Abstract
Immature B cells are targeted to specific areas in the spleen, where a fraction of these cells receive signals that induce them to mature and participate in the immune response. In this study, we show that the C-C chemokine receptor 2 (CCR2) is transcribed in immature B cells, while its message is dramatically down-regulated at the mature stage. CCR2-deficient cells exhibit up-regulation of chemokine-induced actin polymerization, migration, and homing to the lymph nodes of immature B cells. In addition, we demonstrate that control of homing by CCR2 is mediated by its ligand, CCL2/JE, which is secreted by B cells and down-regulates the stromal derived factor-1 (SDF-1) signaling cascade. Thus, this study describes an additional, previously uncharacterized, role for CCR2 and its ligand as negative regulators of the homing of immature B cells.
Introduction
B-cell development involves the ordered progression of a stem cell through a number of stages, ultimately resulting in a mature B cell. In the bone marrow, B-cell development can be divided into different stages according to the rearrangement status of the immunoglobulin H (IgH) and IgL chain loci1,2 and the expression of intracellular and surface-bound markers. The first cells expressing IgM at their surface during this developmental process are the immature B cells,3 which leave the bone marrow and migrate to the spleen for their final maturation.4,5
One of the most fundamental and important aspects of the immune system is its ability to recognize and respond to virtually any foreign antigen while maintaining strict unresponsiveness to self-antigens. For B cells, antigens encountered during the immature stage are likely to be self-antigens, and reactive clones are, therefore, subjected to negative selection through deletion,6-8 receptor editing,9,10 and anergy.11 The selection process occurring within the immature B-cell compartments is also extended to the periphery, allowing deletion of virtually all B cells recognizing peripheral self-antigens outside of the bone marrow.12
Immature B cells leave the bone marrow and migrate to the spleen where they can mature prior to antigen encounter. Like other naive lymphocytes, before their arrival in the spleen immature B cells might recirculate to nonsplenic secondary lymphoid organs, which are specialized tissues for collecting antigens,13 or to sites of infection and inflammation. In these secondary lymphoid organs, where differentiation to mature cells does not occur, antigen encounter would lead to the death of the immature B cells and elimination of effective clones because of the negative selection process. Thus, it is imperative for B cells to home to the spleen and undergo a splenic maturation step before their release into the periphery.
Homing of immature B cells to the spleen proceeds through the terminal branches of central arterioles to blood sinusoids of the marginal zone.4,14 These immature B cells then penetrate the marginal zone sinus and reside in the outer zone of the periarteriolar lymphoid sheath (PALS),15 where they become part of the B-cell–rich follicular areas.15,16 At this site in the spleen, B cells are still immature and can be distinguished from their mature counterparts.4,14,17 The transition from immature to mature B cell is characterized by a series of changes in surface marker expression and the activities of these B cells.
Two populations of splenic B cells were recently identified as precursors of mature cells. Transitional B cells of type 1 (T1) are the recent immigrants from the bone marrow. Migrating T1 cells expressing B-cell receptors (BCRs) with high affinity to soluble self-antigens (Ags) in the blood are likely to die by negative selection by way of Ag-induced death. On entry to the spleen, T1 cells remain at the PALS, where additional bloodborne self-Ags trapped by the spleen may further drive negative selection. The remaining T1 cells develop into transitional B cells of type 2 (T2), which are found exclusively in the primary follicles of the spleen.18 Situated in the microenvironment of the splenic follicles, T2 cells are shielded from the soluble antigens to which T1 cells are exposed. Instead, T2 cells likely encounter a unique set of Ags, possibly on follicular dendritic cells, which results in positive selection.19
Previously, we demonstrated that immature B cells can inhibit their own integrin-mediated adhesion to the extracellular matrix and consequently suppress their own migration into nonsplenic sites. This inhibition is mediated by interferon gamma (IFN-γ), which is transcribed and secreted at low levels by immature B cells and is down-regulated at the mature stage.20 IFN-γ secretion is regulated by the Ly49D receptor, which is expressed on immature B cells and recognizes major histocompatibility complex (MHC) class I on peripheral tissues. This interaction induces secretion of low levels of IFN-γ by immature B cells, thereby down-regulating their homing to the lymph nodes or to sites of inflammation.21 The inhibitory signal of IFN-γ is transmitted through the IFN-γ receptor, whose engagement leads to inhibition of cytoskeletal rearrangement required for promoting integrin-mediated adhesion and migration of B cells.20,22
To ensure complete control of their homing to the lymph nodes, immature B cells might use additional signals to regulate their migration. These cells may secrete specific cytokines or chemokines, which can be down-regulated in the mature stage, to enable targeted B-cell migration to the spleen. In addition, immature B cells might express specific receptors that are down-regulated after splenic maturation. To identify such possible shifts in expression, we analyzed genes that are differentially expressed in immature B cells and are down-regulated in the mature stage. We show here that immature B cells, but not mature cells, express the CC chemokine receptor 2 (CCR2). We further demonstrate that this receptor is involved in the homing regulation of immature B cells and their targeting to the spleen for their final maturation.
Materials and methods
Mice
C57BL/6, invariant chain-deficient (Ii–/–),23 and CCR2–/–24 mice were used at 6 to 8 weeks of age. The Animal Research Committee at the Weizmann Institute approved all animal procedures.
Cells and separation of B cells
Spleen and lymph node cells were obtained from the various mice at 6 to 8 weeks of age as previously described.25 Control and CCR2–/–IgD– cells were separated from the IgD+ B cells by using the magnetic cell sorter (MACS) system as described.26 Ii–/– B cells were purified by using CD45R beads. To separate the IgD–, CD21+, and CD21– populations, IgD– cells were separated according to their CD21 expression by using the MACS system, as previously described.26 To isolate T1, T2, and mature B cells we followed the procedure described previously.27 The murine pre–B-cell-like lymphoma line, 70Z/3,28 was grown in suspension culture at 37° C in RPMI-1640 medium containing 10% (vol/vol) fetal calf serum (FCS) and 200 μM β-mercaptoethanol.
DNA chip arrays
The Affymetrix genechip expression analysis system was used for differential expression analysis. The Weizmann Institute has an Affymetrix-based service facility, which routinely performs RNA labeling, hybridization, and data analysis. RNA from control or Ii–/– B spleen cells were reverse transcribed to cDNA in the presence of biotinylated nucleotides. The target cDNA was then hybridized to the genechip under stringent conditions. The hybridized probe array was stained with a streptavidin-phycoerythrin conjugate and scanned by using the GeneArray scanner.
