Abstract
Amphoterin (HMGB1) is a 30-kD heparin-binding protein involved in process extension and migration of cells by a mechanism involving the receptor for advanced glycation end products (RAGE). High levels of amphoterin are released to serum during septic shock. We have studied the expression of amphoterin in monocytes and the role of amphoterin and RAGE in monocyte transendothelial migration. Un-activated monocytes in suspension did not reveal amphoterin on their surface, but adherent monocytes exported amphoterin to the cell surface. Immunohistochemical staining of arterial thrombi in vivo revealed amphoterin in mononuclear cells and in surrounding extracellular matrix. Amphoterin was secreted from phorbol ester and interferon-γ (IFN-γ)-activated macrophages, and the secretion was inhibited by blocking the adenosine 5′-triphosphate (ATP)-binding cassette transporter-1, a member of the multidrug resistance protein family. Amphoterin was specifically adhesive for monocytes in peripheral blood leukocyte adhesion assay. Adhesion caused an extensive spreading of cells, which was inhibited by the dominant-negative RAGE receptor (soluble ectodomain of RAGE), and adhesion up-regulated chromogranin expression in monocytes, also suggesting a RAGE-dependent interaction. Monocyte transendothelial migration was efficiently inhibited by anti-amphoterin and anti-RAGE antibodies and by the soluble RAGE. We suggest that amphoterin is an autocrine/paracrine regulator of monocyte invasion through the endothelium. (Blood. 2004;104:1174-1182)
Introduction
Circulating monocytes adhere to sites of vascular injury where they participate together with other cells in the regulation of blood clotting, inflammation, and wound healing. Adhesion to other cells and extracellular matrix components is a prerequisite for migration and tissue recruitment of monocytes.1,2 The knowledge of molecules involved in monocyte transendothelial migration is rapidly increasing. However, the overall picture of the transendothelial migration mechanism is not completely understood.2
Amphoterin is a 30-kD heparin-binding protein widely expressed in humans and other organisms, and it is abundantly expressed in the developing brain as well as in various immature and transformed cell lines.3-6 It was isolated as an extracellular neurite outgrowth-promoting protein, but its amino acid sequence turned out to be identical to high-mobility group-1 protein.5,7 In a new nomenclature of high-mobility group proteins amphoterin and other proteins identical in the cDNA sequence are called as HMGB1 (high-mobility group B-1).8 We have used the designation amphoterin for the protein occurring in the extracellular space and interacting with the cell surface.5
Surface-bound amphoterin is adhesive for neural cells and platelets, and it induces extension of membrane processes in adherent cells.3,9,10 Amphoterin binds to plasma membrane lipids, mainly to phosphatidylserine and sulfatide, and enhances and localizes plasminogen activation.6,9,11-13 In neurons, neurite outgrowth on amphoterin surface is mediated by receptor for advanced glycation end products (RAGE), a member of the immunoglobulin superfamily transmembrane receptors.14,15 Consistent with a potential role of RAGE-amphoterin interaction, RAGE and amphoterin were shown to colocalize during brain development, and their signaling induces neurite outgrowth by way of the guanosine triphosphatases (GTPases) rac and Cdc42 and up-regulates expression of chromogranins.14-16 Neurite outgrowth and tumor cell migration can be inhibited with sRAGE (soluble RAGE), antibodies against amphoterin, and by antisense oligonucleotides transfected into cells.14,17,18
Amphoterin can be detected on the cell surface and substratum-attached material under cell culture conditions and in vivo.4,6,9,19-21 Because amphoterin lacks a classical signal sequence, its export mechanism is currently poorly understood. It has been shown that amphoterin is secreted by way of an endolysosomal vesicle-mediated pathway and not by way of the classical endoplasmic reticulum (ER)/Golgi-mediated pathway.22,23 Amphoterin resembles in this respect other proteins, which lack a classical signal sequence but are considered to have an extracellular function, like basic fibroblast growth factor and interleukin-1β (IL-1β).24
Macrophages and monocytes have been shown to secrete amphoterin after induction with cytokines or endotoxin, and high amounts of amphoterin have been detected in sera from patients with endotoxic or hemorrhagic shock.19,25-27 Amphoterin mediates neutrophil migration and development of lung edema in endotoxin-induced lung inflammation.28 Exogenous amphoterin causes cultured human umbilical vein endothelial cell (HUVEC) activation, inducing intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin up-regulation, and increased adhesion of neutrophils, which can be partly inhibited by anti-RAGE antibodies.29,30
Although recent studies have highlighted the role of amphoterin/HMGB1 protein as a monocyte-activating molecule, the physiologic function of amphoterin/HMGB1 in monocyte biology is not fully understood. Because adhesive interactions, motility, and plasminogen activation are important for the physiologic function of monocytes and macrophages, we have studied the expression of amphoterin and its receptors in monocyte/macrophages. Further, we have studied mechanism of amphoterin secretion and the role of extracellular amphoterin and RAGE in transendothelial migration of monocytes. Our results suggest that amphoterin is secreted by way of a pathway requiring a multidrug resistance protein, and amphoterin-RAGE interactions play an essential role in the migration of monocytes through the endothelium.
Materials and methods
Materials
Recombinant amphoterin (recAtn), affinity-purified polyclonal anti-recombinant amphoterin (anti-recAtn), and antipeptide antibodies I to V were produced as described before.6 The recAtn preparations did not contain detectable endotoxin (< 0.05 IU/μg) as studied by the Limulus Amebocyte Lysate assay Pyrogent (BioWhittaker, Walkersville, MD). Anti-Atn was produced in chicken with use of recAtn as an immunogen (AgriSera AB, Vännäs, Sweden). Immunoglobulin Y (IgY) was isolated, and antibodies were affinity purified with use of a recAtn column.6,31 Ficoll-Hypaque and Dextran T 500 were from Pharmacia Biotech (Uppsala, Sweden). Alkaline phosphatase-conjugated (AFOS) rabbit antichicken and goat antimouse immunoglobulins, aprotinin, low-molecular-weight (LMW) heparin, heparin, bovine serum albumin (BSA), poly-L-lysine, sulphobromophtaleine (BSP), 4-phorbol-12-myristate-13 acetate (PMA), 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS), and formyl-methionylleucyl-phenylalanine (fMLP) were from Sigma (St Louis, MO). Glyburide reagents were from Sigma and ICN Biomedicals (Aurora, OH). Fibronectin was from Sigma and Alexis Corporation (Läufel-fingen, Switzerland). Vitronectin was from Alexis Corporation. Alkaline phosphatase-conjugated swine antirabbit and the rhodamine isothiocyanate (TRITC)-conjugated antimouse antibodies were from Dakopatts (Glostrup, Denmark). Horseradish peroxidase (HRP)-conjugated goat antirabbit IgG and rabbit antigoat IgG were from Bio-Rad (Hercules, CA). HRP-conjugated rabbit anti-chicken IgY was from Zymed (San Francisco, CA). Mouse monoclonal anti-CD14 was from CLB (Amsterdam, the Netherlands). Fluorescein isothiocyanate (FITC)-conjugated antichicken was from Jackson Immuno Research (West Grove, PA). Goat anti-RAGE antiserum was from Chemicon International (Temecula, CA). Human plasma albumin (HSA) was from the Finnish Red Cross Blood Transfusion Service (Helsinki, Finland). Bovine lung soluble RAGE was purified as described by using hydroxylapatite-, heparin-Sepharose, Mono S, and gel filtration chromathographies.32,33 Anti-P300 and anti-P301 were produced as described.29 Anti-RAGE N-16 was from Santa Cruz Biotechnology (Santa Cruz, CA). Interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α) were from Roche (Basel, Switzerland).
