeled neutrophil release was greatly augmented compared with either KC or low-dose antibody infusion alone (Figure 6B).
The CXCR2 chemokine KC induces neutrophil release from marrow and this is significantly augmented by low doses of CXCR4 neutralizing antibody. To measure the mobilization of marrow-sequestered neutrophils by the CXC chemokine KC and the synergistic effect of low-level CXCR4 blockade 2 experiments were performed. (A) Mice were treated with KC, 2 μg intravenously (♦) or buffer control (□) 4 hours after labeled marrow neutrophils were sequestered to the marrow, as described. Subsequent marrow content of labeled neutrophils was assayed at 30, 60, and 120 minutes after chemokine infusion. Results are expressed as percentage decrease compared with control-treated animals. (B) Mice were treated with low-dose CXCR4 blocking antibody (0.02 μg intravenously), KC (2 μg intravenously), both antibody and KC, or buffer control 4 hours after labeled marrow neutrophils were sequestered to the marrow, as described. Subsequent marrow content of labeled neutrophils was assayed 60 minutes after chemokine/antibody infusion. Results are expressed as percentage decrease compared with control-treated animals. Data points are the means of 3 to 5 separate experiments (± SEM). *Significantly different when compared with control-treated animals, P = .02. **Significantly different when compared with control, KC, or low-dose blocking antibody alone, P < .05; ns indicates not significant.
The CXCR2 chemokine KC induces neutrophil release from marrow and this is significantly augmented by low doses of CXCR4 neutralizing antibody. To measure the mobilization of marrow-sequestered neutrophils by the CXC chemokine KC and the synergistic effect of low-level CXCR4 blockade 2 experiments were performed. (A) Mice were treated with KC, 2 μg intravenously (♦) or buffer control (□) 4 hours after labeled marrow neutrophils were sequestered to the marrow, as described. Subsequent marrow content of labeled neutrophils was assayed at 30, 60, and 120 minutes after chemokine infusion. Results are expressed as percentage decrease compared with control-treated animals. (B) Mice were treated with low-dose CXCR4 blocking antibody (0.02 μg intravenously), KC (2 μg intravenously), both antibody and KC, or buffer control 4 hours after labeled marrow neutrophils were sequestered to the marrow, as described. Subsequent marrow content of labeled neutrophils was assayed 60 minutes after chemokine/antibody infusion. Results are expressed as percentage decrease compared with control-treated animals. Data points are the means of 3 to 5 separate experiments (± SEM). *Significantly different when compared with control-treated animals, P = .02. **Significantly different when compared with control, KC, or low-dose blocking antibody alone, P < .05; ns indicates not significant.
CXCR2 ligand KC desensitizes marrow neutrophil response to SDF-1
Because the data shown in Figure 6B suggested that KC augmented the effects of the blocking antibody to CXCR4, we questioned whether KC might modify signal transduction through CXCR4. In human neutrophils, IL-8 has been suggested to modify signaling through CXCR4, but by an action ascribed to IL-8 effects on CXCR1.32 Because mice lack CXCR1, it was of interest to determine whether a CXCR2 ligand such as KC might alter signaling through CXCR4. Accordingly, 2 types of experiments were carried out. In the first, marrow neutrophils labeled with Indo-1 were exposed to KC (10 nM) or vehicle for 5 minutes before exposure to SDF-1 (10 nM). As seen in Figure 7A, the peak response to SDF-1 was only slightly diminished by prior exposure to KC. Surprisingly, however, the segment of the calcium transient because of influx of extracellular calcium was systematically diminished. Area under the curve calculations suggested that there was a 40% decrease in that portion of the curve in 7 different experiments. We interpret this as a form of heterologous desensitization, although to our knowledge this precise effect on extracellular influx of calcium has not been previously shown.
