Abstract
Recombinant activated protein C (APC), a well-defined anticoagulant enzyme, reduced mortality in severe sepsis patients in a phase 3 trial. However, 2 potent anticoagulants, antithrombin III and recombinant tissue factor pathway inhibitor, failed to do so, implying the physiologic relevance of APC's less well-defined anti-inflammatory and antiapoptotic activities. Recombinant APC therapy conveys an increased risk of serious bleeding complications due to APC anticoagulant activity. To generate recombinant APC variants with reduced risk of bleeding due to reduced anticoagulant activity, we dissected APC's anticoagulant activity from its cytoprotective activity by site-directed mutagenesis. Using staurosporine-induced endothelial cell apoptosis assays, we show here that Ala mutations (RR229/230AA and KKK191_ 193AAA) in 2 APC surface loops that severely reduce anticoagulant activity result in 2 APC variants that retain normal antiapoptotic activity that requires protease activated receptor-1 and endothelial cell protein C receptor. Thus, it is possible to reduce anticoagulant activity while preserving antiapoptotic activity of recombinant APC variants. We suggest that therapeutic use of such APC variants may reduce serious bleeding risks while providing the beneficial effects of APC acting directly on cells. (Blood. 2004;104: 1740-1744)
Introduction
Recombinant activated protein C (APC) reduced all-cause 28-day relative mortality by 19% in patients with severe sepsis.1 Remarkably, 2 potent anticoagulants, antithrombin III and recombinant tissue factor pathway inhibitor, failed to do so in similar phase 3 clinical trails,2,3 implying the physiologic relevance of the less well-defined direct anti-inflammatory and antiapoptotic activities of APC.4-6 Whereas extensive information is available about the mechanisms that underlie APC's anticoagulant activity, limited information is available about the molecular mechanisms responsible for APC's direct anti-inflammatory and antiapoptotic effects. In vitro tissue culture studies show that APC directly modulates gene expression with notable effects on anti-inflammatory and cell survival genes.4,5,7 In human brain endothelial cells subjected to hypoxia, an early result of APC signaling is the inhibition of increases in the levels of the proapoptotic transcription factor, p53 5 ; moreover, APC down-regulates levels of the proapoptotic factor, Bax, while it up-regulates levels of the antiapoptotic factor, Bcl-2.5 APC directly stimulates intracellular fluxes of Ca2+ ions from the endoplasmic reticulum.8 As first reported by Riewald et al,7 the direct effects of APC on endothelial cells require protease activated receptor-1 (PAR-1)9 and endothelial protein C receptor (EPCR).10 Similarly, the in vitro inhibition of staurosporine-induced apoptosis by APC11 and the in vivo neuroprotective effects of APC in a murine ischemic stroke model require PAR-1 and EPCR.5 Other in vitro and in vivo studies described the cytoprotective direct effects of APC on neurons that were subjected to N-methyl-D-aspartate (NMDA)-induced excitotoxicity and showed that the neuronal protective effects of APC required PAR-1 and PAR-3.12 In baboons, EPCR is required for APC-dependent reduction of mortality in Escherichia coli-induced sepsis.13
APC's potent anticoagulant activity involves cleavages of factors Va and VIIIa, especially a rapid cleavage at Arg506 in factor Va.14,15 For this Arg506 cleavage, a positively charged surface on the protease domain of APC is required for normal interactions of APC with factor Va.16-20 This positive region for binding factor Va on APC is generally located in an area similar to the anion binding exosite I of thrombin and includes loop 37 (protein C residues 190-193 equivalent to chymotrypsin [CHT] residues 36-39), the Ca++-binding loop (residues 225-235, CHT 70-80), and the autolysis loop (residues 301-316, CHT 142-153).21 All available data for APC's direct effects on cells imply that APC activates PAR-1 (see previous paragraph). Assuming cleavage at Arg41 in the N-terminal tail of PAR-1 has different APC exosite requirements than cleavage at Arg506 in factor Va, we sought APC mutants that lack normal anticoagulant activity but retain normal antiapoptotic activity with the goal of identifying mutants for APC therapy that would convey less risk of serious bleeding while providing APC's beneficial cytoprotective effects.