Production of recombinant fusion proteins
The entire extracellular coding region of mouse RAGE was amplified by using mouse RAGE specific primers (5′primer, 5′-TACTAGCTAGCGCCCGGATTGGAGAGCCACTTG-3′; 3′ primer, 5′-ATAGTTTAGCGGCCGCCCCAGACTCACCCACAGAG-3′), and the RAGE V1 domain was amplified with use of human RAGE specific primers (5′ primer, 5′-TACTAGCTAGCGCCCGGATTGGCGAGCCAC-3′; 3′ primer, 5′-ATAGTTTAGCGGCCGCCTGGTAGACACGGACTC-3′). The RAGE fragments were cloned into the modified pRMHA3 vector containing the CD33 signal sequence and human IgG Fc-part, and immunoglobulin fusion proteins were produced and purified as described.34 The mouse AMIGO extracellular part was cloned into modified pRMHA3 vector containing CD33 signal sequence and Strep-tagII fusion part. The soluble fusion protein was purified from S2 insect cell medium with strep-tactin Sepharose according to the manufacturer's guidelines (IBA GmbH, Göttingen, Germany).34
Cells
RAW264.7, U937, K562, and HL-60 cell lines were obtained from the American Type Culture Collection (ATCC; Rockville, MD), and cells were grown according to ATCC's guidelines. HUVECs were obtained from BioWhittaker and grown as described.35
Rat leukocytes were isolated from blood in ethylenediaminetetraacetic acid (EDTA) by Ficoll-Hypaque centrifugation for 30 minutes at 500g.
Glial cells were isolated from postnatal rat brain (P5-7) hypothalamus and midbrain areas as described previously.36 The cells were cultured for 2 to 6 weeks, and microglia was isolated by using extensive overnight shaking. Detached microglia was used for protein or RNA isolations or cell-spreading assays within 24 hours.
For immunofluorescence staining assays monocytes were isolated from whole blood or buffy coats with use of the Nycoprep 1.068 (Nycomed Pharma AS, Oslo, Norway) density centrifugation method or by dextran sedimentation (0.55% [wt/vol] dextran for 0.5 hours) to obtain leukocyterich plasma. The amount of platelets was minimized by centrifuging the monocytes or the leukocyte-rich plasma through a layer of 10% HSA-PBS (phosphate-buffered saline) for 10 minutes at 50g to 200g. The cells were then washed with RPMI by centrifugation.
Immunoblotting
Cells were lysed, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting were done as described.6 Monocyte lysates for anti-RAGE, anti-P300, and anti-P301 Western blotting experiments were obtained from recAtn (20 μg/mL) coated plastic adherent mononuclear cells. Cells were adhered for 1 hour, and nonadherent cells were washed away. Adherent cells were lysed in hot reducing Laemmli sample buffer.
Fluorescence microscopy
For immunostaining of cells in suspension, the cells were fixed with 2% paraformaldehyde-PBS, washed, and blocked with 1% HSA-PBS. For double-immunostaining, the cells were incubated with anti-recAtn detected by FITC-antichicken and then stained with anti-CD14 detected with TRITC-antimouse. The cells were centrifuged onto polylysine-coated object slides and examined with immunofluorescence microscopy as described elsewhere.9
For immunostaining of adherent cells, coverslips were coated with 10% autologous serum or purified proteins and saturated with albumin. The cells were kept on the coverslips for 1 hour, unbound cells were removed by washing, and the adherent cells were either immunostained immediately with anti-recAtn and anti-CD14 or cultured up to 18 hours.
Immunohistochemistry of in vivo thrombi
This study was approved by an institutional ethical committee of the Department of Surgery, Helsinki University Central Hospital, and informed consent was obtained from the study subjects. Embolectomy samples were obtained from patients treated for an acute lower limb arterial occlusion at the Department of Vascular Surgery of Helsinki University Central Hospital. The embolectomy specimens were taken within 24 hours of the onset of the symptoms, snap-frozen, and stored under liquid nitrogen. Frozen sections were fixed with cold acetone (-20°C) for 10 minutes and rinsed in PBS. The sections were incubated with affinity-purified anti-recAtn or nonspecific chicken IgY, both at 3 μg/mL, and the bound antibodies were visualized with the ABC complex/HRP method (Dakopatts).
Macrophage secretion assay
Trypsinized RAW 264.7 cells in 10% fetal calf serum (FCS) RPMI were plated (1.5 × 106 cells/well) to 6-well plates (Corning, Corning, NY) for 1 hour. Nonadherent cells were washed away. The cells were cultured in 1.5 mL OPTI-MEM I (Invitrogen Corporation, Carlsbad, CA) containing 10 μg/mL aprotinin and possible activators and inhibitors. At different time points medium was collected, 1 mL of 10 μg/mL LMW heparin in PBS was added to the cells, incubated for 1 minute, and pooled to the medium. The medium was centrifuged for 5 minutes at 700g, and the supernatants were used for lactate dehydrogenase (LDH) and amphoterin quantifications. LDH activity was detected with Cytotoxicity Detection Kit (Roche) using L-LDH from rabbit muscle (Roche) as a standard. Amphoterin was detected by Western blotting. A 1.5-mL sample of the medium sample was concentrated with Microcon 10 (Millipore, Billerica, Spain), lyophilized, and analyzed with use of anti-recAtn Western blotting. Optical density of the amphoterin bands was analyzed by using Tina 2.0 software (Raytest Isotopenmessgerate GmbH, Staubenhardt, Germany).
