Abstract
Erythropoietin (EPO), a hypoxia-inducible cytokine, is required for survival, proliferation, and differentiation of erythroid progenitor cells. EPO can also stimulate proliferation and angiogenesis of endothelial cells that express EPO receptors (EPORs). In this study we investigated the EPO response of vascular endothelial cells at reduced oxygen tension (5% and 2%), in particular the effect of EPO on nitric oxide (NO) release. Endothelial nitric oxide synthase (eNOS) produces NO, which maintains blood pressure homeostasis and blood flow. We find that EPOR is inducible by EPO in primary human endothelial cells of vein (HUVECs) and artery (HUAECs) and cells from a human bone marrow microvascular endothelial line (TrHBMEC) to a much greater extent at low oxygen tension than in room air. We found a corresponding increase in eNOS expression and NO production in response to EPO during hypoxia. Stimulation of NO production was dose dependent on EPO concentration and was maximal at 5 U/mL. NO activates soluble guanosine cyclase to produce cyclic guanosine monophosphate (cGMP), and we observed that EPO induced cGMP activity. These results suggest that low oxygen tension increases endothelial cell capacity to produce NO in response to EPO by induction of both EPOR and eNOS. This effect of EPO on eNOS may be a physiologically relevant mechanism to counterbalance the hypertensive effects of increased hemoglobin-related NO destruction resulting from hypoxia-induced increased red cell mass. (Blood. 2004;104:2073-2080)
Introduction
Erythropoietin (EPO), required for the proliferation and differentiation of erythroid progenitor cells to yield red blood cells, elicits a response in numerous tissues depending on the level of expression of its receptors. In addition to hematopoietic precursor cells, EPO receptor (EPOR) expression and biologic response to EPO have been observed in endothelial, neural, muscle, cardiac, and other cell types.1-6 During erythroid differentiation, EPORs are minimally expressed on hematopoietic stem cells. EPORs are induced in erythroid progenitor cells as they become more responsive and dependent on EPO for survival, are down-regulated in late erythroid cells, and are absent on mature red blood cells.7,8 In neuronal cells, EPORs are inducible by hypoxia resulting in increased EPO sensitivity.9 Through binding to EPORs, EPO maintains several activities in the cardiovascular system. In cardiac cells, EPO protects cardiomyocytes against ischemic injury, without an increase in hematocrit, demonstrating that EPO can directly protect the ischemic and infarcted heart.10 In endothelial cells, EPO can stimulate dose-dependent cellular proliferation and chemotaxis in vitro and induces angiogenesis in vivo in select model systems such as the chick chorioallantoic membrane and mouse uterine endometrium.1,5,11,12 In an in vitro angiogenesis assay, the stimulation of capillary outgrowth by EPO is comparable with that of vascular endothelial growth factor (VEGF).13,14 EPO has been proposed to increase neovascularization induced by inflammation or ischemia associated with coronary heart disease, possibly via mobilizing endothelial progenitor cells from the bone marrow.15
EPO is produced primarily in the fetal liver and adult kidney in a hypoxia-dependent manner. Low oxygen tension increases activity of hypoxia-inducible factor 1 (HIF-1) that binds to cis-acting DNA hypoxia response elements (HREs) to activate transcription of hypoxia-responsive genes such as EPO, VEGF, glucose transporters, and glycolytic enzymes.16 Clinically, recombinant EPO is used for treatment of anemia in patients with chronic renal failure. However, long-term administration has been associated with hypertension, thought initially to be due to rising hematocrit.17-19 Subsequent studies revealed that EPO-induced hypertension may result from a hematocrit-independent, vasoconstriction-dependent response.20-23 Other factors suggested as contributory to hypertension induced by long-term EPO administration include increased cytoplasmic calcium concentrations leading to a blunted response to the vasodilator nitric oxide (NO), increased endothelin production, and an imbalance in local vasoactive agents.22,23 Infusion of EPO into the lower limbs of healthy volunteers suggested that EPO may impair synthesis of endothelial NO resulting in vasoconstriction.24 Incubation with EPO of human coronary artery endothelial cells to 24 hours inhibited NO production and endothelial nitric oxide synthase (eNOS) expression while stimulating DNA synthesis.25 However, short-term EPO stimulation of vascular endothelial cells in culture generally has not shown modification of NO synthesis or regulation of expression of endothelin-1 or nitric oxide synthase, data interpreted as suggesting that the hypertensive effect of EPO is not a direct effect on endothelial cells.26,27
Conflicting evidence for EPO modification of NO and eNOS and its role in vascular function was provided from analysis of isolated human vessel preparations wherein EPO stimulated relaxation of arterial segments in an endothelium-dependent manner that was abrogated by inhibition of nitric oxide synthase.28 Moreover, transgenic mice overexpressing EPO exhibit increased eNOS expression, bioavailability of NO, and NO relaxation without any accompanying increase in hypertension, stroke, myocardial infarction, or thromboembolism, in spite of increases in hematocrit up to 80%.29 The mice also exhibit increased erythrocyte deformability.30 The increase in NO production in these animals appears to counteract in part the effect of high hematocrit and increased blood viscosity as well as increased expression of the potent vasoconstrictor endothein-1 (ET-1) and thus to prevent cardiovascular dysfunction.29,31 The increases in eNOS expression and NO production observed in transgenic mice with elevated EPO expression, elevated hematocrit, and normal blood pressure are in marked contrast with the association between EPO treatment and hypertension in patients with chronic renal failure. This raises the possibility that EPO may have some direct effect in stimulating vascular endothelium to increase NO bioavailability.