Materials and methods
Materials
Human α-thrombin was purchased from Enzyme Research Laboratories (South Bend, IN). Normal human citrate-anticoagulated plasma was from George King Bio-Medical (Overland Park, KS). The chromogenic substrate l-pyroglutamyl-l-prolyl-l-arginine-p-nitroaniline hydrochoride (S-2366) was obtained from Chromogenix (Franklin, OH).
Preparation of recombinant activated protein C
Mutant protein C expression vectors were constructed, and recombinant protein C mutants were purified from conditioned media as described.17,22 Purified protein C was activated by thrombin.17,22 Briefly, protein C in HBS (50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 150 mM NaCl) with 2 mM EDTA (ethylenediaminetetraacetic acid) and 0.5% bovine serum albumin (BSA), pH 7.4, at a concentration of 600 μg/mL was incubated for 2.5 hours with 12 μg/mL thrombin at 37°C followed by the addition of 1.1 units of hirudin per unit of thrombin to inactivate the thrombin. Subsequently, thrombin was removed by anion-exchange chromatography with NaCl gradient elution.23 Residual thrombin, as determined by fibrin clotting, accounted for less than 0.00025% (moles of thrombin per moles of APC) of the protein. Concentrations of recombinant wild-type (rwt) APC and APC mutants were determined by active-site titration adapted from Chase and Shaw24 using APC at about 8 μM in HBS and p-nitrophenol-guanidino benzoate at 0.1 mM and using an extinction coefficient for p-nitrophenol of 11 400 M-1cm-1 (at pH 7.4) as described.17 The concentration of S360A-APC was determined by Asserachrom Protein C enzyme-linked immunosorbent assay (ELISA) from American Bioproducts (Parsippany, NJ).22
APC activity assays
Amidolytic (S-2366) assays were performed as described.16,22 Activated partial thromboplastin time (APTT) clotting assays were performed according to the following procedure. Plasma (50 μL) was incubated for 1 minute with kaolin/cephalin (50 μL) (C.K. Prest 2; Diagnostica Stago, Parsippany, NJ) at 37°C, and then 25 μL APC in HBS with 0.5% BSA was added at final APC concentrations from 0.5 nM to 32 nM and incubated for an additional 3 minutes. Clotting was then initiated by adding 50 μL of 50 mM CaCl2 in HBS, and the clotting time was recorded using an Amelung KC 4a micro coagulometer (Sigma Diagnostics, St Louis, MO).
APC's cytoprotective effects were determined in assays of staurosporine-induced endothelial cell (EA.hy926) apoptosis as described.11 APC (0.16-100 nM) was incubated with cells for 5 hours prior to induction of apoptosis by staurosporine (10 μM, 1 hour) unless otherwise indicated, and apoptosis was assessed by Apopercentage dye from Biocolor (Belfast, United Kingdom), which measures phosphatidylserine translocation to the outside surface of the cell membrane. Blocking antibodies against PAR-1 (WEDE-15 and ATAP-2 kindly provided by Dr L. Brass, University of Pennsylvania, Philadelphia, PA) and against EPCR (Zymed, South San Francisco, CA) were used as described.11 For activated caspase-3 immunofluorescence staining and DAPI (4,6 diamidino-2-phenylindole) nuclear staining (5 μg/mL) of staurosporine-treated (2 μM, 4 hours) EA.hy926 endothelial cells that had been incubated with APC (25 nM, 5 hours) prior to apoptosis induction, the manufacturer's instructions were followed using a rabbit antiactivated caspase-3 antibody (Promega, Madison, WI) and Alexa-fluor 568-labeled secondary goat antirabbit (Molecular Probes, Eugene, OR). Microscopy and photography were conducted using a Leica DMLB fluorescence microscope (20 × objective, 0.4 NA) fitted with a Spot RT digital color camera (Diagnostic Instruments, Sterling Heights, MI). Dako fluorescent mounting medium (Dako, Carpinteria, CA) was used. Images were prepared for presentation using Adobe Photoshop.