Inhibition of IL-1β secretion by ABC-1 inhibitors
RAW 264.7 cells (1.5 × 106 cells/well) on 6-well plate were activated with IFN-γ (20 ng/mL) for 3 hours. In some wells BSP, DIDS, and glyburide were used as inhibitors. IL-1β content in the culture medium was analyzed with enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN) by using recombinant mouse IL-1β (Roche) as a standard (n = 3).
Cell adhesion ELISA
The cell ELISA assay was performed as described before37,38 with the following modifications. Peripheral blood mononuclear cells in RPMI containing 0.1% HSA were incubated in the protein-coated microwells for 1 hour. The microwells were washed, and the bound cells were fixed and blocked. The cells were incubated with anti-CD14, washed, and incubated with AFOS-conjugated antimouse for 1 hour. The color development with AFOS substrate was measured.
Cell spreading studies
Central areas of plastic plates PD-LD, 3.5 cm in diameter (Greiner Bio-One, Longwood, FL) were coated with 20 μg/mL recAtn or fibronectin for 2 hours and blocked with 1% BSA-PBS. Monocytes were isolated by using RosetteSep-method (StemCell Technologies, Vancouver, Canada), and platelets were depleted with 10% BSA-PBS centrifugation. Monocytes or microglial cells were suspended in 0.1% BSA-RPMI and adhered for 1 hour, and nonadherent cells were washed away. The cell area was measured by taking digital pictures (Olympus IX70 microscope, 40 × objective, DP-10 digital camera; Olympus Optical, Tokyo, Japan) from adherent cells, which were analyzed using BioRad Quantity One software (Bio-Rad).
Expression of chromogranins
Human peripheral blood monocytes (isolated with the RosetteSep-method) or rat brain microglia in OPTI-MEM I were adhered to recAtn or fibronectin (20 μg/mL) coated plastic. Expression of chromogranis was analyzed by reverse transcriptase polymerase chain reaction (RT-PCR).39 Primer pairs for chromogranin cDNA amplifications were matched to different exons to avoid genomic DNA amplification. The primer sequences were as follows: 5′-CTCTTCTCAGAATGGCGTGTCTTCA-3′ and 5′-CGCTGCATCATTGAGGTCCTCTC-3′ for human chromogranin B; 5′-AGGAATATGCTGTGGAGCTCCTCT-3′ and 5′-CTTTTGGTGCAGACTGAGGCTCAT-′3 for human chromogranin C; 5′-CCTCTCAAATGCCCTATCCAAGTCCAGT-3′ and 5′-TATCTTTGCTTCTTTGGACACTTGTTGGTT-3′ for rat chromogranin B; and 5′-CTTGGAGCCTTCCACACAATATAAGA-3′ and 5′-TTCAGCTTGCCTCAAAGCCTCAAGT-3′ for rat chromogranin C. Chromogranin B and C cDNAs were amplificated by using program containing 45 cycles. cDNA content of rat samples were equalized according to RT-PRC results of porphobilinogen deaminase cDNA.40 All cDNA amplifications were made using DynaZyme DNA polymerase (Finnzymes, Espoo, Finland).
Monocyte chemotaxis assay
Human peripheral blood monocytes (5 × 104 cells; isolated with the RosetteSep-method) in 10% FCS-RPMI were placed to the upper compartment of the Transwell chambers (3-μm pore size). fMLP (100 nM) or recAtn (40 nM) was added to the lower well when indicated, and the cells were allowed to migrate for 3 hours. Migrated cells in the lower wells were quantified by using CyQuant (Molecular Probes, Leiden, The Netherlands).
Transendothelial migration
HUVECs (5 × 105/well) were grown overnight on fibronectin-coated (50 μg/mL) porous membranes in a Transwell chamber (Corning) of 6.5-mm diameter and 5-μm pore size and activated for 1 day with 0.5 ng/mL TNF-α.41 Unadherent HUVECs were washed away with PBS, and the Transwells were placed in RPMI 1640 + 10% FCS + 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH = 7.2. Monocytes (5-7 × 104) in 100 μL medium, with inhibitors when indicated, were added to the upper chamber. In transendothelial chemotaxis assay serum-free Dulbecco modified Eagle medium (DMEM) was used as a medium in both compartments, and fMPL was used as a positive control.42 After 3 hours the plates were transferred to 4°C for 30 minutes, and the migrated cells were counted microscopically.
Statistics
P values were calculated using Student unpaired t test in Microsoft Excel2000 program (Microsoft Corporation, Redmond, WA).
Results
Identification of amphoterin and RAGE
Western blotting of cell lysates using affinity-purified anti-recAtn antibodies revealed amphoterin in U937, HL-60, polymorphonuclear cells, and monocytes (Figure 1A). Amphoterin was detected in similar amounts in monocytes as in the immature myeloid cell lines HL-60 and U937 (Figure 1A). According to semiquantitative Western blotting amphoterin corresponded to 0.5% of total cellular protein in these cells and in K562 cells. In granulocytes the amount of amphoterin per total protein was about half of that in monocytes (data not shown). Thus, the proportion of amphoterin of total protein was about 50-fold higher in leukocytes than in platelets.9 No detectable amphoterin was present in erythrocytes (data not shown). The 30-kD protein in the monocyte lysate was also recognized with affinity-purified polyclonal antibodies raised against 5 synthetic peptides covering different parts of the amphoterin sequence (Figure 1B). All antibodies recognized the 30-kD band in the monocyte lysate (Figure 1B). Further, the 30-kD protein from the monocyte lysate bound to Heparin-Sepharose and eluted at a similar salt concentration (0.7-0.9 M NaCl) as the recombinant protein and was recognized by anti-recAtn (data not shown). Anti-recAtn and anti-peptide II, which have been shown to detect only amphoterin and not any other highly homologous 28- to 29-kDa proteins,6 detected a 30-kD band in rat brain microglia lysate (Figure 1C).
Western blotting analysis of amphoterin and RAGE. (A) Leukocytes were lysed with 1% SDS, and 10-g samples of total cellular protein or 40 ng recAtn were run under reducing conditions on SDS-PAGE and transferred to a nitrocellulose filter. The filter was immunostained with anti-recAtn antibodies. PMN indicates polymorphonuclear leukocytes. (B) Monocyte lysates (40 μg protein) and recAtn (80 ng) were run in SDS-PAGE and transferred to nitrocellulose filters. The filters were immunostained with 5 affinity-purified antibodies raised against different amphoterin peptides (I-V). (C) Rat brain microglia lysates were analyzed in Western blotting with anti-recAtn and antipeptide II. Both antibodies recognized a single 30-kD band from the lysate. (D) sRAGE was purified from bovine lung acetone powder. Purified sRAGE migrated as a single band in 12% SDS-PAGE stained with Coomassie blue (lane i). sRAGE was detected by 4 different anti-RAGE antibodies in 10% to 20% SDS-PAGE and Western blotting experiment: anti-P300 (lane ii), anti-P301 (lane iii), anti-RAGE (lane iv), and anti-RAGE N-16 (lane v). (E) Western-blotting of monocyte RAGE. RAGE was detected from 1-hour amphoterin adherent human monocytes or rat leukocytes using anti-P300 (lane i), anti-RAGE (lane ii), or anti-P301 (lane iii). Lanes i-ii: human cells. Lane iii: rat cells.