RNA isolation and reverse transcription
Total RNA was isolated from cells by using the Tri reagent kit (Molecular Research Center, Cincinnati, OH). Reverse transcription was carried out by using Superscript II RT (Gibco-BRL, Grand Island, NY). The primers used included: CCL2/JE, 5′-CCCACTCACCTGCTGCTACT-3′ and 5′-AAGTGCTTGAGGTGGTTGTG-3′; IFN-γ, 5′-CATTGAAAGCCTAGAAAGTCTG-3′ and 5′-CTCATGAATGCATCCTTTTTCG-3′, CCR2, 5′-ATGTTACCTCAGTTCATCCAC-3′ and 5′-GCCCACAAAACCAAAGATGAAT-3′; hypoxanthine phosphoribosyl-transferase (HPRT), 5′-GAGGGTAGGCTGGCCTATGGCT-3′ and 5′-GTTGGATACAGGCCAGACTTTGTTG-3′.
Immunofluorescence and flow cytometry
Staining was performed on freshly isolated splenocytes as previously described.29 The following antibodies were used: anti-CD45R/B220, AMS 9.1 anti-IgD, 2B11 anti-CXCR4, and R6-60.2 anti-IgM (Pharmingen, San Diego, CA).
Transwell migration
Chemotaxis was assayed as previously described.22 Briefly, about 3 × 106 cells were treated with or without JE (PeproTech, Rocky Hill, NJ) at a concentration of 1 μg/mL or 0.1 μg/mL. The migration toward the chemokine, stromal derived factor-1 (SDF-1; PeproTech), residing in the lower part of the apparatus, was analyzed after 3 hours by FACSort.
Lysis of cells and Western blot analysis
JE detection. B220+ cells were collected from control or Ii–/– mice and were lysed as previously described.25 Lysates were separated by 10% (wt/vol) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE); the proteins were transferred onto nitrocellulose and probed with anti-JE (PeproTech) followed by horseradish peroxidase–conjugated anti-mouse or rabbit IgG (Jackson Labs, West Grove, PA).
ERK detection. Cells were lysed as previously described.21 Lysates were separated by 10% (wt/vol) SDS-PAGE. Proteins were electrotransferred to nitrocellulose membranes and reacted with antiphospho-specific extracellular signal-regulated kinase (ERK) 1 or 2 (a kind gift from Dr Rony Seger, Weizmann Institute, Rehovot, Israel) followed by peroxidase-labeled goat antimouse (Jackson Labs), or reacted with polyclonal anti-ERK1/2 (provided by R. Seger, Weizmann Institute) followed by peroxidase anti-rabbit (Jackson Labs).
Cytoskeleton rearrangement
Mature or immature B cells from control or CCR2–/– mice were analyzed without any pretreatment; 70Z/3 cells or immature B cells from Ii–/– mice were pretreated with JE or conditioned medium derived from mature B cells (described in “Cell supernatant”). Cytoskeleton rearrangement was analyzed by flow cytometry following staining with fluorescein isothiocyanate (FITC)–phalloidin, as previously described.22
Cell supernatant
B220+ cells from control mice were plated at a concentration of 3 × 107 (concentrated) or 3 × 106 (diluted) cells/mL in a 96-well plate. After 30 minutes, their supernatant was collected. The supernatant was treated with anti-JE (PeproTech) or with anti-CD8 (Pharmingen) antibodies overnight at 4° C.
Tracking of cells in vivo
Purified immature B cells (IgD– B220+) from control and CCR2–/– mice were labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFDA-SE; Molecular Probes, Leiden, the Netherlands) for 15 minutes at room temperature. The cells were injected into control C57BL/6 mice. Homing of immature B cells to the spleen and the lymph nodes (LNs) was analyzed as previously described.20
Measurement of calcium signaling
70Z/3 cells were pretreated in the presence or absence of JE/CCL2 1 ng/mL for 30 minutes. The cells were then washed 4 times with Tyrode buffer (137 mM NaCl, 2.7 mM KCL, 0.4 mM NaH2PO4, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 5.6 mM glucose, 0.5 mM MgCl2, 2 mM CaCl2, and 1 g/mL bovine serum albumin [BSA], pH 7.3) and incubated for 30 minutes at 37° C with Tyrode buffer and 3 μM Fura2 am (Pentaacetylmonoester of 1-[2-(5-Carboxyoxazol-2-yl)-6-aminobenzofuran-5-oxyl]-2-(2′-amino-S′-methylphenoxy)-ethan-N, N, N′N′-tetra acetic acid; Molecular Probes). The cells were then washed 4 times, and the fluorescence was monitored on a Perkin-Elmer (Boston, MA) LS50B luminescence spectrometer at emission wavelength of 510 nm and at alternate excitation wavelength of 340 nm for baseline fluorescence. After 1 minute, 0.5 μg/mL SDF-1 was added to the cuvette, and the fluorescence monitoring continued.
Results
CCR2 expression is developmentally regulated in B cells
Differentiation of immature to mature B cells involves changes in the expression of a large series of genes. To identify genes that are down- or up-regulated during this maturation stage, we used the Affymetrix Genechip Expression Analysis system to compare the expression pattern of RNA from immature B cells to that of RNA from mature cells. In this analysis, RNA from immature B cells from control (C57BL/6) mice or immature B cells from Ii–/– mice, whose B cells are arrested at the immature stage,29 were compared with RNA derived from (C57BL/6) mature B cells. Many genes were found to be differentially expressed in these populations, including the cytokine IFN-γ, which was shown in our previous studies to be expressed in the immature stage and not in mature cells.20 Another protein that was found to be differentially expressed was CCR2, which was expressed in immature B cells from either control or Ii–/– B cells and whose expression was down-regulated in the mature cells. We speculated that this receptor might be a candidate for an additional molecule that regulates homing of immature B cells.
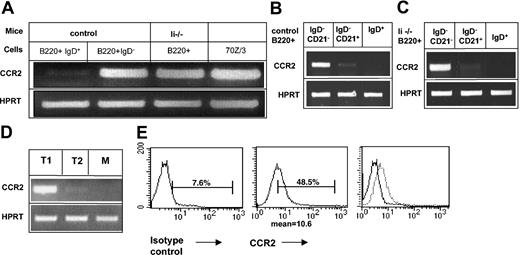
To confirm the down-regulation of CCR2 expression in mature B cells, we first analyzed its transcription in immature and mature B populations. B220+ IgD– from either control (C57BL/6) or Ii–/– mice and mature (B220+ IgD+) B cells from control mice were isolated, and CCR2 message in each population was analyzed by reverse transcriptase–polymerase chain reaction (RT-PCR). As can be seen in Figure 1A, although CCR2 mRNA appeared in the immature B populations from both control and Ii–/– mice, its expression was dramatically down-regulated in the mature stage. Newly emigrant immature B cells can be separated from the marginal zone cells by the expression of the CD21 marker. To determine which population transcribes CCR2 message, we analyzed CCR2 mRNA in the CD21+ and CD21– IgD– populations. As can be seen in Figure 1B-C, CCR2 was expressed in immature B cells (B220+ IgD– CD21–) from both control (Figure 1B) and Ii–/– (Figure 1C) mice. Its expression was dramatically decreased in marginal zone (B220+ IgD– CD21+) B cells. To further follow CCR2 expression we purified T1, T2, and mature populations according to markers described previously.27 As can be seen in Figure 1D, CCR2 is transcribed at the T1 stage, and its message is almost absent in the T2 population, suggesting down-regulation of its expression during this differentiation step. To further demonstrate that B cells transcribe CCR2, we analyzed its message in the cell line 70Z/3.28 As can be seen in Figure 1A, CCR2 mRNA was detected in 70Z/3 pre-B cells.