PAR-1 peptide cleavage
The interactions of rwt APC and APC variants (500 nM) with PAR-1 N-terminal tail peptide (TR33-62) were studied using a synthetic peptide representing PAR-1 residues 33-62 (Bio Synthesis, Lewisville, TX). The peptide sequence was A33TNATLDPR41SFLLRNPNDKYEPFWEDEEKN62 and was cleaved byAPC between Arg41 and Ser42. The substrate peptide and the 2 peptide products of thrombin or APC cleavage at Arg41 (TR33-41 and TR42-62) were resolved and analyzed by high-performance liquid chromatography (HPLC) and quantitated essentially as described.25 All TR33-62 cleavage experiments with APC contained 5 nM hirudin to assure that the observed cleavage was not due to thrombin contamination.
Results
APC variants with reduced anticoagulant activity but normal antiapoptotic activity
Available information indicates that APC's direct cytoprotective action on cells requires EPCR and PAR-1. EPCR on the endothelial surface binds to the Gla domain of APC,26 and this is assumed to position APC's active site proximate to the PAR-1 activating cleavage site at Arg41. Cleavage of PAR-1 at Arg41 by APC presumably initiates anti-inflammatory and antiapoptotic reactions. We sought to identify APC variants with reduced anticoagulant activity but with normal cytoprotective activity. Following initial screening of various APC protease domain variants with low anticoagulant activity (data not shown), we decided to study in detail 2 APC variants, designated 229/230-APC (Arg229Ala and Arg230Ala in the Ca++-binding loop) and 3K3A-APC (Lys191Ala, Lys192Ala, and Lys193Ala in loop 37).16,17,20
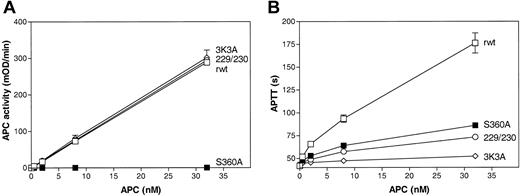
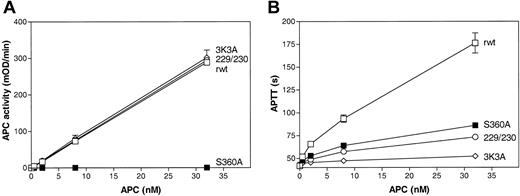
The antiapoptotic, anticoagulant, and amidolytic activities of 229/230-APC and 3K3A-APC were determined and compared with the activities of recombinant wild-type (rwt) APC and of a hydrolytically inactive mutant, S360A-APC, containing Ala in place of the active site residue, Ser360. The 2 APC protease domain loop variants, 229/230-APC and 3K3A-APC, had the same enzymatic activity against a small chromogenic substrate, S-2366, as rwt APC (Figure 1A), indicating the structural and functional preservation of the APC active site, whereas these variants had markedly decreased anticoagulant activity (Figure 1B) that was due to impaired cleavage at Arg506 in factor Va (Table 1). Although S360A-APC had no chromogenic activity (Figure 1A), the anticoagulant activity of S360A-APC was about 23% in the conditions of the APTT assay (Figure 1B). As previously described, in contrast to normal rwt APC, this anticoagulant activity is independent of the incubation time of APC with plasma22 and appears to involve binding of APC exosites to factor Va such that there is inhibition of factor Xa and prothrombin binding to factor Va.
Amidolytic and anticoagulant activity of rwt APC and APC variants. (A) Amidolytic activity of rwt APC and APC variants against the small chromogenic substrate, S-2366. (B) Anticoagulant activity of rwt APC and APC variants determined using activated partial thromboplastin time (APTT) assays. Each point represents the mean ± SEM from at least 3 independent experiments. □ indicates rwt APC; ○, 229/230-APC; ⋄, 3K3A-APC; and ▪, S360A-APC.