To clarify the response of endothelium to EPO stimulation, we examined the levels of EPOR, eNOS, and NO release in cultured endothelial cells at normoxia and at reduced oxygen levels that may be experienced physiologically or pathologically. We found that EPO induces endothelial cell responses, especially at low oxygen tension, explained in part by induction of EPOR after treatment with EPO. EPO stimulation generally increased NO and cyclic guanosine monophosphate (cGMP) production and had an additive effect to that of hypoxia on induction of eNOS.
Materials and methods
Cells and cell culture
Transformed human bone marrow endothelial cells, TrHBMECs,32 were grown on gelatin-coated 35-mm culture dishes in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS), 3 mM glutamine, 1 μg/mL folic acid, 50 μg/mL penicillin, and 50 μg/mL streptomycin in 5% CO2-95% room air. Before exposure to reduced oxygen, cells were trypsinized, washed with phosphate-buffered saline (PBS), and cultured in medium consisting of DMEM with 2% FBS, 3 mM glutamine, 1 μg/mL folic acid, 50 μg/mL penicillin, and 50 μg/mL streptomycin. Human umbilical vein endothelial cells (HUVECs; Clonetics, Walkersville, MD) were cultured in endothelial basal media (EBM-2) containing 2% FBS and cytokines (human endothelial growth factor [hEGF], VEGF, human fibroblast growth factor [hFGF], and insulin-like growth factor 1 [IGF-1]; Clonetics) under 5% CO2 with balanced 95% room air. Before cells were exposed to low oxygen tension (5% or 2% oxygen), cells were washed in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer and plated in EBM-2 containing 2% FBS and cytokines. Human umbilical artery endothelial cells (HUAECs; Clonetics) were cultured with FBS increased to 5% during the initial growth phase.
Real-time RT-PCR
Cells were plated at a density of 6000 cells in 35-mm culture dishes for 2 days. After incubation at different oxygen tensions, cells were harvested. RNA was isolated using STAT 60 (Tel-TEST, Friendswood, TX) and treated with RNase-Free DNase (Promega, Madison, WI). Total RNA (1 μg) from each sample was used for first-strand cDNA synthesis using murine leukemia virus (MuLV) reverse transcriptase (RT) and oligo d(T)16 (Applied Biosystems, Foster City, CA). Quantitative real-time RT-polymerase chain reaction (PCR) analyses were performed using a 7700 Sequence Detector and Taqman oligonucleotide probes (Applied Biosystems) spanning adjacent exons of specific genes and forward and reverse PCR primers from the upstream and downstream exon regions, respectively (Table 1). The oligonucleotide probes were fluorescently labeled on the 5′ end with FAM (6-carboxy-fluorescein) and on the 3′ end with TAMRA (6-carboxy-tetramethyl-rhodamine). PCR reaction conditions consisted of 50°C for 2 minutes and 95°C for 4 minutes, followed by cycling between a melting temperature of 95°C for 15 seconds and an anneal-extension temperature of 60°C for 1 minute, repeated for 40 cycles. Quantification of the corresponding mRNA transcript was determined by comparison with gene-specific cDNA standards. β-Actin was used as an internal control for the total amount of RNA analyzed.
Protein analysis
Cells were washed 2 times with cold PBS, treated with lysis buffer, and scraped from the plate. The cell lysate was centrifuged for 10 minutes and the supernatant was transferred to a new tube. For EPOR analysis, 1 mg protein was incubated with 1:1000 dilution of EPOR rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. The immunocomplex was captured by adding protein A-Agarose (Santa Cruz Biotechnology), rocked at 4°C for 2 hours, and centrifuged, and the immunoprecipitate washed 2 times with cold PBS. The protein in the sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. The membrane was blocked with 5% milk-0.1% Tween 20 for 1 hour at room temperature and probed with EPOR rabbit polyclonal antibody 1:1000 (Santa Cruz Biotechnology) overnight at 4°C. Horseradish peroxide-labeled anti-rabbit immunoglobulin G (IgG) was used as the secondary antibody. Hyperfilm was used to visualize the secondary antibody by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). For analysis of eNOS phosphorylation, TrHBMECs were washed with cold PBS, left in serum-free media for 6 hours, and treated with EPO for 15 minutes, 30 minutes, and one hour. Then, they were lysed and proteins were incubated with anti-eNOS monoclonal antibody (1-2 μg/mL; BD Transduction Laboratories, San Jose, CA) overnight. The proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed using as a primary antibody a specific antibody for eNOS phosphorylated at serine 1177 (Ser-1177; BD Transduction Laboratories) 1:1000 and incubated at 4°C overnight. The labeled phosphorylated eNOS was visualized by chemiluminescence. Membrane was then stripped for one hour at 55°C, washed, blocked, and incubated with anti-eNOS monoclonal antibody overnight at 4°C and then visualized by chemiluminescence.