Western blotting analysis of amphoterin and RAGE. (A) Leukocytes were lysed with 1% SDS, and 10-g samples of total cellular protein or 40 ng recAtn were run under reducing conditions on SDS-PAGE and transferred to a nitrocellulose filter. The filter was immunostained with anti-recAtn antibodies. PMN indicates polymorphonuclear leukocytes. (B) Monocyte lysates (40 μg protein) and recAtn (80 ng) were run in SDS-PAGE and transferred to nitrocellulose filters. The filters were immunostained with 5 affinity-purified antibodies raised against different amphoterin peptides (I-V). (C) Rat brain microglia lysates were analyzed in Western blotting with anti-recAtn and antipeptide II. Both antibodies recognized a single 30-kD band from the lysate. (D) sRAGE was purified from bovine lung acetone powder. Purified sRAGE migrated as a single band in 12% SDS-PAGE stained with Coomassie blue (lane i). sRAGE was detected by 4 different anti-RAGE antibodies in 10% to 20% SDS-PAGE and Western blotting experiment: anti-P300 (lane ii), anti-P301 (lane iii), anti-RAGE (lane iv), and anti-RAGE N-16 (lane v). (E) Western-blotting of monocyte RAGE. RAGE was detected from 1-hour amphoterin adherent human monocytes or rat leukocytes using anti-P300 (lane i), anti-RAGE (lane ii), or anti-P301 (lane iii). Lanes i-ii: human cells. Lane iii: rat cells.
RAGE was detected in human and rat monocytes by immunoblotting of cells after adhesion to amphoterin. Functionality of the antibodies was tested by detecting sRAGE from bovine lung (Figure 1D). RAGE in human monocytes was detected with anti-RAGE and anti-P300 against human RAGE and with anti-P301 in rat monocytes (Figure 1E). Anti-RAGE detected an additional band of 45-kD size (Figure 1E).
Cell surface immunostaining of amphoterin in monocytes
The surface localization of amphoterin in monocytes was studied by double-immunofluorescence staining with anti-recAtn and anti-CD14 antibodies. When freshly isolated peripheral blood leukocytes were fixed in solution, CD14+ cells were not stained with anti-recAtn antibodies (Figure 2A-B). When the cells were kept on serum-coated coverslips for 1 hour, a minor proportion of the adherent cells were stained (data not shown), and after culture for 18 hours more than half of the CD14+ cells were stained with anti-recAtn antibodies (Figure 2C-D). Staining was more intense for cells cultured for 18 hours than after 1-hour attachment (data not shown). Characteristic for the staining was the occurrence of 1 or 2 intense patches localized mainly at the edges of the cells and staining of cellular processes. Control stainings using anti-α-actinin or nonspecific chicken IgG antibodies confirmed that the plasma membrane remained impermeable, and that the staining was specific for amphoterin (data not shown).
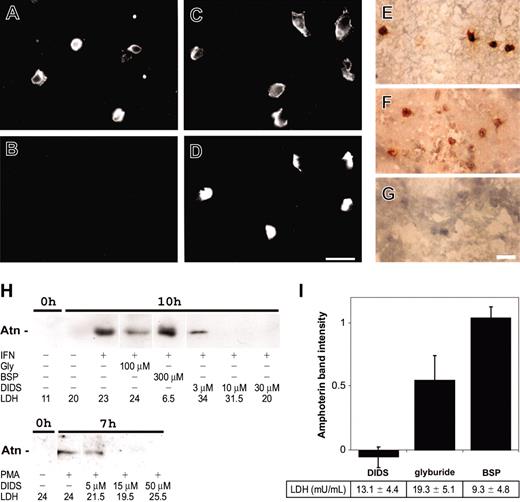
Secretion of amphoterin from mononuclear cells. (A-D) Freshly isolated peripheral blood leukocytes were double-immunostained in suspension (A-B) and after adhesion and culture on coverslips for 18 hours in the presence of 10% autologous serum (C-D). Staining for CD14 is shown in panels A and C and for amphoterin in panels B and D. Cells were fixed with 2% paraformaldehyde for 15 minutes and double-stained with anti-CD14 and TRITC-conjugated antimouse immunoglobulins followed by anti-recAtn and FITC-conjugated antichicken immunoglobulins. (E-G) Embolectomy sample taken within 24 hours of the onset of symptoms for lower-limb arterial occlusion was snap-frozen in liquid nitrogen. Frozen sections were fixed with cold acetone, incubated with anti-recAtn (E-F) or nonspecific chicken IgY (G), and detected with peroxidase-labeled antichicken IgY. Scale bar 20 μm (A-G). (H) Secretion of amphoterin from RAW 264.7 cells is induced by IFN-γ and PMA and inhibited with ABC-1 inhibitors DIDS and glyburide. Cells were treated with 20 ng/mL IFN-γ or 10 nM PMA, and medium samples (0, 7, or 10 hours) were collected. Medium (1.5 mL) was concentrated to 15 L and analyzed in anti-recAtn Western blotting. Amphoterin was detected from activated cell culture medium after 7 or 10 hours of culture. Secretion was dose dependently inhibited with DIDS and to a lower extent with glyburide. BSP did not inhibit secretion. Medium samples were analyzed for activity (in mU/mL) of LDH by enzyme activity measurement. The results shown are from a representative experiment of at least 3 experiments. IFN indicates IFN-γ. (I) Optical density (OD) of the amphoterin band in Western blotting of IFN-γ activated (7 hours) cell culture supernatants, and lactate dehydrogenase activity in supernatants was measured. OD of bands from noninhibited samples was defined as 1 (± SD, n = 3).