Amidolytic and anticoagulant activity of rwt APC and APC variants. (A) Amidolytic activity of rwt APC and APC variants against the small chromogenic substrate, S-2366. (B) Anticoagulant activity of rwt APC and APC variants determined using activated partial thromboplastin time (APTT) assays. Each point represents the mean ± SEM from at least 3 independent experiments. □ indicates rwt APC; ○, 229/230-APC; ⋄, 3K3A-APC; and ▪, S360A-APC.
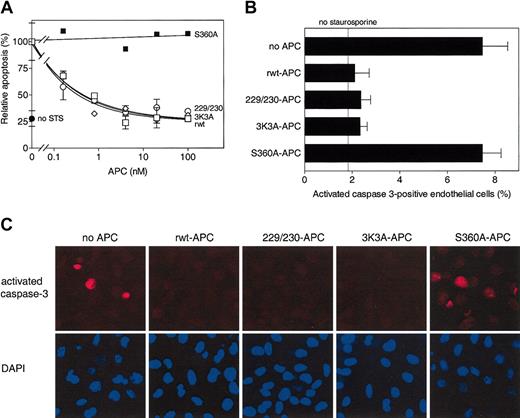
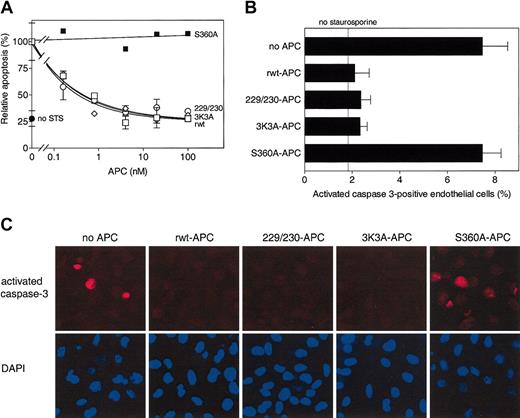
To determine cytoprotective activity of these APC variants, staurosporine-induced endothelial cell apoptosis4,11 was studied. APC-mediated inhibition of staurosporine-induced apoptosis is time dependent and dose dependent, and it requires APC's active site, PAR-1, and EPCR.11 Half-maximum inhibition of staurosporine-induced apoptosis by rwt APC was achieved at 0.16 nM under the conditions employed (Figure 2A). Dose-dependent inhibition of apoptosis by 229/230-APC and 3K3A-APC was indistinguishable from that of rwt APC with half-maximum inhibition at 0.17 nM and 0.14 nM, respectively (Figure 2A). No inhibition of apoptosis by an APC mutant lacking the active site serine, S360A-APC,22 was observed (Figure 2A-C). The ability of rwt APC and APC variants to inhibit generation of activated caspase-3 in endothelial cells exposed to staurosporine was monitored immunohistochemically. Recombinant wild-type APC and the variants, 229/230-APC and 3K3A-APC, each similarly reduced activated caspase-3-positive cells by approximately 70%, whereas the active site mutant, S360A-APC, had no effect (Figure 2B-C). Thus, certain protease domain residues essential for normal anticoagulant activity of APC—namely, Arg229, Arg230, Lys191, Lys192, and Lys193—are not required for normal antiapoptotic activity of APC.
Antiapoptotic activity of rwt APC and anticoagulantly impaired APC variants. (A) Inhibition of staurosporine (STS)-induced apoptosis by APC (see “Materials and methods”). Percentage of apoptotic endothelial cells observed in the absence of added APC (18% of all cells) was taken as 100%. Each point represents the mean ± SEM from at least 3 independent experiments. □ indicates rwt APC; ○, 229/230-APC; ⋄, 3K3A-APC; ▪, S360A-APC; and •, no staurosporine. (B-C) Reduction of activated caspase-3-positive cells by rwt APC and APC variants (25 nM, 5 hours) upon induction of apoptosis by staurosporine (2 μM, 4 hours). (B) Activated caspase-3-positive cells expressed as a percentage of the total number of cells present. As indicated by the “no staurosporine” thin line, approximately 2% of the endothelial cells were positive for activated caspase-3 in the absence of staurosporine. Each bar represents the mean ± SEM of 2 to 4 independent experiments. (C) Immunofluorescence analysis of activated caspase-3-positive cells using an activated caspase-3 specific antibody (red) and DAPI nuclear staining (blue). Columns represent identical fields. Original magnification × 200.