Measurement of nitrite levels in supernatant of cell culture
Cells were washed with PBS and the medium was replaced with Dulbecco modified Eagle medium (DMEM) with 3 mM glutamine, 1 μg/mL folic acid, 50 μg/mL penicillin, and 50 μg/mL streptomycin or HEPES buffer (135 mM NaCl, 2.7 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 11.9 mM NaHCO3, 0.36 mM NaH2PO4, 14.7 HEPES, and 11 mM glucose). Supernatant media from treated cells was immediately frozen on dry ice and stored at -80°C for future measurement of nitrite. Frozen samples of treated medium were thawed and then injected into an I3-solution actively purged with a helium stream in line with an NO chemiluminescence analyzer (Sievers, Boulder, CO). Nitrite was measured by reduction in acidified NaI: 7 mL glacial acetic acid, 2 mL distilled water, 50 mg NaI, an antifoaming agent, and a crystal of iodine was added to yield a concentration of 6 to 20 μM.33
cGMP measurement
Cells were washed with PBS, left in serum-free media for 2 hours, pretreated with 1 mM l-N(omega)-nitro-l-arginine methyl ester (l-NAME) for 30 minutes, and then stimulated with 5 U/mL EPO for 15 minutes to 4 hours. At selected time points, medium from the cells was aspirated and 0.1N HCl was added to lyse the cells. After incubation for 10 minutes, the culture plates were scraped, cell lysates were centrifuged, and the supernatants were used for the measurement of cGMP by immunoassay (R&D Systems, Minneapolis, MN).
Statistical analysis
The one-way analysis of variance (ANOVA) and Tukey posttest were used in Prism software (Graph-Pad Software, San Diego, CA).
Results
EPOR and eNOS expression in TrHBMECs
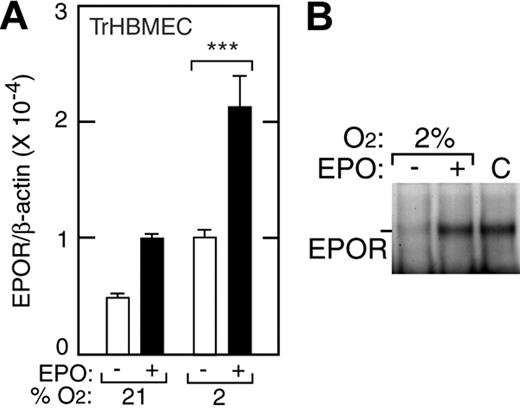
To assess EPO stimulation of endothelial cells, we used a human bone marrow microvascular endothelial cell line (TrHBMEC) that exhibits typical differentiated characteristics of primary HBMECs and has been useful for studying signal transduction, heparin activation, response to growth factors, and interactions between endothelium and hematopoietic progenitor cells.32,34-36 Studies of erythropoiesis indicate that responses to EPO by erythroid precursor cells correlate with their level of EPOR expression. We used real-time quantitative RT-PCR and primers and Taqman probe specific for human EPOR to determine the level of EPOR expression. We found that EPOR expression in TrHBMECs increased when EPO (5 U/mL) was added and further increased when oxygen tension was reduced, with and without EPO present (Figure 1A). At 48 hours of culture, a maximal 5-fold induction of EPOR mRNA was observed at 2% O2 with EPO (P < .001). Western blotting confirmed that EPO treatment of TrHBMECs induced EPOR protein up to 2-fold (Figure 1B). In TrHBMECs, induction of EPOR by hypoxia alone may contribute to increased sensitivity of response to EPO at 2% O2. Although EPO has been reported present in mouse endothelial cells following cerebral ischemia,37 no EPO expression was detectable in any human endothelial cells we studied with or without hypoxic culture conditions (data not shown).
Induction of EPOR by hypoxia in TrHBMECs. (A) EPOR expression was determined by quantitative real-time RT-PCR in TrHBMECs at 21% and 2% O2 without (□) and with (▪) EPO (5 U/mL). Compared with the EPOR level in 21% O2, we observed a 5-fold maximal induction of the EPOR after 48 hours of treatment with EPO at 2% oxygen (***P < .001). Results were normalized to β-actin expression. (B) Western blotting confirmed EPO induction of EPOR protein for cells cultured at 2% O2 for 48 hours. Error bars represent SD.