CCR2 is expressed in immature B cells and is down-regulated as part of the transition between immature and mature B cells. (A-D) RT-PCR of (A) immature (IgD– B220+) cells derived from control or invariant chain-deficient (Ii–/–) mice; control mature (IgD+ B220+) cells and 70Z/3 cells were purified. Total RNA was isolated, and reverse transcription was carried out by using Superscript II RT. (B-C) B cells (B220+) from control (B) or invariant chain deficient (Ii–/–) (C) mice were separated into IgD+ and IgD– cells; IgD– cells were separated according to their CD21 expression using the MACS system. Total RNA was isolated, and reverse transcription was carried out by using Superscript II RT. The results presented are representatives of 5 different experiments. (D) T1, T2, and mature (M) B cells were separated by fluorescence activated cell sorting (FACS). Total RNA was isolated, and reverse transcription was carried out by using Superscript II RT. (E) FACS analysis of CCR2 expression on 70Z/3 cells. 70Z/3 cells were stained with anti-CCR2 or a control antibody. The results presented are representative of 3 different experiments. Brackets indicate percentage of CCR2-positive cells.
CCR2 is expressed in immature B cells and is down-regulated as part of the transition between immature and mature B cells. (A-D) RT-PCR of (A) immature (IgD– B220+) cells derived from control or invariant chain-deficient (Ii–/–) mice; control mature (IgD+ B220+) cells and 70Z/3 cells were purified. Total RNA was isolated, and reverse transcription was carried out by using Superscript II RT. (B-C) B cells (B220+) from control (B) or invariant chain deficient (Ii–/–) (C) mice were separated into IgD+ and IgD– cells; IgD– cells were separated according to their CD21 expression using the MACS system. Total RNA was isolated, and reverse transcription was carried out by using Superscript II RT. The results presented are representatives of 5 different experiments. (D) T1, T2, and mature (M) B cells were separated by fluorescence activated cell sorting (FACS). Total RNA was isolated, and reverse transcription was carried out by using Superscript II RT. (E) FACS analysis of CCR2 expression on 70Z/3 cells. 70Z/3 cells were stained with anti-CCR2 or a control antibody. The results presented are representative of 3 different experiments. Brackets indicate percentage of CCR2-positive cells.
To determine cell surface protein expression of CCR2 in immature B cells, we first tried to analyze receptor expression on immature B splenocytes by using FACS analysis. However, it was difficult to demonstrate specific staining because of the high background binding of the secondary antibody (not shown). We, therefore, analyzed the expression of CCR2 on 70Z/3 cells, which do not express surface immunoglobulin. As can be seen in Figure 1E, CCR2 protein was detected on 70Z/3, indicating translation of CCR2 in early B cells.
Thus, CCR2 is expressed in early stage B cells and its expression is down-regulated during maturation.
CCR2 regulates chemokine-induced migration of immature B cells in vitro
To determine whether CCR2 participates in a pathway that regulates homing of immature B cells, we followed the behavior of a CCR2-deficient population. First, we compared the B-cell population in spleens derived from control and CCR2–/– mice. As can be seen in Figure 2A, similar levels of the various B-cell populations were detected in both mice. Next, the chemokine induced integrin-mediated in vitro migration of immature B cells derived from control versus CCR2-deficient (CCR2–/–) mice was compared. Purified immature B (B220+ IgD–) cells from either control (C57BL/6) or CCR2–/– mice (Figure 2B) were placed in the upper chamber of a transwell plate, and the migration toward the chemokine SDF-1 residing in the lower chamber was evaluated by FACS after 3.5 hours. A substantial elevation in CCR2–/– cell migration toward SDF-1 compared with control cells was observed (Figure 2C).
CCR2-deficient immature B cells exhibit elevated actin polymerization and chemokine-induced migration. (A) Splenocytes from control and CCR2–/– mice were triple stained with anti-B220 and anti-IgD or anti-IgM, anti-CD23, anti-CD21, and anti-CD5. Dot plots show the expression of the markers on B220+ cells. M indicates mature B cells; T1, transitional B cells 1, T2, transitional B cells 2; MZ, marginal zone B cells. Histograms show the expression of CD5 on B220 cells. Numbers represent percentage of each population. The results presented are representative of 5 different experiments. (B) Purification of immature B cells. IgD– splenocytes from control and CCR2–/– mice were separated by anti-B220 magnetic beads as described in “Materials and methods.” Numbers represent percentage of CD5-positive cells. (C) Transwell migration assay. Immature (IgD– B220+) B cells from control or CCR2–/– mice were placed in the upper well of a 24-well transwell plate in the presence or absence of SDF-1 (1000 ng/mL). The number of the migrating cells found in the lower chamber was evaluated after 3 hours of FACS analysis. Percentage migration was calculated as the number of migrating cells in the lower chamber as a fraction of the input cells in the upper chamber. The results presented are representative of 3 different experiments. (D) Cytoskeleton rearrangement. Immature B cells from control and CCR2–/– mice were stimulated with 500 ng/mL SDF-1 for 15 seconds, fixed, and permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. The change in polymerized actin was analyzed by FACS. Percentage increase in actin polymerization was calculated as the polymerization of actin in the presence of SDF-1–polymerization of actin without SDF-1/polymerization of actin without SDF-1. The results presented are representative of 5 different experiments. Error bars represent standard error of the results of all the experiments used to calculate the average.
CCR2-deficient immature B cells exhibit elevated actin polymerization and chemokine-induced migration. (A) Splenocytes from control and CCR2–/– mice were triple stained with anti-B220 and anti-IgD or anti-IgM, anti-CD23, anti-CD21, and anti-CD5. Dot plots show the expression of the markers on B220+ cells. M indicates mature B cells; T1, transitional B cells 1, T2, transitional B cells 2; MZ, marginal zone B cells. Histograms show the expression of CD5 on B220 cells. Numbers represent percentage of each population. The results presented are representative of 5 different experiments. (B) Purification of immature B cells. IgD– splenocytes from control and CCR2–/– mice were separated by anti-B220 magnetic beads as described in “Materials and methods.” Numbers represent percentage of CD5-positive cells. (C) Transwell migration assay. Immature (IgD– B220+) B cells from control or CCR2–/– mice were placed in the upper well of a 24-well transwell plate in the presence or absence of SDF-1 (1000 ng/mL). The number of the migrating cells found in the lower chamber was evaluated after 3 hours of FACS analysis. Percentage migration was calculated as the number of migrating cells in the lower chamber as a fraction of the input cells in the upper chamber. The results presented are representative of 3 different experiments. (D) Cytoskeleton rearrangement. Immature B cells from control and CCR2–/– mice were stimulated with 500 ng/mL SDF-1 for 15 seconds, fixed, and permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. The change in polymerized actin was analyzed by FACS. Percentage increase in actin polymerization was calculated as the polymerization of actin in the presence of SDF-1–polymerization of actin without SDF-1/polymerization of actin without SDF-1. The results presented are representative of 5 different experiments. Error bars represent standard error of the results of all the experiments used to calculate the average.