Antiapoptotic activity of rwt APC and anticoagulantly impaired APC variants. (A) Inhibition of staurosporine (STS)-induced apoptosis by APC (see “Materials and methods”). Percentage of apoptotic endothelial cells observed in the absence of added APC (18% of all cells) was taken as 100%. Each point represents the mean ± SEM from at least 3 independent experiments. □ indicates rwt APC; ○, 229/230-APC; ⋄, 3K3A-APC; ▪, S360A-APC; and •, no staurosporine. (B-C) Reduction of activated caspase-3-positive cells by rwt APC and APC variants (25 nM, 5 hours) upon induction of apoptosis by staurosporine (2 μM, 4 hours). (B) Activated caspase-3-positive cells expressed as a percentage of the total number of cells present. As indicated by the “no staurosporine” thin line, approximately 2% of the endothelial cells were positive for activated caspase-3 in the absence of staurosporine. Each bar represents the mean ± SEM of 2 to 4 independent experiments. (C) Immunofluorescence analysis of activated caspase-3-positive cells using an activated caspase-3 specific antibody (red) and DAPI nuclear staining (blue). Columns represent identical fields. Original magnification × 200.
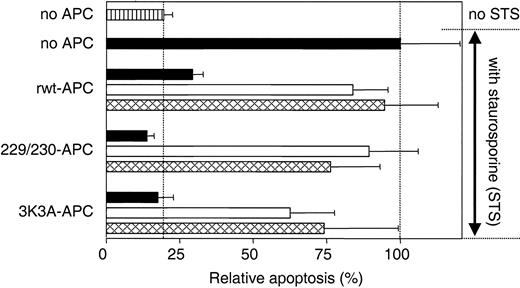
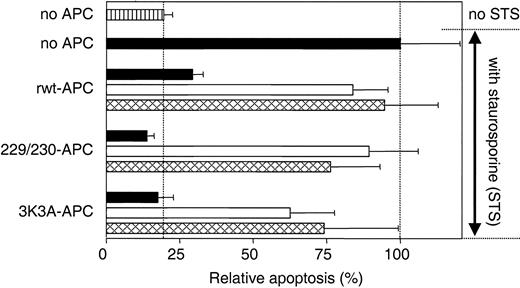
APC antiapoptotic effects require PAR-1 and EPCR.5,11 Similarly, the antiapoptotic activity of 229/230-APC and 3K3A-APC in assays of staurosporine-induced endothelial cell apoptosis required PAR-1 and EPCR because the cytoprotective activity of each APC variant was inhibited by 72% and 69% in the presence of antibodies against EPCR that block binding of APC to the receptor and by 88% and 55% in the presence of blocking anti-PAR-1 antibodies, respectively (Figure 3). These results indicate that interactions between cells and the 2 APC variants, like rwt APC, require PAR-1 and EPCR.
Inhibition of apoptosis by rwt APC and APC variants requires PAR-1 and EPCR. PAR-1 and EPCR dependence for inhibition of staurosporine-induced endothelial cell apoptosis by rwt APC and anticoagulantly impaired APC variants was studied using blocking antibodies against PAR-1 (□; combination of WEDE-15 at 20 μg/mL and ATAP-2 at 15 μg/mL) or EPCR ( ; rabbit anti-EPCR at 20 μg/mL). ▪ represents no antibodies added. Cells were incubated with rwt APC or APC variants (5 nM) 5 hours prior to induction of apoptosis by staurosporine (10 μM, 1 hour). Apoptosis was analyzed by the uptake of Apopercentage dye and expressed as a percentage relative to the percentage of apoptotic cells observed in the absence of added APC (20% of all cells), which was set as 100%. ▥ represents relative apoptosis in the absence of APC and staurosporine. Each bar represents the mean ± SEM from at least 3 independent experiments.