Induction of EPOR by hypoxia in TrHBMECs. (A) EPOR expression was determined by quantitative real-time RT-PCR in TrHBMECs at 21% and 2% O2 without (□) and with (▪) EPO (5 U/mL). Compared with the EPOR level in 21% O2, we observed a 5-fold maximal induction of the EPOR after 48 hours of treatment with EPO at 2% oxygen (***P < .001). Results were normalized to β-actin expression. (B) Western blotting confirmed EPO induction of EPOR protein for cells cultured at 2% O2 for 48 hours. Error bars represent SD.
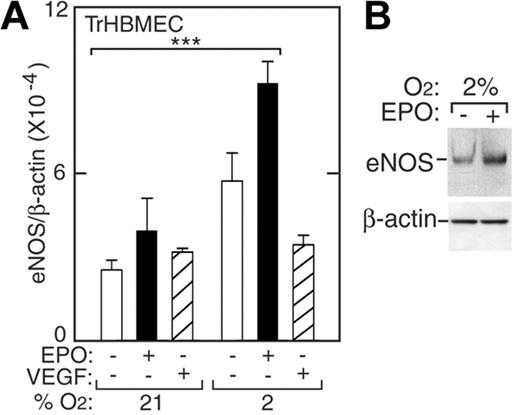
In endothelial cells, eNOS is a constitutively produced protein, required for NO production, that functions to maintain blood pressure homeostasis. To examine eNOS mRNA levels in TrHBMECs we used real-time quantitative RT-PCR and probe and primers specific for human eNOS. We observed induction of eNOS mRNA and protein at low oxygen tension. When normoxic TrHBMECs were treated with EPO (5 U/mL) for 48 hours, eNOS mRNA modestly increased (Figure 2A, closed bars). However, at 2% O2, eNOS expression was more strongly increased by 3-fold (P < .001). The induction of eNOS protein by EPO was demonstrated by Western blotting showing 2-fold induction (Figure 2B). Under normoxia, EPO induction of eNOS protein was observed at 6 hours, but induction was not sustained and was no longer detected at 24 hours (data not shown). At 2% O2, EPO addition to TrHBMECs resulted in the persistent elevation of eNOS mRNA for up to 48 hours and continued induction of eNOS protein for up to 24 hours. To rule out the possibility that these effects might be mediated by VEGF, which is a hypoxia-inducible endothelial cell proliferative factor, VEGF was also added directly to TrHBMEC cultures at various oxygen tensions. VEGF did not increase eNOS expression and therefore did not mediate induction of eNOS by EPO (Figure 2A, striped bars). These data suggest that bone marrow microvascular endothelial cells at hypoxic conditions respond to EPO by inducing eNOS expression—a phenomenon we also observed with large vessel HUVECs and HUAECs.
Induction of eNOS by EPO in TrHBMECs. (A) eNOS expression was determined by quantitative real-time RT-PCR without (□) or with (▪) EPO (5 U/mL) or VEGF (5 pg/mL; ▨) at 21% O2 and 2% O2 for 48 hours. eNOS expression was induced following treatment with EPO at 2% O2 compared with expression at 21% (***P < .001) but was not induced following treatment with VEGF. Results were normalized to β-actin expression. (B) Western blotting confirmed greater induction of eNOS protein in cells cultured for 24 hours at 2% O2 in the presence of EPO. Error bars represent SD.
Induction of eNOS by EPO in TrHBMECs. (A) eNOS expression was determined by quantitative real-time RT-PCR without (□) or with (▪) EPO (5 U/mL) or VEGF (5 pg/mL; ▨) at 21% O2 and 2% O2 for 48 hours. eNOS expression was induced following treatment with EPO at 2% O2 compared with expression at 21% (***P < .001) but was not induced following treatment with VEGF. Results were normalized to β-actin expression. (B) Western blotting confirmed greater induction of eNOS protein in cells cultured for 24 hours at 2% O2 in the presence of EPO. Error bars represent SD.