The CXCR2 ligand KC desensitizes marrow neutrophil response to SDF-1. The effects of pretreatment with KC on neutrophil calcium response to SDF-1α were examined by using murine neutrophils loaded with Indo-1, as described, that were exposed to 10 nM SDF-1 with or without 10 nM KC pretreatment. (A) Untreated cells exposed to SDF-1 alone (blue line) show a robust calcium transient with a prolonged plateau phase because of extracellular calcium influx. Pretreatment with KC (red line) 5 minutes before exposure to SDF1 results in an SDF-1 response lacking the secondary plateau phase. (B) Response of cells exposed to 0.5 nM SDF-1 for 45 minutes at 37°C before analysis. Cells subsequently stimulated with 10 nM SDF-1 alone (blue line) show an attenuated response (compare with blue line in panel A), but, if treated with 10 nM KC 5 minutes before (red line), they show a decrement in the peak calcium transient. Note that exposure to 0.5 nM SDF-1 for 45 minutes had no effect on the response to KC, but the scale of the y-axis was changed to better depict the changes in response to SDF-1.
The CXCR2 ligand KC desensitizes marrow neutrophil response to SDF-1. The effects of pretreatment with KC on neutrophil calcium response to SDF-1α were examined by using murine neutrophils loaded with Indo-1, as described, that were exposed to 10 nM SDF-1 with or without 10 nM KC pretreatment. (A) Untreated cells exposed to SDF-1 alone (blue line) show a robust calcium transient with a prolonged plateau phase because of extracellular calcium influx. Pretreatment with KC (red line) 5 minutes before exposure to SDF1 results in an SDF-1 response lacking the secondary plateau phase. (B) Response of cells exposed to 0.5 nM SDF-1 for 45 minutes at 37°C before analysis. Cells subsequently stimulated with 10 nM SDF-1 alone (blue line) show an attenuated response (compare with blue line in panel A), but, if treated with 10 nM KC 5 minutes before (red line), they show a decrement in the peak calcium transient. Note that exposure to 0.5 nM SDF-1 for 45 minutes had no effect on the response to KC, but the scale of the y-axis was changed to better depict the changes in response to SDF-1.
In a second series of experiments, we attempted to mimic the proposed exposure of developing neutrophils to SDF-1 in the marrow. Although SDF-1 is among the most potent chemoattractants at the induction of homologous desensitization,12,33 it must nonetheless be able to exert tonic effects on neutrophils if it is to be responsible for retaining cells in the marrow. Accordingly, we determined whether 45 minutes of exposure to 0.5 nM SDF-1 would completely abolish responses to 10 nM SDF-1. As seen in Figure 7B, although there is a diminution of the response to SDF-1, it remains capable of inducing a calcium transient. Under these circumstances (in the setting of tonic exposure to low concentrations of SDF-1), exposure of the neutrophils to KC (10 nM) 5 minutes before high dose (10 nM) SDF-1 further attenuated the SDF-1 response. Thus, interposing KC in the process of SDF-1-induced homologous desensitization further increased desensitization.
Discussion
Neutrophils in circulation, although typically thought of as a uniform population, may in fact be quite heterogeneous even under homeostatic conditions.34 Such heterogeneity appears to reflect elements of ongoing functional and phenotypic maturation,35 as well the subsequent development of senescence. Control of circulating neutrophil heterogeneity is of critical importance, as the inappropriate release of immature cells or the failure to remove activated or senescent cells might have deleterious consequences to the organism. Although work has demonstrated the role of liver in the retention and clearance of damaged, activated, or possibly senescence circulating neutrophils, the role of the marrow in the regulation of the circulating neutrophil population appears to be more complex.2,4 Even as the bone marrow is recognized as the primary site for the release of neutrophils, evidence suggests it may also function in the selective retention and even subsequent re-release of circulating neutrophils.2,3 Considered in this fashion, the marrow may thus represent a compartment that is in dynamic equilibrium with circulating cells.