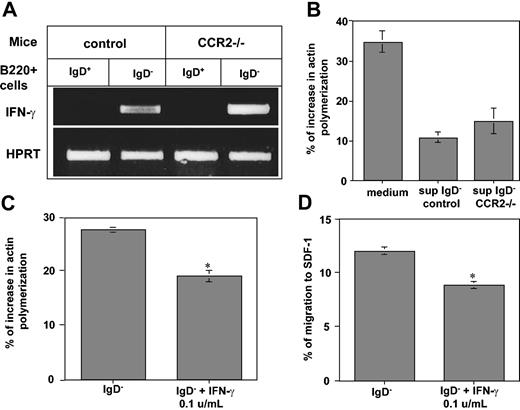
Among the requirements for integrin-mediated migration are an increased rate of actin polymerization and extensive reorganization of the actin-based cytoskeleton. To determine whether the increased migration of immature B cells from CCR2–/– is associated with an increase in their cytoskeleton rearrangement, we analyzed actin polymerization in immature B cells derived from control or CCR2–/– mice. Immature B cells (B220+ IgD–) from both control and CCR2–/– mouse strains were analyzed for their cytoskeleton rearrangement following SDF-1 stimulation. As can be seen in Figure 2D, CCR2–/– immature B cells substantially increased their actin polymerization in response to SDF-1 stimulation compared with control cells (9.16% control versus 16.92% CCR2–/–; P = .006). Thus, CCR2 has an inhibitory role on cytoskeleton rearrangement and migration of immature B cells. In its absence, immature B cells enhance their response to chemokine stimulation.
CCR2-induced inhibitory pathway is IFN-γ independent
Previously, we showed that immature B cells down-regulate their homing to the LN and to antigen-enriched sites by autocrine secretion of low levels of IFN-γ. To determine whether the IFN-γ and CCR2 inhibitory pathways are coupled, we first analyzed IFN-γ message in immature (B220+ IgD–) B cells from control and CCR2–/– mice by using RT-PCR. As can be seen in Figure 3A, IFN-γ message was detected at similar levels in both immature B-cell populations, showing that in the absence of CCR2, the IFN-γ gene is still transcribed. Our previous studies demonstrated that immature B cells secrete into their conditioned medium low levels of IFN-γ, which inhibit B- and T-cell chemokine-induced actin polymerization and migration.20,30 To determine whether similar low levels of IFN-γ are secreted from CCR2-deficient cells, the activity of conditioned medium from CCR2–/– cells was analyzed. Mature B cells (that do not secrete IFN-γ) from control mice were treated for 30 minutes with medium alone, or conditioned medium derived from immature B cells from either control or CCR2–/– mice. Actin polymerization in the treated B cells was analyzed before and after SDF-1 stimulation. As can be seen in Figure 3B, conditioned medium derived from control or CCR2–/– immature B cells similarly inhibited the ability of B cells to polymerize actin in response to SDF-1 stimulation. To determine whether low levels of IFN-γ down-regulate the CCR2–/– immature B-cell response to chemokine-induced stimulation, we analyzed the actin polymerization and transwell migration of immature CCR2–/– B cells that were preincubated with or without low levels of IFN-γ. Low levels of IFN-γ inhibited the SDF-1–induced actin polymerization (Figure 3C; 26.89% IgD– versus 18.1% IgD– plus IFN-γ; P = .0003) and transwell migration (Figure 3D; 12% IgD– versus 8.8% IgD– plus IFN-γ; P = .002) of CCR2–/– immature B cells. These results indicate that CCR2-deficient immature B cells transcribe IFN-γ, which similarly inhibits B-cell cytoskeleton rearrangement and migration. Thus, the inhibitory effect of CCR2 on cytoskeleton rearrangement and migration of immature B cells is IFN-γ independent.
The inhibitory control of CCR2 on migration is independent of the IFN-γ regulated pathway. (A) IFN-γ transcription in CCR2–/– cells. Mature (IgD+ B220+) and immature (IgD– B220+) B cells derived from control or CCR2–/– mice were purified, total RNA was isolated, and reverse transcription was carried out by using Superscript II RT. (B-C) Actin polymerization assay. Mature B cells were treated for 30 minutes with medium or supernatant collected from immature B cells of control or CCR2–/– mice (B). Immature CCR2–/– B cells were pretreated with IFN-γ (0.1 U/mL) for 30 minutes (C). The cells were then stimulated with 50 μg/mL SDF-1 for 15 seconds, fixed, and permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. Percentage increase in actin polymerization was calculated as described in Figure 2. (D) Transwell migration assay. Immature CCR2–/– B cells were pretreated with IFN-γ (0.1 U/mL) for 30 minutes and then placed in the upper well of a 24-well transwell plate in the presence or absence of SDF-1. After 3 hours, the number of the migrating cells found in the lower chamber was evaluated by FACS analysis. Percentage migration was calculated as described in Figure 2. The results presented are representative of 3 different experiments. Error bars represent the standard error of the results of all the experiments used to calculate the average. *Significant at a 98% level.
The inhibitory control of CCR2 on migration is independent of the IFN-γ regulated pathway. (A) IFN-γ transcription in CCR2–/– cells. Mature (IgD+ B220+) and immature (IgD– B220+) B cells derived from control or CCR2–/– mice were purified, total RNA was isolated, and reverse transcription was carried out by using Superscript II RT. (B-C) Actin polymerization assay. Mature B cells were treated for 30 minutes with medium or supernatant collected from immature B cells of control or CCR2–/– mice (B). Immature CCR2–/– B cells were pretreated with IFN-γ (0.1 U/mL) for 30 minutes (C). The cells were then stimulated with 50 μg/mL SDF-1 for 15 seconds, fixed, and permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. Percentage increase in actin polymerization was calculated as described in Figure 2. (D) Transwell migration assay. Immature CCR2–/– B cells were pretreated with IFN-γ (0.1 U/mL) for 30 minutes and then placed in the upper well of a 24-well transwell plate in the presence or absence of SDF-1. After 3 hours, the number of the migrating cells found in the lower chamber was evaluated by FACS analysis. Percentage migration was calculated as described in Figure 2. The results presented are representative of 3 different experiments. Error bars represent the standard error of the results of all the experiments used to calculate the average. *Significant at a 98% level.