EPO affects NO production in TrHBMECs
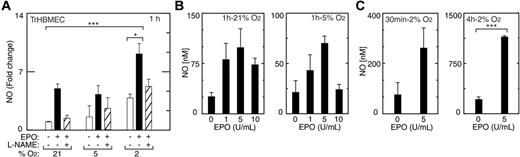
To assess whether eNOS induced in TrHBMECs by EPO and hypoxia represented functional enzyme, cells treated with EPO at different oxygen tensions were monitored for NO release using nitrite levels as an index of NO production. In addition to inducing eNOS mRNA and protein, EPO rapidly activated NO production in TrHBMECs (Figure 3A). An increase in NO production in the presence of EPO (5 U/mL) was detected at one hour of treatment in both normoxic and hypoxic cultures (P < .05). The induction of NO in cells kept at 2% O2 was still detectable after 24 hours of treatment with EPO (data not shown). Addition of the eNOS inhibitor, L-N(omega)-nitro-l-arginine methyl ester (L-NAME), decreased the EPO induction of NO. These data demonstrate a direct, prompt, and persistent synergistic effect of hypoxia and EPO on NO production in endothelial cells. Stimulation of NO production by EPO was dose and oxygen tension dependent. Comparing different concentrations of EPO added to cultures exposed to normoxia or reduced oxygen tension, we observed that NO production was maximal at 5 U/mL at all oxygen tensions evaluated (Figure 3B). At 5 U/mL EPO and one-hour incubation under normoxic conditions, NO release measured more than 3-fold that released in the absence of EPO and further increased with hypoxic conditions. Absolute NO production was greatest under hypoxic conditions (2% O2) at all time points tested up to 4 hours using 5 U/mL EPO (Figure 3B-C), representing a 3-fold increase compared with no EPO at 30 minutes, 6-fold at 1 hour (data not shown), and 6-fold at 4 hours (P < .001). In TrHBMECs, induction of EPOR by hypoxia appears to contribute to the greater sensitivity to EPO at 2% O2, compared with normoxia.
Induction of NO by EPO in TrHBMECs. (A) An increase in NO production was observed at one hour in cells cultured at 21% and 2% O2 in the presence of EPO (5 U/mL) (▪) compared with no EPO (□) (***P < .001; *P < .05), and was inhibited by L-NAME (1 mM; ▨), an inhibitor of NO synthase activity. (B) NO production was determined after one hour of treatment with different concentrations of EPO (1, 5, and 10 U/mL) at 21%, 5%, and 2% O2. Response was most pronounced at 2% O2. EPO induction of NO was maximal at 5 U/mL. (C) NO production at 2% O2 with EPO after 30 minutes and 4 hours of EPO stimulation was also maximal at 5 U/mL EPO (***P < .001). Error bars represent SD.
Induction of NO by EPO in TrHBMECs. (A) An increase in NO production was observed at one hour in cells cultured at 21% and 2% O2 in the presence of EPO (5 U/mL) (▪) compared with no EPO (□) (***P < .001; *P < .05), and was inhibited by L-NAME (1 mM; ▨), an inhibitor of NO synthase activity. (B) NO production was determined after one hour of treatment with different concentrations of EPO (1, 5, and 10 U/mL) at 21%, 5%, and 2% O2. Response was most pronounced at 2% O2. EPO induction of NO was maximal at 5 U/mL. (C) NO production at 2% O2 with EPO after 30 minutes and 4 hours of EPO stimulation was also maximal at 5 U/mL EPO (***P < .001). Error bars represent SD.
Activity of cGMP levels in and eNOS phosphorylation in TrHBMECs
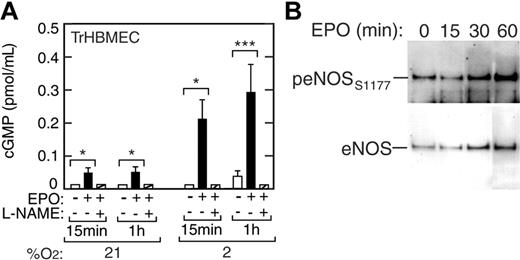
In endothelial cells, guanylate cyclase acts as a second messenger in signal transduction pathways. Induction of eNOS leads to production of NO, which in turn can activate guanylate cyclase to drive cGMP production from guanosine triphosphate (GTP). We examined whether EPO stimulation of NO production in TrHBMECs would result in increased cGMP synthesis (Figure 4A). When TrHBMECs were cultured in the presence of EPO at normoxia and with reduced oxygen tension we observed a hypoxiaand EPO-dependent induction of cGMP. EPO increased cGMP levels by 3-fold at 21% O2 (P < .05) and by 10-fold at 2% O2 (P < .001) (Figure 4A), starting as early as 15 minutes following EPO stimulation. L-NAME inhibited EPO induction of cGMP in these cells, providing evidence that EPO stimulation leads to increased cGMP via the eNOS pathway and NO release.
Induction of cGMP and eNOS phosphorylation in TrHBMECs. (A) The cGMP levels were increased 3-fold by EPO (▪) at 21% O2 and up to 10-fold by EPO at 2% O2 as early as 15 minutes following EPO stimulation compared with the no EPO control (□) (*P < .05; ***P < .001). The eNOS inhibitor, L-NAME (▨), inhibited EPO induction of cGMP in these cells. (B) Western blot analysis showed induction of eNOS protein and eNOS phosphorylation at serine 1177 (peNOSs1177) for cells cultured with EPO at 2% O2. At 60 minutes, the increase in band intensity of eNOS is 2.5-fold and of peNOSs1177 is 5.3-fold compared with 0 minutes, giving an overall increase of 2-fold in the peNOSs1177/eNOS ratio. Error bars represent SD.