; rabbit anti-EPCR at 20 μg/mL). ▪ represents no antibodies added. Cells were incubated with rwt APC or APC variants (5 nM) 5 hours prior to induction of apoptosis by staurosporine (10 μM, 1 hour). Apoptosis was analyzed by the uptake of Apopercentage dye and expressed as a percentage relative to the percentage of apoptotic cells observed in the absence of added APC (20% of all cells), which was set as 100%. ▥ represents relative apoptosis in the absence of APC and staurosporine. Each bar represents the mean ± SEM from at least 3 independent experiments.
Inhibition of apoptosis by rwt APC and APC variants requires PAR-1 and EPCR. PAR-1 and EPCR dependence for inhibition of staurosporine-induced endothelial cell apoptosis by rwt APC and anticoagulantly impaired APC variants was studied using blocking antibodies against PAR-1 (□; combination of WEDE-15 at 20 μg/mL and ATAP-2 at 15 μg/mL) or EPCR ( ; rabbit anti-EPCR at 20 μg/mL). ▪ represents no antibodies added. Cells were incubated with rwt APC or APC variants (5 nM) 5 hours prior to induction of apoptosis by staurosporine (10 μM, 1 hour). Apoptosis was analyzed by the uptake of Apopercentage dye and expressed as a percentage relative to the percentage of apoptotic cells observed in the absence of added APC (20% of all cells), which was set as 100%. ▥ represents relative apoptosis in the absence of APC and staurosporine. Each bar represents the mean ± SEM from at least 3 independent experiments.
; rabbit anti-EPCR at 20 μg/mL). ▪ represents no antibodies added. Cells were incubated with rwt APC or APC variants (5 nM) 5 hours prior to induction of apoptosis by staurosporine (10 μM, 1 hour). Apoptosis was analyzed by the uptake of Apopercentage dye and expressed as a percentage relative to the percentage of apoptotic cells observed in the absence of added APC (20% of all cells), which was set as 100%. ▥ represents relative apoptosis in the absence of APC and staurosporine. Each bar represents the mean ± SEM from at least 3 independent experiments.
Cleavage of synthetic PAR-1 N-terminal tail by wild-type and variant APC
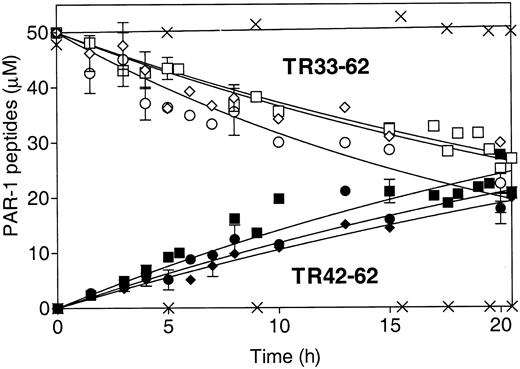
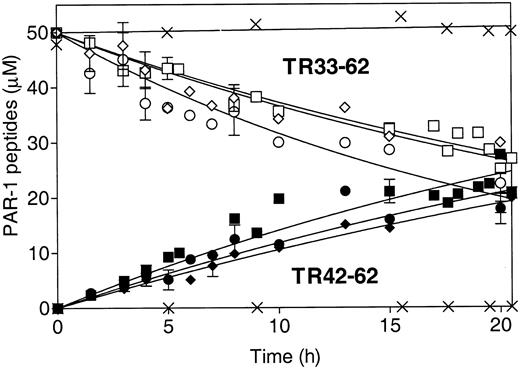
The absence of antiapoptotic activity of S360A-APC and the requirement for PAR-1 imply that a primary mechanistic step for APC's antiapoptotic activity involves PAR-1 proteolytic activation.5,11 To characterize the effects of the mutations in APC on proteolytic activation of PAR-1 due to cleavage at Arg41, a synthetic 30-mer peptide representing the PAR-1 N-terminal sequence (residues 33-62 [TR33-62]) was studied as an APC substrate. This TR33-62 PAR-1 peptide is cleaved at Arg41 by thrombin.25 APC cleaves another PAR-1 synthetic N-terminal peptide at Arg41, the thrombin cleavage site.27 Using HPLC quantitative analysis, we found that rwt APC cleaved the TR33-62 peptide at Arg41 and generated similar fragments as thrombin, TR33-41, and TR42-62 but at approximately a 25 000-fold lower catalytic efficiency based on comparison of kcat/Km for the 2 enzymes (data not shown). When the time course for TR33-62 cleavage was monitored using HPLC to quantitate the disappearance of the peak for the TR33-62 substrate and the appearance of the TR42-62 product, the results showed that there were no substantial differences in the rate of TR33-62 cleavage between the rwt APC, 229/230-APC, and 3K3A-APC (Figure 4). Similarly, no significant differences in APC-induced Ca++-intracellular flux monitored as FURA-2-AM fluorescence changes were observed in EA.hy926 endothelial cells when rwt APC was compared with the 2 antiapoptotic APC variants, 229/230-APC and 3K3A-APC (data not shown). These results are consistent with the hypothesis that APC cleaves PAR-1 at Arg41 and that the mutations in the 2 APC variants described here with reduced anticoagulant activity but with normal antiapoptotic activity did not significantly reduce the ability of the protease domain of APC to cleave PAR-1 at Arg41.
