Comment on Luft et al, page 1066
The strength and persistence of identical signaling pathways determine the capacity of monocyte-derived DCs either to migrate to draining lymph nodes or to secrete locally inflammatory cytokines.
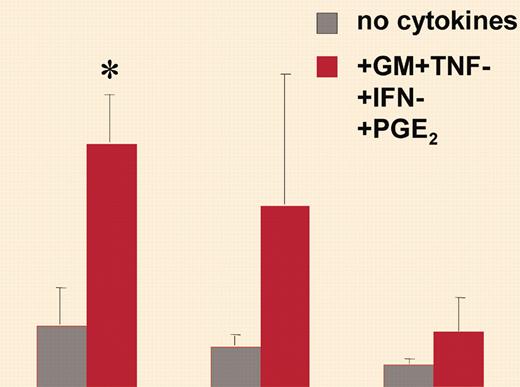
Dendritic cells are now perceived as sentinels to the immune system at the interface between innate and acquired immunity. They are empowered by their ability to recognize pathogens through Toll-like receptors (TLRs) and to alert both the innate arm of the immune system (through the release of inflammatory mediators at the site of infection) and the acquired arm (by eliciting a pathogen-specific immune response through naive T-cell priming following their migration to the draining lymph node). In a series of studies, Luft et al1 have built a model suggesting that, depending on the signal encounter, dendritic cells (DCs), in particular DCs derived from monocytes, may acquire the capacity either to produce high levels of inflammatory cytokines or to migrate to the draining lymph node, these 2 capacities being mutually exclusive. The capacity of DCs to migrate to draining lymph nodes relies on their ability to respond to CC chemokine receptor 7 (CCR7) ligand chemokines, which drive their emigration out of the inflammatory site and their entry into the lymph stream.2 The authors have shown that monocyte-derived DCs activated by certain types of activation, such as tumor necrosis factor (TNF) + prostaglandin E2 (PGE2), have a high propensity to respond to CCR7 ligands while producing low levels of inflammatory cytokines. In contrast, stronger stimuli, such as trimeric forms of CD40 ligand (CD40L), induce monocyte-derived DCs to secrete high levels of interleukin-12p70 (IL-12p70) but prevent their response to CCR7 ligands. In a report in this issue, Luft and colleagues use monomeric or trimeric forms of CD40L or intact microbes applied during controlled time lapse to further show that the strength and persistence of identical signaling pathways determine the capacity of monocyte-derived DCs either to develop strong migratory ability toward CCR7 ligand (weak and transient signaling) or to secrete inflammatory cytokines (strong and persistent signaling). Indeed, they found that the strength and persistence of signaling induced by these different activators, as measured by the levels of extracellular signal-regulated kinase 1/2 (ERK1/2) and p38K phosphorylation and nuclear factor–κB (NF-κB) binding activity, were predictive determinants of the functional profile expressed by monocyte-derived DCs. ERK1/2 and p38K activation synergistically mediated cytokine secretion, whereas migration was enhanced by p38K activation but reduced by persistent ERK1/2 activity. The predominant migratory-type functional profile of CD1c+ peripheral blood DCs (PBDCs) can be explained according to their model by nonpersistent signaling induced in this population through CD40 ligation.FIG1
The initial activation stimulus commits MoDCs irreversibly to express a specific functional phenotype. See the complete figure in the article beginning on page 1066.
The initial activation stimulus commits MoDCs irreversibly to express a specific functional phenotype. See the complete figure in the article beginning on page 1066.
These observations are reminiscent of those by Rescigno and colleagues (Rotta et al3 ) showing that during virulent bacterial infection induced inflammation monocytes cannot differentiate into DCs and cannot migrate to the draining lymph node.
Thus, depending on the local environment, monocyte-derived DCs can either develop their inflammatory function or migrate to the draining lymph node, while other pre-conditioned populations, such as the CD1c+ PBDCs or resident DCs (Langerhans cells [LCs], interstitial DCs), can perceive only transient signals, allowing them to migrate and to induce an acquired immune response even in the case of acute and persistent infection.
According to this model, persistent signaling may condition the local environment during pathogen invasion, when large amounts of IL-12p70 activate innate effector cells, such as natural killer (NK) cells. In contrast, DCs migrating out of epicenters of infection to draining lymph nodes will produce attenuated levels of cytokines more suitable for the priming and stimulation of antigen-specific T cells and avoiding bystander activation.