Induction of cGMP and eNOS phosphorylation in TrHBMECs. (A) The cGMP levels were increased 3-fold by EPO (▪) at 21% O2 and up to 10-fold by EPO at 2% O2 as early as 15 minutes following EPO stimulation compared with the no EPO control (□) (*P < .05; ***P < .001). The eNOS inhibitor, L-NAME (▨), inhibited EPO induction of cGMP in these cells. (B) Western blot analysis showed induction of eNOS protein and eNOS phosphorylation at serine 1177 (peNOSs1177) for cells cultured with EPO at 2% O2. At 60 minutes, the increase in band intensity of eNOS is 2.5-fold and of peNOSs1177 is 5.3-fold compared with 0 minutes, giving an overall increase of 2-fold in the peNOSs1177/eNOS ratio. Error bars represent SD.
Induction of NO production was detected at 30 minutes and one hour after EPO treatment. The sensitivity of this increase to addition of L-NAME points to a direct contribution from increased eNOS activity that can result from several factors including transcriptional and posttranslational modification, intracellular localization, cofactors, and phosphorylation.38 Western blot analysis of short-term TrHBMEC cultures at 2% O2 was made using antibodies to eNOS and antibodies specific for eNOS phosphorylated at serine 1177 that is associated with increased eNOS activity. This analysis showed an increase in the ratio of phosphorylated eNOS to total eNOS by 2-fold or more by 30 minutes following EPO stimulation (Figure 4B). The results also indicate that eNOS protein increases by 2.5-fold after exposure to EPO.
EPOR and eNOS expression in HUVECs
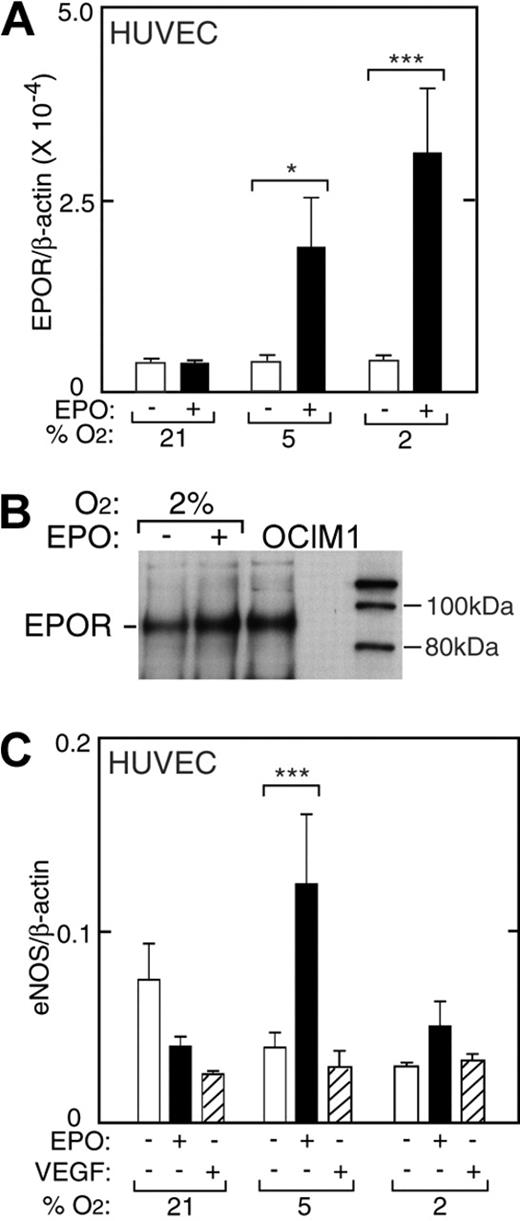
HUVECs express EPOR and exhibit a mitogenic and chemotactic response to EPO.1,12 Under normal culture conditions in the absence of hypoxia and EPO, HUVECs express EPOR at levels comparable with TrHBMECs (Figure 5). We cultured low-passage HUVECs for 48 hours at reduced oxygen tension and examined the ability of EPO to induce EPOR. In contrast to induction of EPOR in TrHBMECs, reduced oxygen tension itself had little or no effect on EPOR expression in HUVECs. However, the combination of low oxygen tension (5% and 2% O2) with EPO (5 U/mL) resulted in hypoxia-dependent increase in EPOR mRNA expression up to 9-fold (P < .001) (Figure 5A). Western blot analysis confirmed EPO induction of EPOR 1.7-fold (Figure 5B).
EPO induction of EPOR in HUVECs. (A) EPO (▪) in combination with reduced oxygen tension (5% and 2% O2) increased EPOR expression in primary HUVECs as measured by quantitative RT-PCR. No change in EPOR was observed with reduced oxygen tension in the absence of EPO (□). Results are normalized to β-actin expression (*P < .05; ***P < .001). (B) Western blot analysis confirmed induction of EPOR protein for HUVECs cultured with EPO at 2% O2. OCIM-1 cell extract was used as a positive control (OCIM1). (C) Expression of eNOS in HUVECs at different oxygen tensions. In the absence of EPO (open bars), expression of eNOS at 2% O2 was less than one-half of the value under normoxia. However, addition of EPO (▪) reversed the decrease in eNOS expression at low oxygen tension, increasing eNOS by 2-fold or more in HUVECs (***P < .001). VEGF (▨) did not induce eNOS expression. Error bars represent SD.