Secretion of amphoterin from mononuclear cells. (A-D) Freshly isolated peripheral blood leukocytes were double-immunostained in suspension (A-B) and after adhesion and culture on coverslips for 18 hours in the presence of 10% autologous serum (C-D). Staining for CD14 is shown in panels A and C and for amphoterin in panels B and D. Cells were fixed with 2% paraformaldehyde for 15 minutes and double-stained with anti-CD14 and TRITC-conjugated antimouse immunoglobulins followed by anti-recAtn and FITC-conjugated antichicken immunoglobulins. (E-G) Embolectomy sample taken within 24 hours of the onset of symptoms for lower-limb arterial occlusion was snap-frozen in liquid nitrogen. Frozen sections were fixed with cold acetone, incubated with anti-recAtn (E-F) or nonspecific chicken IgY (G), and detected with peroxidase-labeled antichicken IgY. Scale bar 20 μm (A-G). (H) Secretion of amphoterin from RAW 264.7 cells is induced by IFN-γ and PMA and inhibited with ABC-1 inhibitors DIDS and glyburide. Cells were treated with 20 ng/mL IFN-γ or 10 nM PMA, and medium samples (0, 7, or 10 hours) were collected. Medium (1.5 mL) was concentrated to 15 L and analyzed in anti-recAtn Western blotting. Amphoterin was detected from activated cell culture medium after 7 or 10 hours of culture. Secretion was dose dependently inhibited with DIDS and to a lower extent with glyburide. BSP did not inhibit secretion. Medium samples were analyzed for activity (in mU/mL) of LDH by enzyme activity measurement. The results shown are from a representative experiment of at least 3 experiments. IFN indicates IFN-γ. (I) Optical density (OD) of the amphoterin band in Western blotting of IFN-γ activated (7 hours) cell culture supernatants, and lactate dehydrogenase activity in supernatants was measured. OD of bands from noninhibited samples was defined as 1 (± SD, n = 3).
Expression of amphoterin in mononuclear cells and extracellular matrix of arterial thrombi
Because adhesion induced amphoterin secretion from monocytes, we studied amphoterin secretion in vivo using an easily available model of adherent monocytes. Frozen sections of arterial thrombi, which contained adherent monocytes, were immunostained with anti-amphoterin antibodies. The presence of amphoterin in mononuclear cells in vivo was analyzed from thromboemboli obtained from lower limb arteries. Immunohistochemical staining of the thrombi demonstrated typical morphology with layers of tangled fibrin strands intermingled by platelet aggregates.43 Strong amphoterin reactivity was seen in mononuclear cells visible among the fibrin strands (Figure 2E). Fainter extracellular reactivity was seen in regions populated by mononuclear cells (Figure 2F-G). The staining pattern differed from that seen with the platelet-specific markers GPIIbIIIa and P-selectin which stained large platelet aggregates (data not shown).
Secretion of amphoterin from mononuclear cells
The mouse macrophage cell line RAW 264.7 secreted amphoterin after treatment with 20 ng/mL IFN-γ or 10 nM PMA.23,25 Secretion did not correlate to LDH-leakage (Figure 2H). Amphoterin secretion was dose dependently inhibited by the ABC-1 inhibitor DIDS44,45 (Figure 2H). At 100 μM concentration DIDS inhibited amphoterin secretion totally (Figure 2I). IL-1β secretion was, as well, inhibited by DIDS (1.57 ± 0.03 ng/mL and 0.55 ± 0.28 ng/mL, noninhibited and 50 μM DIDS inhibited [± SD], respectively (n = 3, P = .034). Another ABC-1 inhibitor, glyburide, inhibited both amphoterin (Figure 2H-I) and IL-1β (1.23 ± 0.05 ng/mL, n = 3, P = .006) release. However, even at high concentrations of glyburide, inhibition of amphoterin release was only partial (Figure 2H-I). BSP inhibited IL-1β secretion at 300 μM concentration (0.54 ± 0.09 ng/mL, n = 3, P = .0007), but it did not inhibit amphoterin secretion (Figure 2H-I).
Adhesion and spreading of monocytes to immobilized amphoterin
Morphology of the CD14+ cells adhering to amphoterin-coated coverslips was strikingly different when compared with cells adhering to fibronectin- or vitronectin-coated coverslips. On the latter proteins, CD14+ cells exhibited little spreading during 1 hour (Figure 3A-B). In contrast, cells adhering to amphoterin demonstrated remarkably flattened morphology and large lamellipodia already after 1 hour (Figure 3C). In many cases the lamellipodia surrounded the cells in a halolike fashion. Staining with rhodaminephalloidin revealed the presence of actin filaments in the cytoplasm of cells kept on amphoterin and fibronectin for 1 hour. The surface area of amphoterin-adherent cells was clearly larger than that of fibronectin-adherent cells (Figure 3C). sRAGE inhibited monocyte spreading on amphoterin, whereas the control immunoglobulin superfamily protein, sAMIGO, had no effect (Figure 3D). Rat brain microglial cells studied as a representative of tissue-derived macrophages did not display differential spreading on amphoterin and fibronectin (217 ± 69 versus 258 ± 63 mm2 × 10-6 cellular area in cells adhering on amphoterin and fibronectin, respectively [mean ± SD], n = 3, P = NS).
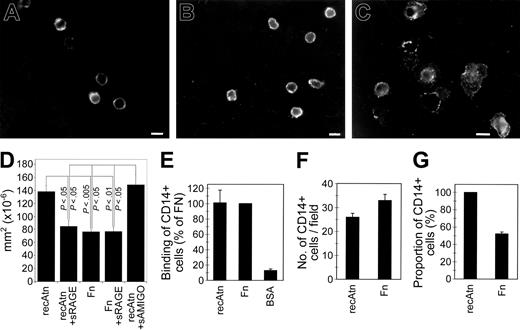
Adhesion and spreading of monocytes on amphoterin and extracellular matrix proteins. (A-C) Peripheral blood leukocytes were kept on coverslips coated with vitronectin (A), fibronectin (B), or recAtn (C) for 1 hour in serum-free medium. The coverslips were washed, fixed with 2% paraformaldehyde, and immunostained with anti-CD14 and TRITC-labeled antimouse immunoglobulins. Scale bar (A-C) 10 μm. (D) Measurement of amphoterin and fibronectin adherent monocyte areas. Monocytes were adhered to recAtn or fibronectin-coated plastic for 1 hour, and adherent cells were fixed. Digital microscopy pictures were taken, and cell areas were measured. Mean of areas was calculated. Amphoterin-adherent cells spread strongly compared with fibronectin-adherent cells, and spreading was inhibited with 100 g/mL sRAGE but not with sAMIGO. Error bars represent ± SD (n = 3). (E) Peripheral blood mononuclear cells in serum-free medium were incubated in microwells coated with recAtn, fibronectin (Fn), or albumin (BSA) for 1 hour, and the bound CD14+ cells were detected with anti-CD14 and ELISA. (F-G) Peripheral blood leukocytes were incubated on recAtn or Fn-coated coverslips for 1 hour in serum-free medium, and the bound cells were immunostained with anti-CD14 and TRITC-labeled second antibody. The number of CD14+ cells (F) and their proportion of total cells (G) were determined by fluorescence and phase-contrast microscopy. The mean and SEM of 3 different experiments (E) or 12 fields of 2 different experiments (F-G) are shown.