B cells express the CCR2 ligand, CCL2/JE, which inhibits their actin polymerization, migration, and homing
The major ligand of CCR2 is CCL2, also known as monocyte chemoattractant protein 1 (MCP-1 in human or JE in mice).31 This chemokine is produced by endothelial cells, smooth muscle cells, macrophages,32 and early and late B cells.33 To analyze the activation of CCR2 and its inhibitory pathway in B cells, we monitored CCL2 mRNA transcription and translation in control immature (B220+ IgD–) and mature (B220+ IgD+) B cells. As can be seen in Figure 4, CCL2/JE message (Figure 4A) and protein (Figure 4B) were detected in both immature and mature populations at similar levels. Thus, B cells indeed transcribe and translate CCL2/JE, the CCR2 ligand.
JE mRNA and protein are expressed in both mature and immature B cells and inhibit migration of immature B cells by interacting with CCR2. (A) Mature (IgD+ B220+) and immature (IgD– B220+) cells from control mice were purified. Total RNA was isolated, and reverse transcription was carried out by using Superscript II RT. (B) Western blot analysis. Levels of JE in total cell lysates of IgD+ 220+ and IgD– B220+ control cells. The band representing JE is indicated. The results presented are representative of 3 separate experiments. (C) Transwell migration. IgD– B220+ cells were isolated from control or CCR2–/– mice and pretreated for 30 minutes in 10 mL medium with or without JE. The cells were then washed and placed in the upper well of a 24-well transwell plate in the presence of SDF-1. The number of migrating cells found in the lower chamber was evaluated after 3 hours by FACS analysis. Migration was calculated as described in Figure 2, and the inhibition of migration was calculated as the migration with JE/migration without JE × 100. The results presented are representative of 3 different experiments. Error bars represent the standard error of the results of all the experiments used to calculate the average. *Significant at a 98% level.
JE mRNA and protein are expressed in both mature and immature B cells and inhibit migration of immature B cells by interacting with CCR2. (A) Mature (IgD+ B220+) and immature (IgD– B220+) cells from control mice were purified. Total RNA was isolated, and reverse transcription was carried out by using Superscript II RT. (B) Western blot analysis. Levels of JE in total cell lysates of IgD+ 220+ and IgD– B220+ control cells. The band representing JE is indicated. The results presented are representative of 3 separate experiments. (C) Transwell migration. IgD– B220+ cells were isolated from control or CCR2–/– mice and pretreated for 30 minutes in 10 mL medium with or without JE. The cells were then washed and placed in the upper well of a 24-well transwell plate in the presence of SDF-1. The number of migrating cells found in the lower chamber was evaluated after 3 hours by FACS analysis. Migration was calculated as described in Figure 2, and the inhibition of migration was calculated as the migration with JE/migration without JE × 100. The results presented are representative of 3 different experiments. Error bars represent the standard error of the results of all the experiments used to calculate the average. *Significant at a 98% level.
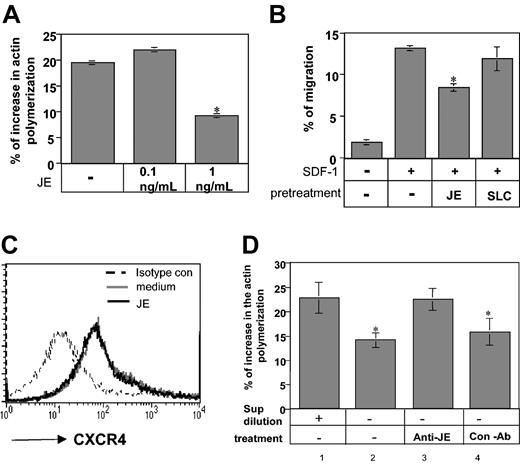
To determine whether soluble CCL2/JE transmits an inhibitory signal through its receptor, CCR2, we analyzed whether JE has an inhibitory effect on the migration of immature B cells derived from control or CCR2–/– mice. Our previous studies20 and results presented earlier suggest that immature B cells express 2 independent inhibitory pathways that autoregulate their migration, IFN-γ and CCR2. Previously, we showed that 5-fold dilution of immature B-cell–conditioned medium results in abolishment of its inhibitory activity.20 Therefore, to eliminate the inhibitory effect of low levels of IFN-γ secreted by the cells on cytoskeletal rearrangement and migration of immature B cells, immature B cells were cultured at low density, and the effect of CCL2/JE was analyzed. Immature B cells from CCR2–/– and control mice grown in diluted culture were pretreated with CCL2/JE, and their migration toward SDF-1 was followed. As can be seen in Figure 4C, although CCL2/JE pretreatment reduced migration of control immature B cells by about 35%, CCL2/JE treatment had almost no inhibitory effect on CCR2-deficient cells (35.66% control versus 9% CCR2–/–; P = .01). These results indicate that JE down-regulates B-cell homing by binding to the CCR2 receptor expressed on immature B cells.
To analyze whether CCL2/JE is involved in the inhibitory effect of CCR2 on homing of immature Ii–/– B cells, its role in actin polymerization and migration was further evaluated. As can be seen in Figure 5A, 1 ng/mL CCL2 down-regulated the rearrangement of actin by about 30% in diluted immature Ii–/– B cells (19.47% control versus 9.17% JE; P = 1.47 × 10–6), showing that CCL2/JE has an inhibitory effect on this process. We then analyzed the effect of CCL2/JE on the transwell migration of immature B cells. Ii–/– B cells cultured at low density were pretreated with medium or CCL2/JE for 30 minutes. The cells were then washed and placed in the upper well of a transwell setup, and their migration toward SDF-1 was evaluated. As can be seen in Figure 5B, low levels of JE decreased the migration of immature B cells toward SDF-1 (13.03% SDF versus 8.49% SDF ± JE; P = 3.4 × 10–6), whereas a control chemokine, secondary lymphoid-tissue chemokine (SLC), did not inhibit their migration. Previous reports indicated that the chemotactic responses of cells toward chemokines could be affected by other cytokines as a result of modulation of chemokine receptor expression on the cell surface.34,35 To determine whether CCL2/JE-induced inhibition of SDF-1 chemotaxis was caused by altered cell surface expression of the CXCR4 receptor, we examined CXCR4 receptor expression on immature B cells pretreated in the presence or absence of JE/CCL2. As shown in Figure 5C, CCL2 did not alter the level of CXCR4 expression on B cells.