EPO induction of EPOR in HUVECs. (A) EPO (▪) in combination with reduced oxygen tension (5% and 2% O2) increased EPOR expression in primary HUVECs as measured by quantitative RT-PCR. No change in EPOR was observed with reduced oxygen tension in the absence of EPO (□). Results are normalized to β-actin expression (*P < .05; ***P < .001). (B) Western blot analysis confirmed induction of EPOR protein for HUVECs cultured with EPO at 2% O2. OCIM-1 cell extract was used as a positive control (OCIM1). (C) Expression of eNOS in HUVECs at different oxygen tensions. In the absence of EPO (open bars), expression of eNOS at 2% O2 was less than one-half of the value under normoxia. However, addition of EPO (▪) reversed the decrease in eNOS expression at low oxygen tension, increasing eNOS by 2-fold or more in HUVECs (***P < .001). VEGF (▨) did not induce eNOS expression. Error bars represent SD.
Quantification of eNOS mRNA in HUVECs showed a progressive decrease in eNOS mRNA expression after 48 hours of culture with decreasing percent of O2 in the absence of EPO (Figure 5C, open bars). The tendency for eNOS expression to decrease at reduced oxygen tension in HUVECs was in contrast to the increasing levels determined in TrHBMECs. Expression of eNOS mRNA at 2% O2 was less than one half the value at normoxia. Although eNOS mRNA decreased by 2-fold when EPO was added to cultures at normoxia, EPO reversed the decline in eNOS expression in HUVECs cultured at low oxygen tension, increasing eNOS by up to 3-fold at 5% O2 (P < .001, Figure 5C, closed bars). In contrast, adding VEGF to HUVECs decreased the level of eNOS mRNA at normoxia, but did not reverse the decline in eNOS expression that occurred at low oxygen tension (Figure 5C, striped bars). These data suggest that the stimulation of eNOS by EPO in endothelial cells might also be an important oxygen tension-mediated response in vivo and provide further evidence that this effect of EPO is not mediated via VEGF. The EPO induction of eNOS expression at low oxygen tension was concomitant with EPO induction of EPOR at 5% and 2% O2 (Figure 5A), while no increase in EPOR was observed with EPO treatment at 21% O2, suggesting that EPO induction of eNOS may depend on induction of EPOR. Hypoxia has no effect on EPOR in these cells, and EPOR increases in response to EPO and hypoxia with decreasing O2. In contrast, the increase in eNOS by EPO and hypoxia is more complex due to the decrease in eNOS by hypoxia.
NO production in HUVECs
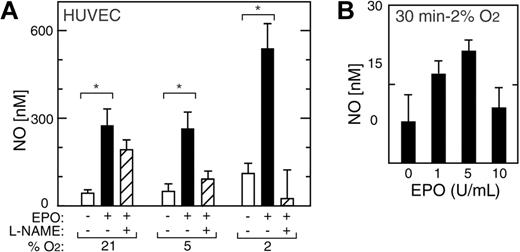
In addition to inducing eNOS mRNA and protein, EPO rapidly activated NO production in HUVECs (Figure 6A). A doubling of NO production in HUVECs was observed after exposure to 2% O2 for one hour in the absence of EPO but was not sustained (Figure 6). Indeed, we observed increased NO production of up to 5- to 6-fold following one hour of EPO treatment at all oxygen tensions between 21% and 2% O2, although eNOS expression decreased after 48 hours of EPO treatment at normoxia (Figure 6). The greatest increase in NO upon EPO treatment was observed at 2% O2 (P < .05); this was markedly diminished by the addition of the eNOS inhibitor L-NAME. Induction of NO production in HUVECs by EPO at 2% O2 was observed both in very short-term (30 minutes) and long-term (21 hours) exposure to EPO, peaked at 5 U/mL of EPO, and was blunted at 10 U/mL (Figure 6B).
EPO induction of NO in HUVECs. (A) NO production was measured in HUVECs as released nitrite after one hour of stimulation by EPO (5 U/mL) (▪) and compared with no EPO (□) (*P < .05). L-NAME suppressed the EPO response (▨). (B) In HUVECs cultured at 2% O2, EPO induction of NO was observed within 30 minutes with a maximal effect at 5 U/mL. Error bars represent SD.
EPO induction of NO in HUVECs. (A) NO production was measured in HUVECs as released nitrite after one hour of stimulation by EPO (5 U/mL) (▪) and compared with no EPO (□) (*P < .05). L-NAME suppressed the EPO response (▨). (B) In HUVECs cultured at 2% O2, EPO induction of NO was observed within 30 minutes with a maximal effect at 5 U/mL. Error bars represent SD.