Adhesion and spreading of monocytes on amphoterin and extracellular matrix proteins. (A-C) Peripheral blood leukocytes were kept on coverslips coated with vitronectin (A), fibronectin (B), or recAtn (C) for 1 hour in serum-free medium. The coverslips were washed, fixed with 2% paraformaldehyde, and immunostained with anti-CD14 and TRITC-labeled antimouse immunoglobulins. Scale bar (A-C) 10 μm. (D) Measurement of amphoterin and fibronectin adherent monocyte areas. Monocytes were adhered to recAtn or fibronectin-coated plastic for 1 hour, and adherent cells were fixed. Digital microscopy pictures were taken, and cell areas were measured. Mean of areas was calculated. Amphoterin-adherent cells spread strongly compared with fibronectin-adherent cells, and spreading was inhibited with 100 g/mL sRAGE but not with sAMIGO. Error bars represent ± SD (n = 3). (E) Peripheral blood mononuclear cells in serum-free medium were incubated in microwells coated with recAtn, fibronectin (Fn), or albumin (BSA) for 1 hour, and the bound CD14+ cells were detected with anti-CD14 and ELISA. (F-G) Peripheral blood leukocytes were incubated on recAtn or Fn-coated coverslips for 1 hour in serum-free medium, and the bound cells were immunostained with anti-CD14 and TRITC-labeled second antibody. The number of CD14+ cells (F) and their proportion of total cells (G) were determined by fluorescence and phase-contrast microscopy. The mean and SEM of 3 different experiments (E) or 12 fields of 2 different experiments (F-G) are shown.
When peripheral blood leukocytes were allowed to adhere to immobilized amphoterin, fibronectin, or albumin, CD14+ cells bound to a similar extent to microwells coated with amphoterin or fibronectin, whereas only low binding was observed to wells coated with albumin (Figure 3E). When the cells were kept for 1 hour on protein-coated coverslips, about 30% more CD14-expressing cells were seen on fibronectin-coated coverslips than on amphoterin-coated coverslips (Figure 3F). Interestingly, almost all cells adhering to recAtn were CD14+, whereas half of the cells adhering to fibronectin were CD14- (Figure 3G). The total number of adhered leukocytes was 2- to 3-fold higher on fibronectin than on amphoterin.
Amphoterin-induced chromogranin B up-regulation
Up-regulation of chromogranins has been identified as a hallmark of amphoterin-RAGE interactions in neural cells.16,34 Recently, Tasiemski et al39 showed that monocytes express mRNA coding for chromogranin B but not for chromogranin A. We, therefore, studied whether mononuclear cells also express chromogranin C (secretogranin II) and whether the expression of chromogranins is up-regulated by amphoterin. RT-PCR revealed a band of expected size for chromogranin C in both monocytes and microglial cells (Figure 4A-B). Chromogranin B expression in amphoterin adherent monocytes was not altered after 1 hour, but it was strongly up-regulated after 20 hours (Figure 4A-C). In addition, chromogranin B was up-regulated in amphoterin-adherent microglia (Figure 4B-C). The expression level of chromogranin B in fibronectin-adherent cells remained unaltered (Figure 4A-C). Chromogranin C mRNA was not up-regulated in monocytes or microglia during adhesion (Figure 4A-C).
Detection of chromogranins B and C from monocytes and microglia, and amphoterin-induced up-regulation of chromogranin B. Cells were adhered to amphoterin or fibronectin for 1 or 20 hours. mRNA was isolated and amplified in RT-PCR using primers specific for chromogranins B and C. (A) Amplified monocyte cDNA was analyzed using agarose gel electrophoresis and ethidium bromide staining. Bands of the expected size were obtained for both chromogranin B and C. +RT indicates reverse transcripted RNA; -RT, RNA without reverse transcription. (B) Amplified microglia cDNA from 1-hour fibronectin adherent or 20 hours of amphoterin or fibronectin-adherent microglia was analyzed. Bands specific for chromogranin B and C were detected in agarose gel electrophoresis. (C) Amphoterin induced chromogranin B up-regulation. Optical density of the RT-PCR bands was determined. Optical density of bands from cells adhering for 1 hour on fibronectin was defined as 1. Chromogranin B mRNA was strongly up-regulated in monocytes during 20 hours of adhesion to amphoterin but not to fibronectin. In addition, chromogranin B was up-regulated in amphoterin-adherent microglia. Chromogranin C mRNA was not up-regulated during adhesion. Results of RT-PCR are from 3 different experiments using monocytes from 3 different donors and from 4 different experiments using rat microglia from 4 different littermates. Bars represent ± SD.
Detection of chromogranins B and C from monocytes and microglia, and amphoterin-induced up-regulation of chromogranin B. Cells were adhered to amphoterin or fibronectin for 1 or 20 hours. mRNA was isolated and amplified in RT-PCR using primers specific for chromogranins B and C. (A) Amplified monocyte cDNA was analyzed using agarose gel electrophoresis and ethidium bromide staining. Bands of the expected size were obtained for both chromogranin B and C. +RT indicates reverse transcripted RNA; -RT, RNA without reverse transcription. (B) Amplified microglia cDNA from 1-hour fibronectin adherent or 20 hours of amphoterin or fibronectin-adherent microglia was analyzed. Bands specific for chromogranin B and C were detected in agarose gel electrophoresis. (C) Amphoterin induced chromogranin B up-regulation. Optical density of the RT-PCR bands was determined. Optical density of bands from cells adhering for 1 hour on fibronectin was defined as 1. Chromogranin B mRNA was strongly up-regulated in monocytes during 20 hours of adhesion to amphoterin but not to fibronectin. In addition, chromogranin B was up-regulated in amphoterin-adherent microglia. Chromogranin C mRNA was not up-regulated during adhesion. Results of RT-PCR are from 3 different experiments using monocytes from 3 different donors and from 4 different experiments using rat microglia from 4 different littermates. Bars represent ± SD.
Amphoterin mediates transendothelial migration of monocytes
Expression of amphoterin and RAGE in monocytes and the extensive cell spreading effect of surface-bound amphoterin on monocytes suggested that amphoterin mediates monocyte migration in an autocrine/paracrine manner. We, therefore, tested whether amphoterin and RAGE are involved in migration of monocytes across porous filters. Amphoterin was not chemotactic in migration assays across uncoated or endothelium-coated polycarbonate filters (Table 1).