JE secreted by B cells inhibits immature B-cell actin polymerization and migration. (A) Actin polymerization. Immature B cells were isolated from Ii–/– mice and pretreated for 30 minutes in 10 mL medium with or without JE. The cells were then stimulated with 50 μg/mL SDF-1 for 15 seconds, fixed, and permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. The change in the polymerized actin was analyzed by FACS. Percentage increase in actin polymerization was calculated as described in Figure 2. (B) Transwell migration. Immature B cells were isolated from Ii–/– mice and pretreated for 30 minutes with medium, JE (1 ng/mL), or SLC (1 μg/mL). The cells were washed and then assayed for migration as described earlier. The results presented are representatives of 5 different experiments. (C) FACS analysis of CXCR4 expression on Ii–/– B cells incubated in the presence or absence of CCL2. Ii–/– immature B cells were stained with anti-CXCR4 or a control antibody. The results presented are representative of 3 different experiments. (D) B220+ cells from control mice were isolated, and 2 × 107 (concentrated) or 2 × 106 (diluted) cells were suspended in 10 mL medium for 30 minutes, and their conditioned medium was collected. Concentrated conditioned medium was treated overnight with or without anti-JE or control antibody. The treated and nontreated conditioned media were used for 30 minutes of pretreatment of immature B cells that were isolated from Ii–/– mice. The pretreated immature B cells were then stimulated with 50 μg/mL SDF-1 for 15 seconds, fixed, permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. The change in the polymerized actin was analyzed by FACS and calculated as described in Figure 2. Error bars represent the standard error of the results of all the experiments used to calculate the average. *Significant at a 98% level.
JE secreted by B cells inhibits immature B-cell actin polymerization and migration. (A) Actin polymerization. Immature B cells were isolated from Ii–/– mice and pretreated for 30 minutes in 10 mL medium with or without JE. The cells were then stimulated with 50 μg/mL SDF-1 for 15 seconds, fixed, and permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. The change in the polymerized actin was analyzed by FACS. Percentage increase in actin polymerization was calculated as described in Figure 2. (B) Transwell migration. Immature B cells were isolated from Ii–/– mice and pretreated for 30 minutes with medium, JE (1 ng/mL), or SLC (1 μg/mL). The cells were washed and then assayed for migration as described earlier. The results presented are representatives of 5 different experiments. (C) FACS analysis of CXCR4 expression on Ii–/– B cells incubated in the presence or absence of CCL2. Ii–/– immature B cells were stained with anti-CXCR4 or a control antibody. The results presented are representative of 3 different experiments. (D) B220+ cells from control mice were isolated, and 2 × 107 (concentrated) or 2 × 106 (diluted) cells were suspended in 10 mL medium for 30 minutes, and their conditioned medium was collected. Concentrated conditioned medium was treated overnight with or without anti-JE or control antibody. The treated and nontreated conditioned media were used for 30 minutes of pretreatment of immature B cells that were isolated from Ii–/– mice. The pretreated immature B cells were then stimulated with 50 μg/mL SDF-1 for 15 seconds, fixed, permeabilized, and their intracellular F-actin was stained with FITC-phalloidin. The change in the polymerized actin was analyzed by FACS and calculated as described in Figure 2. Error bars represent the standard error of the results of all the experiments used to calculate the average. *Significant at a 98% level.
To directly show that JE secreted from B cells regulates their migration, we analyzed conditioned medium from mature B cells. Unlike immature B cells that express both IFN-γ and JE, mature B cells were shown to secrete JE (Figure 4),33 whereas they do not express or secrete IFN-γ.20 We first evaluated whether an inhibitory protein exists in the conditioned medium derived from mature B cells and whether dilution of this medium could abolish its effect. Immature B cells expressing CCR2 were preincubated for 30 minutes with concentrated or diluted conditioned medium collected from mature B cells, and their actin polymerization in response to SDF-1 stimulation was evaluated. As can be seen in Figure 5D, concentrated conditioned medium from mature B cells down-regulated the cells' ability to polymerize their actin, whereas dilution of the conditioned medium abrogated this effect (actin polymerization, 22.55% diluted versus 14% concentrated; P = .01). To directly show that CCL2/JE is indeed the protein responsible for this inhibitory effect, the conditioned medium from mature cells was treated with neutralizing antibodies to CCL2/JE (Figure 5D, lane 3) or nonrelevant antibodies (Figure 5D, lane 4). Treatment with anti-JE antibodies almost completely abolished the inhibitory effect of mature cell-conditioned medium, whereas medium incubated with the control antibody maintained its inhibitory effect, demonstrating that CCL2/JE is the inhibitory factor.
To further follow the CCL2/JE downstream signaling cascade that results in inhibition of SDF-1–induced migration, we analyzed the 70Z/3 B-cell lymphoma, which was shown to transcribe and translate CCR2 (Figure 1). We first followed the ability of these cells to mimic the immature B-cell response to CCL2 and to increase actin polymerization and migration as a consequence of chemokine stimulation; we further evaluated the ability of CCL2/JE to inhibit the response of these cells. Both chemokine-induced actin polymerization (Figure 6A; 12.5% SDF versus 5.38% SDF + preincubated JE; P = 4.27 × 10–5) and migration (Figure 6B; without JE 36.24% versus 25.06% with JE; P = 5 × 10–6) of 70Z/3 cells were substantially inhibited by pretreatment of the cells with CCL2/JE, showing that this cell line can serve as an appropriate system to monitor the signaling pathway induced by CCL2/JE. To determine whether CCR2 cross-inhibition is due to early effects, CCL2 was added to the upper transwell chamber. As can be seen in Figure 6A, addition of CCL2 to the chamber inhibited the SDF-1–induced migration, to a similar extent as CCL2 pretreatment, suggesting that the CCL2 downstream cascade results in an immediate inhibitory effect.
JE inhibits SDF-1–induced migration of 70Z/3 cells because of inhibition of ERK phosphorylation. (A) Transwell migration. 70Z/3 cells were pretreated with or without JE for 30 minutes. The cells were then washed, and transwell assay was preformed as described in Figure 2. The results presented are representative of 3 different experiments. (B) Actin polymerization. 70Z/3 cells were pretreated with or without JE for 30 minutes. The change in the cells' actin polymerization was then evaluated as described in panel A. The results presented are representative of 3 different experiments. Error bars represent the standard error of the results of all the experiments used to calculate the average. *Significant at a 98% level. (C) 70Z/3 cells pretreated in the presence (dotted line) or absence (dark line) of CCL2/JE (1 ng/mL). The cells were labeled, and calcium mobilization was monitored before and after SDF-1 stimulation. The results are representative of 3 different experiments (D) 70Z/3 cells were pretreated with or without CCL2/JE (1 ng/mL) for 30 minutes. The cells were then stimulated with SDF-1 for 1 minute. Cells were lysed immediately, and lysates were separated on reducing 10% (wt/vol) SDS-PAGE and immunoblotted with anti-phosphospecific ERK1/2 (p-ERK). Immunoblots were stripped and reprobed with anti-ERK1/2. Ratio calculation is as follows: The intensity of p-ERK band in each treatment was divided by the intensity of the ERK band in each lane. The ratio in the absence of any treatment was normalized to 1, and the ratio in each treatment was calculated as the intensity in treatment relative to 1.