Cleavage of PAR-1 N-terminal TR33-62 peptide at Arg41 by rwt APC and APC variants. HPLC was used to monitor TR33-62 cleavage by APC over time as disappearance of the TR33-62 peptide substrate peak (open symbols) and as appearance of the TR42-62 peptide product peak (solid symbols). ▪ and □ indicate rwt APC; • and ○, 229/230-APC; ♦ and ⋄, 3K3A-APC; and ×, S360A-APC. The pooled data points of 3 to 5 independent experiments are shown for rwt APC and the 2 antiapoptotic APC variants. No cleavage was observed for the S360A-APC that lacks the active site Ser (× denotes no changes in TR33-62 or TR42-62). Error bars indicate SEM.
Cleavage of PAR-1 N-terminal TR33-62 peptide at Arg41 by rwt APC and APC variants. HPLC was used to monitor TR33-62 cleavage by APC over time as disappearance of the TR33-62 peptide substrate peak (open symbols) and as appearance of the TR42-62 peptide product peak (solid symbols). ▪ and □ indicate rwt APC; • and ○, 229/230-APC; ♦ and ⋄, 3K3A-APC; and ×, S360A-APC. The pooled data points of 3 to 5 independent experiments are shown for rwt APC and the 2 antiapoptotic APC variants. No cleavage was observed for the S360A-APC that lacks the active site Ser (× denotes no changes in TR33-62 or TR42-62). Error bars indicate SEM.
Discussion
In vivo data suggested there might be an important distinction between the anticoagulant and cell protective activities of APC.5,12 APC-induced neuroprotective effects in a murine ischemic stroke model were observed at low APC doses that had no effect on fibrin deposition or on restoration of blood flow, indicating that APC's neuroprotective effects, at least in part, were independent of APC's anticoagulant activity.5 Moreover, APC provides direct protection for neurons against NMDA-induced excitotoxic injury.12 Interestingly, heterozygosity for the Arg506Gln mutation in factor V (factor VLeiden), which effectively retards factor Va inactivation by APC and is a significant risk factor for venous thrombosis, is reported to protect against lipopolysaccharide (LPS)-induced sepsis in mice and may also do the same in humans.28 This implies that inactivation of factor Va, the hallmark of APC anticoagulant activity, may not provide the entire explanation for APC-mediated reduction of mortality in patients with severe sepsis.1
To generate recombinant APC variants with reduced risk of bleeding due to reduced anticoagulant activity, we dissected APC's anticoagulant activity from its cytoprotective activity by site-directed mutagenesis. Using staurosporine-induced endothelial cell apoptosis assays, we show here that Ala mutations (RR229/230AA and KKK191_193AAA) in 2 APC surface loops that severely reduce anticoagulant activity result in 2 APC variants that retain normal antiapoptotic activity that requires PAR-1 and EPCR. Moreover, these 2 APC variants retain a normal ability to cleave a PAR-1 N-terminal peptide at Arg41. To compare these 2 APC variants with rwt APC in terms of their relative antiapoptotic and anticoagulant activities, we assigned the observed activity of rwt APC a value of 100% and calculated the percent activity of each APC variant from dose-response data (Figures 1 and 2). This normalization inherently yields a “cytoprotective to anticoagulant” ratio for rwt APC of 1.0 (Table 1). When the ratio of antiapoptotic activity to anticoagulant activity was calculated for the APC mutants (Table 1), the 2 APC variants exhibited 7 times and 25 times greater antiapoptotic activity relative to anticoagulant activity compared with rwt APC, respectively.