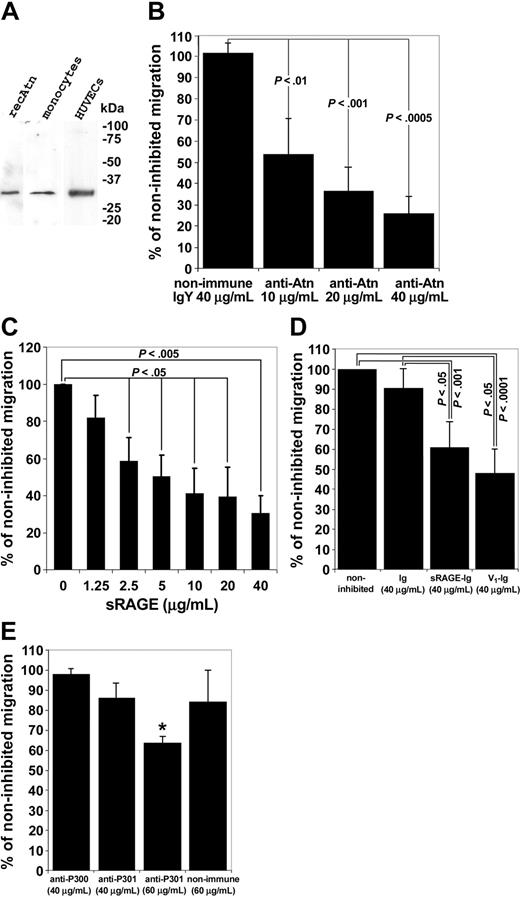
However, transendothelial migration was significantly and dose dependently inhibited by anti-amphoterin and anti-RAGE antibodies and by sRAGE (Figure 5B-D). Furthermore, ligand-binding distal immunoglobulin domain (V1 domain) of RAGE46,47 significantly inhibited transendothelial migration of monocytes (Figure 5E). It, thus, appears that amphoterin/RAGE has an autocrine/paracrine role in transendothelial migration of monocytes rather than a chemotactic role.
Monocyte transendothelial migration is inhibited by anti-amphoterin and anti-RAGE antibodies, and by soluble fragments of RAGE. (A) Western blotting of monocyte and HUVEC lysates with the anti-Atn used in the migration assay. Cells were lysed in hot reducing Laemmli sample buffer, and the lysates were analyzed in Western blotting on 10% to 20% gradient gel. recAtn was used as a standard. Western blotting experiments revealed specifically a 30-kD band. (B) Transendothelial migration assay of monocytes. Monocytes were added to upper chambers of HUVEC-coated Transwell filters. Monocytes were allowed to migrate for 3 hours. Various concentrations of antibodies were added to the upper well at the start of the experiment where indicated. Migrated monocytes were counted microscopically. Noninhibited migration was defined as 100%. Bars represent ± SD (n = 3). (C) Monocyte transendothelial migration is inhibited by sRAGE isolated from bovine lung. Monocytes were added to the upper wells of the Transwell chambers with various concentrations of sRAGE. Noninhibited migration was defined as 100%. Migration assay was done as in Figure 5B. (± SD, n = 3). (D) Monocyte transendothelial migration is inhibited by recombinant sRAGE-Ig and the RAGE V1 domain. Monocytes were added to the upper wells of the Transwell chambers with 40 g/mL sRAGE-Ig, V1-Ig, or Fc-control (Ig). Noninhibited migration was defined as 100%. Migration assay was done as in Figure 5B (± SD, n = 8 for noninhibited, Ig, and sRAGE-Ig wells, and n=4 for V1-Ig wells). (E) Various concentrations of anti-RAGE antibodies (anti-P300 and anti-P301) were added to upper well at the start of the experiment where indicated. Nonimmune IgG was used as a control. Migration assay was done as in Figure 5B. Noninhibited migration was defined as 100% (± SD, n = 4, *P < .00005).
Monocyte transendothelial migration is inhibited by anti-amphoterin and anti-RAGE antibodies, and by soluble fragments of RAGE. (A) Western blotting of monocyte and HUVEC lysates with the anti-Atn used in the migration assay. Cells were lysed in hot reducing Laemmli sample buffer, and the lysates were analyzed in Western blotting on 10% to 20% gradient gel. recAtn was used as a standard. Western blotting experiments revealed specifically a 30-kD band. (B) Transendothelial migration assay of monocytes. Monocytes were added to upper chambers of HUVEC-coated Transwell filters. Monocytes were allowed to migrate for 3 hours. Various concentrations of antibodies were added to the upper well at the start of the experiment where indicated. Migrated monocytes were counted microscopically. Noninhibited migration was defined as 100%. Bars represent ± SD (n = 3). (C) Monocyte transendothelial migration is inhibited by sRAGE isolated from bovine lung. Monocytes were added to the upper wells of the Transwell chambers with various concentrations of sRAGE. Noninhibited migration was defined as 100%. Migration assay was done as in Figure 5B. (± SD, n = 3). (D) Monocyte transendothelial migration is inhibited by recombinant sRAGE-Ig and the RAGE V1 domain. Monocytes were added to the upper wells of the Transwell chambers with 40 g/mL sRAGE-Ig, V1-Ig, or Fc-control (Ig). Noninhibited migration was defined as 100%. Migration assay was done as in Figure 5B (± SD, n = 8 for noninhibited, Ig, and sRAGE-Ig wells, and n=4 for V1-Ig wells). (E) Various concentrations of anti-RAGE antibodies (anti-P300 and anti-P301) were added to upper well at the start of the experiment where indicated. Nonimmune IgG was used as a control. Migration assay was done as in Figure 5B. Noninhibited migration was defined as 100% (± SD, n = 4, *P < .00005).
Discussion
In the present paper we show that amphoterin is secreted from mononuclear cells, specifically induces monocyte spreading as compared with peripheral leukocytes, and mediates transendothelial migration of monocytes. Amphoterin is one of at least 3 closely homologous heparin-binding proteins occurring in tissues.6 The same sequence as amphoterin has been cloned in studies of the DNA-binding HMGB1-protein.7 One highly homologous molecule existing in rat brain is HMGB2.48 The third member of the HMGB family, HMGB3, appears to be highly similar to rat brain heparin-binding protein P28 according to peptide sequences of digested fragments of P28 and results of Western blotting experiments with antipeptide antibodies.6,49 To discern amphoterin from the other homologous proteins, we have used affinity-purified antibodies against recombinant amphoterin and synthetic amphoterin peptides. In an earlier study we have demonstrated that amphoterin can be specifically recognized with these antibodies.6 The findings that all anti-amphoterin antibodies tested recognized the 30-kD monocyte protein and that the 30-kD heparin-binding protein was detected with anti-recAtn confirmed its identity with amphoterin.