JE inhibits SDF-1–induced migration of 70Z/3 cells because of inhibition of ERK phosphorylation. (A) Transwell migration. 70Z/3 cells were pretreated with or without JE for 30 minutes. The cells were then washed, and transwell assay was preformed as described in Figure 2. The results presented are representative of 3 different experiments. (B) Actin polymerization. 70Z/3 cells were pretreated with or without JE for 30 minutes. The change in the cells' actin polymerization was then evaluated as described in panel A. The results presented are representative of 3 different experiments. Error bars represent the standard error of the results of all the experiments used to calculate the average. *Significant at a 98% level. (C) 70Z/3 cells pretreated in the presence (dotted line) or absence (dark line) of CCL2/JE (1 ng/mL). The cells were labeled, and calcium mobilization was monitored before and after SDF-1 stimulation. The results are representative of 3 different experiments (D) 70Z/3 cells were pretreated with or without CCL2/JE (1 ng/mL) for 30 minutes. The cells were then stimulated with SDF-1 for 1 minute. Cells were lysed immediately, and lysates were separated on reducing 10% (wt/vol) SDS-PAGE and immunoblotted with anti-phosphospecific ERK1/2 (p-ERK). Immunoblots were stripped and reprobed with anti-ERK1/2. Ratio calculation is as follows: The intensity of p-ERK band in each treatment was divided by the intensity of the ERK band in each lane. The ratio in the absence of any treatment was normalized to 1, and the ratio in each treatment was calculated as the intensity in treatment relative to 1.
To further analyze the mechanism by which CCL2/JE regulates the migration of B cells, we assessed the calcium mobilization response of SDF-1–induced cells incubated in the presence or absence of CCL2. As shown in Figure 6C, no difference in calcium mobilization was detected in cells incubated in the presence or absence of CCL2, showing that CCL2 does not induce receptor desensitization. Because CCL2-mediated inhibition of SDF-1–induced cytoskeleton changes and migration is independent of CXCR4 receptor internalization or desensitization, it is likely that the effects observed are directly mediated through CCR2 signaling. We, therefore, tested whether exposure of immature B cells to CCL2 would affect a known SDF-1/CXCR4 signaling pathway. We focused on the activation of the mitogen-activated protein kinase (MAPK) pathway, which includes various downstream kinases, such as ERK1/2.36 As can be seen in Figure 6D, SDF-1 induced phosphorylation of ERK1/2 (about 3-fold). However, activation of ERK1/2 was inhibited when the cells were pretreated with CCL2. Thus, the CXCR4–SDF-1 interaction induces a cascade that activates ERK1/2. The CCR2 signaling pathway prevents ERK1/2 phosphorylation, a process that results in inhibition of cytoskeleton rearrangement and chemokine-induced migration.
Elevated in vivo homing of CCR2-deficient immature B cells to the LN
We then evaluated the role of CCR2 in the in vivo homing of immature B cells. The immature (B220+ IgM+IgD–) and mature (B220+ IgM+IgD+) B-cell profile in the spleen and LNs of control and CCR2–/– mice were compared. As can be seen in Figure 7A-C, similar levels of immature and mature populations were detected in the spleens of both mouse strains. However, mice deficient in CCR2 showed an increased (about 50%) presence of (IgM+IgD–) (Figure7A-B) and CD23– (Figure 7C) immature B cells in their LN. Thus, CCR2 regulation of cytoskeletal rearrangement and migration inhibits homing of the cells to the LN. In the absence of this receptor, homing of immature B cells to the LN compartment is enabled.
CCR2 inhibits in vivo homing of immature B cells. (A-B) Splenocytes (spl) and lymph node (LN) cells from control and CCR2–/– mice were triple stained with anti-B220 and anti-IgD or anti-IgM. Dot plots show the expression of the markers on B220+ cells. Numbers represent percentages of immature population. (A). The graph represents the percentage change in the mature and immature B-cell population in CCR2–/– mice compared with the control immature and mature populations, which were regarded as 100% from 7 different experiments (B). (C) Splenocytes (spl) and lymph node (LN) cells from control and CCR2–/– mice were double stained with anti-B220 and anti-CD23. Histograms show CD23 expression on B220+ cells. Brackets and numbers show the percentage of CD23– population. (D) Purification of immature B cells. IgD– splenocytes from control and CCR2–/– mice were then separated by anti-B220 magnetic beads as described in “Materials and methods.” Brackets and numbers show the percentage of IgD+ and B220+ populations. (E-F) Homing of labeled immature B cells to the spleen (E) and LN (F). Labeled immature B cells derived from control and CCR2–/– mice were injected to control mice. After 3.5 hours, the spleen (E) and LN (F) were collected, and the FITC-positive population was analyzed by FACS. Error bars represent the standard error of the results of all the experiments used to calculate the average.
CCR2 inhibits in vivo homing of immature B cells. (A-B) Splenocytes (spl) and lymph node (LN) cells from control and CCR2–/– mice were triple stained with anti-B220 and anti-IgD or anti-IgM. Dot plots show the expression of the markers on B220+ cells. Numbers represent percentages of immature population. (A). The graph represents the percentage change in the mature and immature B-cell population in CCR2–/– mice compared with the control immature and mature populations, which were regarded as 100% from 7 different experiments (B). (C) Splenocytes (spl) and lymph node (LN) cells from control and CCR2–/– mice were double stained with anti-B220 and anti-CD23. Histograms show CD23 expression on B220+ cells. Brackets and numbers show the percentage of CD23– population. (D) Purification of immature B cells. IgD– splenocytes from control and CCR2–/– mice were then separated by anti-B220 magnetic beads as described in “Materials and methods.” Brackets and numbers show the percentage of IgD+ and B220+ populations. (E-F) Homing of labeled immature B cells to the spleen (E) and LN (F). Labeled immature B cells derived from control and CCR2–/– mice were injected to control mice. After 3.5 hours, the spleen (E) and LN (F) were collected, and the FITC-positive population was analyzed by FACS. Error bars represent the standard error of the results of all the experiments used to calculate the average.
Finally, to directly show that CCR2 indeed has a role in homing of immature B cells, control and CCR2–/– immature B cells were purified (Figure 7D), labeled, and injected into control mice. The proportion of labeled cells recovered in the spleen (Figure 7E) and lymph nodes (Figure 7F) was determined 3.5 hours after injection. As can be seen in Figure 7F, although control cells were barely detectable in the LN, efficient homing of CCR2-deficient cells to the LN was observed. Together, these results show that CCR2 negatively regulates homing of immature B cells to the lymph nodes. In its absence, immature B cells efficiently home to these compartments.