Were this hypothesis to be true, then manipulating the signals by which neutrophils are retained within the marrow might not only prevent retention of neutrophils in marrow but also induce the release of cells into the circulation. We have tracked these events separately in mice using neutrophils of different stages of maturation and activation to define the role of the CXCR4/SDF-1 axis in the modulation of both components of this putative equilibrium: release from marrow and retention in marrow. Our data indicate that CXCR4 and its sole ligand, SDF-1, are involved in both release of neutrophils from marrow and retention of neutrophils in marrow.
As we have previously demonstrated,2 marrow retention of mature blood neutrophils appears to account for nearly 50% of their clearance following circulation in a murine model. Such retention appears to be selective for less mature and unstimulated neutrophils, as 60% to 70% of neutrophils isolated from the postmitotic pool of the marrow are retained in the marrow following infusion, whereas only 15% to 20% of activated (exudate) do so with the remainder being retained primarily in the liver. It should be noted that, although the behavior of the neutrophil populations we examined varies appropriately with the degree of maturation and activation of the cells,2 some small degree of activation may occur during isolation. This is typically reflected as greater localization of the infused cells to the liver.2,4,21 It is, therefore, likely that, particularly in the case of infused isolated peripheral neutrophils, we underestimate the true degree of marrow sequestration.
Although multiple competing mechanisms are likely to be responsible for the balance between liver and marrow sequestration, our current work suggests that marrow retention of mature blood neutrophils is largely CXCR4 dependent, as antibody blockade of this receptor leads to a marked inhibition of such retention following circulation (Figure 1). Furthermore, we find that CXCR4 blockade significantly inhibits marrow retention of both mature marrow neutrophils and exudate neutrophils, suggesting that the CXCR4/SDF-1 axis is a common mechanism underlying the marrow homing of all 3 populations of cells (Figure 2).
In efforts to explain the selective homing of less mature and unstimulated cells to the marrow, we next examined SDF-1-induced signaling of the 3 neutrophil populations as measured by calcium flux. This reveals a close correlation between the magnitude of SDF-1 response and degree of marrow homing of the different cell populations following circulation (Figure 3). It was likewise found that cell surface expression of CXCR4 decreases with maturation and activation of the neutrophil populations (Figure 4), suggesting that regulation of CXCR4 expression and signaling is a central mechanism in the control of neutrophil localization following circulation. Such a link between SDF-1-related signaling and expression of CXCR4 surface receptor, although perhaps intuitive, is in contrast to the dissociation of SDF-1 signaling and CXCR4 expression previously demonstrated in both developing B cells36 and hematopoietic stem cells.9
Given the body of evidence supporting a role for CXCR4 in the retention of developing neutrophils in the marrow,10,11,17 and our findings that the receptor is critical for the retention of mature cells from circulation, we hypothesized that the CXCR4/SDF-1 axis might also be involved in the orderly release of maturing neutrophils from the postmitotic pool. By using a technique previously used in our laboratory to observe the release of marrow neutrophils in the setting of inflammation, we examined the effects of CXCR4 blocking antibody in normal animals. Significant release of labeled cells from marrow was found following treatment with antibody (Figure 5), suggesting that neutrophil release may indeed be governed, at least in part, by the CXCR4/SDF-1 axis. Interestingly, the effects of such blockade appear transient, as examination at 16 hours after antibody treatment demonstrates return of the cells to the marrow.