The localization of amphoterin in the surrounding matrix of mononuclear cells observed in arterial thrombi suggests that amphoterin is exported from monocytes in vivo. This suggests that monocytes are at least one source of amphoterin in serum of septic patients. We have previously shown that platelets contain amphoterin mRNA and protein, and they release amphoterin to the extracellular space. However, the concentration of amphoterin in platelets is several fold lower than in monocytes. This may explain why platelet-rich areas in our staining of arterial thrombi were negative for amphoterin. Platelets have been shown to synthesize IL-1β from their mRNA during clot formation.50 Whether platelets use the same mechanism to synthesize new amphoterin polypeptide inside thrombi and, thus, enhance monocyte migration into the areas where plateletrich fibrin clots are formed remains to be clarified.
Various cell-activating agents have been shown to induce amphoterin secretion in culture.19,23,25 However, nicotine and acetylcholine may inhibit the release of proinflammatory cytokines, such as amphoterin/HMGB1, from macrophages expressing α-bungarotoxin-sensitive acetylcholine receptors.51,52 In this study we show that in addition to IFN-γ and PMA activation, adhesion induces amphoterin secretion from monocytes. Inhibition of IFN-γ- and PMA-induced secretion by DIDS suggests that ABC-1 is involved in the secretion process. However, another ABC-1 inhibitor, BSP, did not inhibit amphoterin secretion. Recently, Gardella et al22 showed that IL-1β and amphoterin/HMGB1 are not secreted by an identical mechanism. These results indicate some similarity, but not identity, in IL-1β and amphoterin secretion routes. Secretion of some other leaderless secreted proteins, annexin I and macrophage migration inhibitory factor, are inhibited with ABC-1 inhibitors.53,54 These data suggest that ABC-1 is involved in the secretion of some unrelated leaderless proteins.
The finding that monocytes but not other leukocytes adhered and rapidly spread on immobilized amphoterin suggests that extracellular amphoterin mediates cell-to-cell and cell-to-matrix interactions in monocytes. As the other leukocytes and tissue macrophages did not show corresponding adherence and spreading on amphoterin, this property appears to be specific for mature monocytes. However, in this study inability of rat brain microglia to spread on amphoterin could be due to the selection of poorly adherent microglia to the experiments, because microglia was isolated as a loosely adherent cell population detached by shaking. RAGE has been shown to mediate firm adhesion of monocytes to immobilized glycated albumin.55 Amphoterin-induced monocyte spreading that is inhibited by sRAGE suggests that RAGE is a monocyte receptor for amphoterin.
Our results show that anti-amphoterin and anti-RAGE antibodies and the dominant-negative RAGE receptor effectively inhibit transendothelial migration of monocytes. The finding that amphoterin is highly expressed in monocytes and also secreted by these cells suggests that amphoterin/RAGE belongs to the autocrine machinery enhancing monocyte migration rather than acting through a chemotactic mechanism, which inference is supported by our chemotaxis assays. Taken into account that HUVECs, in addition to monocytes, may release amphoterin, amphoterin-mediated transendothelial migration may also involve a paracrine interaction because of HUVEC-derived amphoterin.
The finding that the tissue-derived sRAGE is a more potent inhibitor than the recombinant sRAGE in the invasion assays may be due to different glycosylation of RAGE in tissue, which was recently shown to affect amphoterin binding.56 The strong inhibition hints at the possibility that blocking of amphoterin/RAGE interactions may inhibit some of the key regulators in the signaling cascade involved in transendothelial migration of monocytes. Rho family of small GTPases, including rac and Cdc42, is involved in RAGE-mediated amphoterin signaling.15 These GTPases are critical mediators of leukocyte invasion through the endothelium.1,57-60 This suggests that the inhibition mechanism of monocyte transendothelial migration by blocking amphoterin/RAGE may involve inhibition of the small GTPase signaling.
Anti-amphoterin antibodies and soluble fragments of RAGE cause a 60% to 70% inhibition of monocyte migration through endothelium. Furthermore, the anti-RAGE antibodies used in our study display some function-blocking activity. On the basis of these experiments the possibility still remains that other amphoterin-binding molecules in addition to RAGE, like proteoglycans61 or sulfoglycolipids,12 could play a role as amphoterin receptors in transendothelial migration.
The ability of amphoterin to bind plasminogen and effectively enhance its activation6 suggests that it may function as a nidus for plasminogen activation on the cell surface and enable directed proteolysis on the surface of monocyte/macrophages during cell migration and tissue repair. Expression of amphoterin at the cell surface of spreading monocytes is compatible with this inference.
Chromogranin B was found to be an amphoterin/RAGE-induced gene in differential display assays, and ligation of hippocampal neuron surface RAGE with amphoterin or with anti-RAGE antibodies up-regulated chromogranin B mRNA expression.16,34 In this study we found that this same gene is up-regulated during monocyte adhesion to amphoterin. This finding, supported by our other results, suggests that interactions of monocytes with amphoterin are mediated by RAGE. Induction of chromogranin B gene because of amphoterin interactions in monocytes suggests a possible role of amphoterin in innate immune response mediated by secretolytin which is an antibacterial peptide derived from the C-terminus of chromogranin B.62 In addition, chromogranin B has been shown to be a neurite outgrowth-enhancing heparin-binding protein.63 It might, thus, act as a cell migration and motility factor such as amphoterin. Whether amphoterin induces release of chromogranin B or secretolytin from monocytes is not currently known.
In this study we show that both monocytes and microglia express RNA coding for chromogranin C. According to our knowledge this is the first time that chromogranin C is identified in mononuclear cells. Chromogranin C is a precursor for the monocyte migration-enhancing peptide secretoneurin.42 Expression of chromogranin C in monocytes and microglia brings out an interesting possibility that chromogranin C and peptides derived from it might act as endogenous migration-enhancing substances in monocytes and microglia.
Prepublished online as Blood First Edition Paper, May 6, 2004; DOI 10.1182/blood-2003-10-3536.
Supported by grants from the Finnish Academy, Finnish Cancer Organizations, and the Sigrid Juselius Foundation (H.R., A.R., J.K-P.) and from the Sigrid Juselius Foundation (R.K.T.).
J.K-P. and E.W. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Kirsti Salmela, Anne Remes, Seija Lehto, Eveliina Saarikalle, and Erja Huttu for excellent technical assistance.