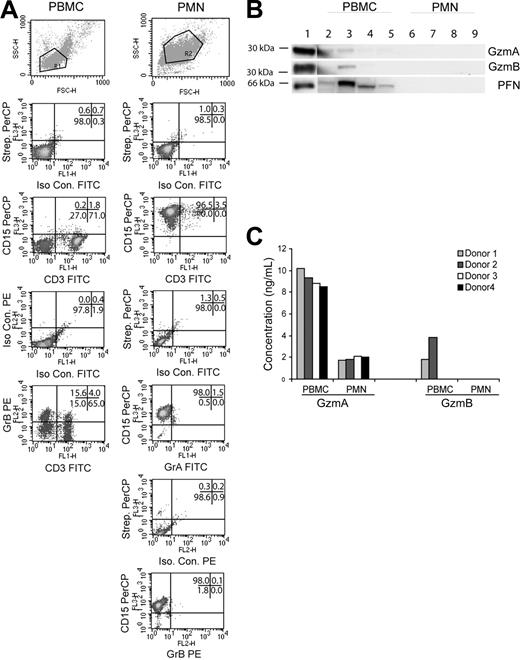
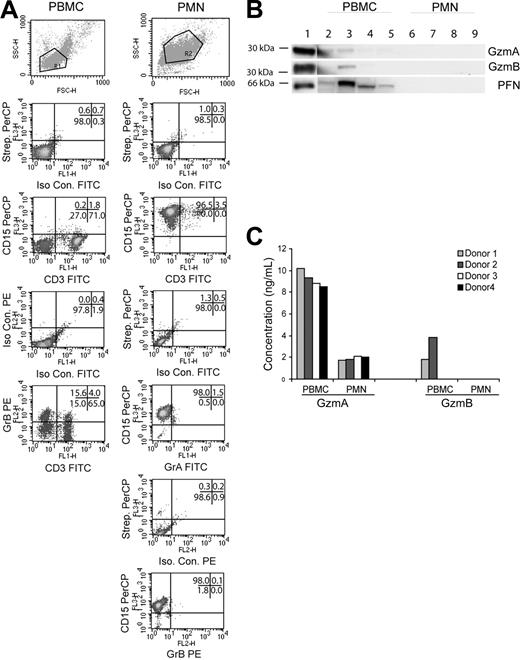
Wagner et al1 and Hochegger et al2 reported that human polymorphonuclear neutrophils (PMNs) express granzyme A (GzmA), granzyme B (GzmB), and perforin (PFN), postulating a role for these granule proteins in PMN-mediated antibody-dependent cellular cytotoxicity. Sayers et al3 had originally observed that PMN lacked these proteins. We thought it would be instructive to assess this disagreement by identifying these proteins with the combination of flow cytometry, Western blot, and enzyme-linked immunosorbent assay (ELISA; EIA).
Human peripheral blood mononuclear cells (PBMCs) were isolated from 4 healthy donors by Ficoll-Paque centrifugation (Amersham Bioscience, Arlington Heights, IL), and PMNs were enriched by hypotonic lysis of red blood cell pellet and a second Ficoll-Paque centrifugation. Cells or lysates from the PBMC and PMN fractions were tested as follows: GzmA and GzmB expression by flow cytometry (n = 2); GzmA, GzmB, and PFN expression by Western blot (n = 4); GzmB enzymatic activity by Ile-glu-Thr-Asp-pNitroaniline assay (n = 4); and GzmA and GzmB expression by EIA (n = 4). For flow cytometry, the cells were rinsed thrice in wash buffer (Hanks balanced salt solution [HBSS] with 0.5% bovine serum albumin [BSA], 0.02% sodium azide, 150 μg/mL human gamma globulin [intravenous immune globulin]; [Gammaguard, Baxter Healthcare, Miami, FL]). After blocking with intravenous immune globulin to reduce nonspecific Fc receptor interactions, intact cells were incubated with CD3–fluorescein isothiocyanate (FITC) (BD Pharmingen, San Diego, CA) and CD15-Biotin (Leinco Technologies, St Louis, MO) followed by Streptavidin peridinin chlorophyll A protein (PerCP; BD Pharmingen) and analyzed on a FACSCalibur (BD Immunocytometry Systems, San Jose, CA). For simultaneous surface and intracellular staining, intact cells were stained, washed, fixed with 4% paraformaldehyde/phosphate-buffered saline (PBS), permeabilized with 0.1% Saponin, and then reacted with GzmA-FITC (clone CB9; BD Pharmingen) or GzmB-PE (clone GB12; Caltag, Burlingame, CA). Relying on directly labeled monoclonal antibodies (mAbs), PMNs were found to contain neither GzmA or GzmB (Figure 1A).
Enriched human PMNs do not express granzymes A, B, or perforin. (A) Scatter and density plots for lineage-specific surface markers (CD3, CD15) and intracellular granzymes (GzmA and GzmB). Purified fractions were stained as indicated and a total of 10 000 events were acquired in the indicated gates. Data are representative of 1 of 2 donors. The numbers in the upper right corner of each panel indicate the percentage of events in the respective quadrants. (B) Purified PBMC (lanes 2-5) and PMN (lanes 6-9) lysates from 4 donors were blotted as indicated. Isolated GzmA, GzmB, or PFN (100 ng) served as positive control (lane 1). (C) PBMC and PMN lysates from 4 donors were assayed for GzmA and GzmB by EIA. Values represent concentration of granzyme (ng/mL) in lysate.
Enriched human PMNs do not express granzymes A, B, or perforin. (A) Scatter and density plots for lineage-specific surface markers (CD3, CD15) and intracellular granzymes (GzmA and GzmB). Purified fractions were stained as indicated and a total of 10 000 events were acquired in the indicated gates. Data are representative of 1 of 2 donors. The numbers in the upper right corner of each panel indicate the percentage of events in the respective quadrants. (B) Purified PBMC (lanes 2-5) and PMN (lanes 6-9) lysates from 4 donors were blotted as indicated. Isolated GzmA, GzmB, or PFN (100 ng) served as positive control (lane 1). (C) PBMC and PMN lysates from 4 donors were assayed for GzmA and GzmB by EIA. Values represent concentration of granzyme (ng/mL) in lysate.
For Western blot, lysates (20 μg) from PBMCs (lanes 2-5) and PMNs (lanes 6-9) were probed with anti-GzmA (GA4; 1:1000), anti-GzmB (2C5, 1:5000), or anti-PFN (2D4Perf, 1:1000; Figure 1B). GzmA, GzmB, and PFN proteins were detected in PBMC lysates of 2, 1, and 4 donors, respectively, but this approach failed to identify the proteins in PMN lysates. Next, a esterolytic assay relatively specific for GzmB (IETD-pNA4 ) yielded similar results (not shown). Finally, GzmA and GzmB levels in lysates were measured by a sensitive EIA.4,5 PBMCs contained GzmA and GzmB in 4 and 2 lysates, respectively. For PMN lysates, GzmA was identified at low levels consistent with the presence of contaminating PBMCs (5%) and GzmB was completely undetectable (Figure 1C).
The failure to adequately block PMN Fc receptors may have contributed to the results observed by Wagner et al1 and Hochegger et al.2 As a cautionary note, the combination of sensitive methodology and uncontrollable cellular contamination risks generating false-positive results that might contribute to the apparent presence of the granzymes in PMNs by reverse transcriptase–polymerase chain reaction (RT-PCR) or as we show here by a sensitive EIA.