Discussion
Cell migration is controlled by multistep processes that include chemoattraction, cell-cell adhesion, and transmigration through cell layers.37,38 Chemotactic signals play important roles in leukocyte navigation by regulating migration from the blood into tissues, as well as subsequent localization within the tissue microenvironment. Two major chemokine subfamilies are distinguished by whether the first 2 conserved cysteine residues occur together (CC) or are separated by another amino acid (CXC). The CC chemokine family members serve as ligands for 7 transmembrane-spanning receptors that induce signaling through a G-protein–linked pathway, resulting in cytoskeletal rearrangement, intracellular calcium increases, adhesion to endothelium or extracellular matrix proteins, T helper 1 (Th1)/Th2 differentiation, costimulation, interleukin 2 (IL-2) production, proliferation, and chemotaxis.39-41 The CC chemokine receptor 2, CCR2, serves as the receptor for CCL2 (MCP-1 in humans or JE in mice) and is expressed on variety of cell types of the immune system, including monocytes, activated T cells, natural killer (NK) cells, and dendritic cells.42,43 CCR2-deficient mice were previously shown to exhibit severe deficits in macrophage recruitment in response to either antigenic or nonantigenic challenge24,44,45 and in the skewing of Th1 and Th2 responses.44,46,47 Furthermore, it was shown that CCR2 is expressed on naive and memory human tonsil B cells but not on germinal center B cells,42,48 and Leishmania major–infected CCR2-deficient mice show increased expression of the B-cell chemokine, BLC, and striking B-cell outgrowth.49
In this study, we describe an additional role for CCR2 in B cells. We have shown that CCR2 is expressed on immature murine B cells, and its expression is down-regulated following differentiation to the mature stage. To determine the physiologic role of CCR2 on these immature cells, the cytoskeletal rearrangement and migration of immature B cells from mice lacking CCR2 were analyzed. CCR2–/– B cells showed a remarkable elevation in their response to SDF-1 stimulation, as demonstrated by their cytoskeletal rearrangement and migration in a transwell assay in vitro. In addition, lymph nodes of CCR2–/– mice were populated with elevated numbers of immature B cells, suggesting the augmented in vivo migration of immature B cells in CCR2–/– mice. These results suggest that CCR2 negatively regulates migration and homing of immature B cells. In its absence, the response of immature B cells to chemokine stimulation is more profound. These results correlate with the negative interaction between CCR2 and the SDF-1 receptor, CXCR4, described previously.50,51
To down-regulate immature B-cell homing, CCR2 expressed on immature B cells is activated by its ligand, CCL2/JE. This chemokine was found to be transcribed and translated at similar levels by both mature and immature B cells and secreted into the conditioned medium. Consistent with this finding, it was recently shown that in the human bone marrow, immature and mature B cells secrete the CCR2 ligand, MCP-1.33 Thus, although both populations secrete CCL2/JE, only immature B cells, which express the CCL2/JE receptor, can respond by down-regulation of cytoskeleton rearrangement and homing.
We have demonstrated that the CCR2 receptor mediates the inhibitory effect induced by JE/CCL2. Our studies show that the inhibitory effect of CCL2 does not induce CXCR4 receptor internalization or desensitization. This receptor transmits a signal that results in the inhibition of actin polymerization and the extensive reorganization of the actin-based cytoskeleton. The SDF-1/CXCR4 signaling pathway activates the MAPK pathway, which includes various downstream kinases, such as ERK1/2,36 resulting in cytoskeleton rearrangement and cell migration. The CCR2 downstream pathway negatively regulates ERK1/2 phosphorylation, a process that results in inhibition in cell migration. Therefore, CCR2 probably activates a signaling process that reduces the CXCR4 signaling cascade. Thus, cross talk between different chemokine receptors on cells might enable the cells to navigate along the different chemotactic gradients in the bloodstream or in specific environments.
Together with our previous studies, these findings suggest that several independent mechanisms control the targeting of immature B cells to the spleen and prevent their premature homing to the lymph nodes. In addition to the previously described secretion of low levels of IFN-γ,20 immature B cells express the chemokine receptor CCR2, which down-regulates their homing to the lymph nodes. We show here that a CCR2-mediated inhibitory mechanism regulates immature B-cell homing independently of the negative regulation by IFN-γ, because in the absence of CCR2 the inhibitory mechanism of IFN-γ remained operative.
The entrance of B cells to the lymph nodes is a process that requires interaction between L-selectins and their ligands on the high endothelial venules. Subsequently, a chemokine-mediated triggering event (mediated by chemokines such as SDF-1, SLC, or EBI1-ligand chemokine [ELC]) causes integrin activation and adhesion.37,38 The integrins lymphocyte function–associated antigen-1 (LFA-1), α4β7, and α4β1 contribute to this process.38,52 Despite our detailed understanding of the steps involved in lymphocyte entry to lymph nodes, relatively little is known about how the cells enter the spleen and the white pulp of this compartment. The entry of B cells to the spleen was regarded until recently as a process for which integrin activation was not required,53-55 but recent results show that integrin activation also has a role in the entry of mature B cells into the splenic white pulp. However, although integrin inhibition causes a decrease in B-cell entry to the white pulp, it has no influence on the total B-cell number in the spleen.56 In addition, although inhibition of a single integrin (LFA-1 or α4β7) results in a dramatic inhibition of homing of T and B cells to the lymph nodes, it has only a minor effect on their entrance to the splenic white pulp. Only dual integrin inhibition can inhibit the entrance to the white pulp.56 The newly arrived immature B cells enter the spleen through the central arteriole and develop into T1 cells, which are located at the outer PALS, outside of the follicle. These cells then further develop to T2 cells, which are situated within primary follicles adjacent to mature cells.18 Our studies have found CCR2 to be expressed in the T1 stage when the cells are located at the PALS but down-regulated at the T2 stage. These findings suggest that to migrate to the T2 area, T1 cells must down-regulate their inhibitory proteins, IFN-γ and CCR2, a process that allows them to home to the follicular area. However, although we have not yet identified the specific integrin controlled by the 2 inhibitory pathways, it is likely that these inhibitory mechanisms negatively regulate a single integrin pathway, preventing entry to the lymph nodes, while homing to the spleen can still occur. Finally, it is still not clear why immature B cells require the dual inhibitory mechanisms of IFN-γ and CCR2. Future studies will further elucidate the interactions between these 2 pathways in the regulation of B-cell homing and maturation.
Prepublished online as Blood First Edition Paper, May 4, 2004; DOI 10.1182/blood-2003-11-4013.
Supported by The Israel Science Foundation founded by the Academy of Sciences and Humanities, the Minerva Foundation, and the European Union Migration and Inflammation grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Matthias Mack for his generous gift of the anti-CCR2 antibodies and Dr Dimitry Gakamsky for his help. We thank Dr R. Seger for providing anti-extracellular signal-regulated kinase 1/2 antibodies. We also thank members of the Shachar lab for discussions and comments on this manuscript. I.S. is the incumbent of the Alvin and Gertrude Levine Career Development Chair of Cancer Research.