Thus, these data imply that the RR229/230AA and KKK191_ 193AAA mutations in APC, which cause decreased cleavage at Arg506 in factor Va, do not impair cleavage at Arg41 in PAR-1.
The most notable serious adverse event associated with APC treatment of severe sepsis patients is serious bleeding.1,29 Administration of APC in the Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial was associated with an increase in serious bleeding events, most of which occurred during the 4-day APC infusion period (2.1% [20 of 940] versus 0.7% [6 of 881], APC versus placebo; P < .01).29 Although APC reduced mortality in severe sepsis, at least some of APC's lifesaving effects may have been counteracted by an increase in hemorrhage as cause of death (2.5% [6 of 236] versus 0.7% [2 of 273], APC versus placebo; P = .1).29 Safer APC variants such as 229/230-APC and 3K3A-APC therefore not only might reduce bleeding complications associated with the current APC regimen but also might achieve an additional reduction in mortality from increased dosage and/or prolonged infusion periods.
In summary, here we provide 2 examples of APC mutants—namely, 3K3A-APC and 229/230-APC—that have a substantial reduction in anticoagulant activity but retain normal antiapoptotic activity. These findings indicate that genetic engineering strategies aimed at reducing APC's anticoagulant activity while preserving EPCR-dependent ability of APC to signal cells via PAR-1 activation are feasible. We suggest that therapeutic use of such APC variants are likely to reduce serious bleeding events associated with clinical use of rwt APC while providing the beneficial effects of APC acting directly on cells.
Prepublished online as Blood First Edition Paper, June 3, 2004; DOI 10.1182/blood-2004-01-0110.
Supported in part by an American Heart Association Fellowship (L.O.M.) and by grant R37HL52246 from the National Heart, Lung, and Blood Institute (J.H.G.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr L. Brass (University of Pennsylvania, Philadelphia, PA) for PAR-1 antibodies, Dr C. J. S. Edgell (University of North Carolina, Chapel Hill) for the EA.hy926 endothelial cell line, and Ms Xiao Xu for expert technical assistance.


 ; rabbit anti-EPCR at 20 μg/mL). ▪ represents no antibodies added. Cells were incubated with rwt APC or APC variants (5 nM) 5 hours prior to induction of apoptosis by staurosporine (10 μM, 1 hour). Apoptosis was analyzed by the uptake of Apopercentage dye and expressed as a percentage relative to the percentage of apoptotic cells observed in the absence of added APC (20% of all cells), which was set as 100%. ▥ represents relative apoptosis in the absence of APC and staurosporine. Each bar represents the mean ± SEM from at least 3 independent experiments.
; rabbit anti-EPCR at 20 μg/mL). ▪ represents no antibodies added. Cells were incubated with rwt APC or APC variants (5 nM) 5 hours prior to induction of apoptosis by staurosporine (10 μM, 1 hour). Apoptosis was analyzed by the uptake of Apopercentage dye and expressed as a percentage relative to the percentage of apoptotic cells observed in the absence of added APC (20% of all cells), which was set as 100%. ▥ represents relative apoptosis in the absence of APC and staurosporine. Each bar represents the mean ± SEM from at least 3 independent experiments.