In addition to the controlled release of neutrophils at maturity, an important series of studies have suggested that release of immature neutrophils from the bone marrow (the left shift) accompanies pneumonia.37,38 Rabbits were given experimental streptococcal pneumonia, and neutrophils were tracked using nuclear labeling of dividing cells by infused 5′-bromo-2′-deoxyuridine (BrdU, a thymidine analog). These workers demonstrated shortened transit times of neutrophils through the marrow with rapid release of segmented and band forms,39 whereas examination of the labeled neutrophil population revealed defective diapedesis to the airspace of infected lungs and increased lysozyme release37,40 indicative of immaturity. A series of studies has suggested that potential mediators of marrow release are chemoattractants. Pioneering work of Jagels and Hugli1 indicated the ability of C5a to induce leukocytosis. More recently, the CXC chemokine IL-8 (CXCL8) was shown to be involved in signaling release from marrow, although the mechanisms involved remain obscure. Interestingly, studies from Richardson et al32 suggest that IL-8 exerts a partial heterologous desensitization effect on CXCR4 signaling, through an effect ascribed to protein kinase C (PKC).
In this context, we were interested in determining to what extent CXC chemokines in mice might mediate release from marrow. We demonstrated that the CXCR2 ligand KC induces release of previously sequestered neutrophils, similar to other CXCR2 ligands that have been shown to induce leukocytosis.41 Furthermore, KC induced a unique form of heterologous desensitization to the effects of SDF-1. As seen in Figure 7, prior exposure to KC led to a SDF-1 response that, although quantitatively similar in the initial phase, lacked a component ascribed to extracellular calcium influx. This novel type of desensitization might be expected to modulate a variety of neutrophil functions, because extracellular calcium is required for chemotaxis as well as primary granule release. One of the paradoxes of the role of SDF-1 in retaining neutrophils in the marrow is the profound ability of SDF-1 to induce homologous desensitization.12,33 Accordingly, we questioned whether exposure to KC might also modify the response to chronic low-level exposure to SDF-1 as might occur in the marrow. We, therefore, tested the effect of SDF-1 and KC on neutrophils incubated in the presence of a low-concentration (0.5 nM) SDF-1 for 45 minutes. As seen in Figure 7, the subsequent response to 10 nM SDF-1 was diminished, but not abolished, by this exposure, suggesting that neutrophils exposed chronically to SDF-1 (as we propose is the case in the marrow) are actually undergoing continual, but incomplete, homologous desensitization. Under these circumstances, exposure to KC further attenuated the calcium response to 10 nM SDF-1, suggesting that KC might thus be able to enhance the process of homologous desensitization induced by SDF-1. Either of these responses might be expected to contribute to release of neutrophils from the marrow; in support of this hypothesis is the finding that low-dose antibody blockade appears synergistic with KC treatment in the release of labeled neutrophils from the marrow in vivo (Figure 6B). Recently, work that examined the effects of granulocyte colony-stimulating factor (G-CSF) on the mobilization of CD34+ stem cells from marrow has suggested that G-CSF may act on the CXCR4/SDF-1 axis both directly (through the regulation of CXCR4 expression) and indirectly through the induction of neutrophil elastase release and subsequent cleavage of both marrow SDF-1 and cell surface CXCR4.42,43 A similar mechanism for G-CSF-mediated neutrophil release has been suggested,43,44 although the mechanism appears to be more complex as such mobilization appears to be normal in elastase-deficient mice.44
In the setting of a bona fide inflammatory response, many chemoattractants will be generated (in addition to KC) that could be expected to reach marrow. Although we have not examined the effects of other chemoattractants that act through G-protein-coupled, 7 transmembrane-spanning receptors, there is considerable evidence that similar events modifying SDF-1 signaling may exist. In human neutrophils, IL-8 appears to modify SDF-1 signaling through CXCR1.32 Our data imply the existence of general mechanisms through which chemoattractants, especially those acting through CXCR2, may exert potent effects on SDF-1 signaling, and thereby induce mobilization of neutrophils from marrow. Considerable work will be necessary to define whether this more general theory is true, but such work may substantially illuminate the mechanisms of leukocytosis and the consequences for the inflammatory response.
Prepublished online as Blood First Edition Paper, April 6, 2004; DOI 10.1182/blood-2003-10-3638.
This work was supported by grants K08 HL04 499, 1R01 HL68 876, and NCRR COBRE P20 RR-155557 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.