EPO stimulation of HUAECs
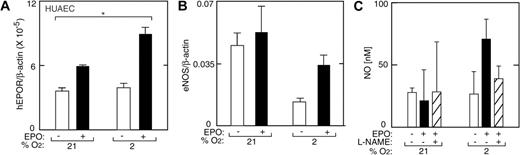
We used HUAECs as a source of primary arterial endothelial cells derived from a large artery. At normoxia, these cells express EPOR at higher levels than HUVECs (Figure 7A). Incubation of HUAECs at low oxygen tension (2% O2) did not alter EPOR expression. However, EPO significantly induced EPOR after 48 hours at low oxygen tension (P < .05), although relative induction of EPOR by EPO in HUAECs at low oxygen tension was markedly less than that observed in HUVECs.
EPO induction of EPOR, eNOS, and NO in HUAECs. (A) EPO (▪) in combination with low oxygen tension (2% O2) increased EPOR expression in primary HUAECs compared with no EPO (□) at 21% O2 (*P < .05). Results were normalized to β-actin expression. (B) In the absence of EPO, expression of eNOS at 2% O2 was less than one-half of the value under normoxia. However, EPO (solid bars) reversed the decrease in eNOS expression at low oxygen tension. Results were normalized to β-actin expression. (C) NO production was measured in HUAECs as released nitrite after one hour of stimulation with EPO (5 U/mL) (▪) and without EPO (open bars). L-NAME (▨) suppressed the EPO response. Error bars represent SD.
EPO induction of EPOR, eNOS, and NO in HUAECs. (A) EPO (▪) in combination with low oxygen tension (2% O2) increased EPOR expression in primary HUAECs compared with no EPO (□) at 21% O2 (*P < .05). Results were normalized to β-actin expression. (B) In the absence of EPO, expression of eNOS at 2% O2 was less than one-half of the value under normoxia. However, EPO (solid bars) reversed the decrease in eNOS expression at low oxygen tension. Results were normalized to β-actin expression. (C) NO production was measured in HUAECs as released nitrite after one hour of stimulation with EPO (5 U/mL) (▪) and without EPO (open bars). L-NAME (▨) suppressed the EPO response. Error bars represent SD.
Culture of HUAECs at 2% O2 resulted in decreased eNOS expression (Figure 7B), as was observed in HUVEC cultures at low oxygen tension. At normoxia, addition of EPO to the cultures did not alter eNOS expression or NO production. In contrast, at low oxygen tension (2% O2) EPO attenuated the decrease in eNOS caused by hypoxia, similarly to HUVECs. HUAECs exposed to hypoxia and EPO also produced increased NO, and that production was blocked by L-NAME (Figure 7C). The lesser induction of EPOR in HUAECs by hypoxia and EPO compared with HUVECs may be reflected in the more modest NO production response of HUAECs.
Discussion
The intact endothelial lining produces the vasodilator, NO, and other vasoactive products that maintain blood pressure homeostasis and vasomotor activity in blood vessels. Impairment of endothelium-dependent vasorelaxation occurs in hypertension in part because of the loss of endothelial-derived NO. At low oxygen tension in vivo a set of adaptive responses is induced at both the systemic and cellular level: erythropoiesis, vasodilatation, angiogenesis, and glycolysis. Hypoxia-stimulated erythropoiesis can raise blood viscosity, to which the vascular endothelium responds by up-regulation of eNOS.39 Exogenous erythropoietin administration, used to induce erythrocytosis in rats, has also been associated with increased NO production in vivo.40,41 Constitutive overexpression of EPO in transgenic mice also results in erythrocytosis increasing hematocrit to 80%, as plasma EPO levels rise from 0.022 U/mL in normal littermates to more than 10-fold higher in mice carrying the transgene. In addition, these mice exhibit increased eNOS activity and NO production that appear to protect against arterial hypertension and thromboembolism.29 The increase in eNOS activity and NO production induced by EPO may be essential in maintaining NO-mediated vascular regulatory mechanisms since hemoglobin, whether free in plasma or contained in the red cells, rapidly destroys NO or traps NO via direct reaction of NO with hemoglobin. Elevated eNOS activity observed in rodents with elevated EPO levels has been attributed to increased shear stress created by the increase in hematocrit, since shear stress can stimulate eNOS activity by a variety of mechanisms including increasing eNOS mRNA expression and/or stability, or eNOS protein phosphorylation.42,43
However, in patients with polycythemia—who are not receiving exogenous EPO (and who may have very low EPO levels)—high hematocrit appears to inhibit endothelium-dependent vasodilatation in response to acetylcholine. This process can be reversed by hemodilution and normalization of blood hemoglobin concentration.44 This suggests that the increased hemoglobin itself in polycythemic patients may rapidly destroy NO. Thus, in mice and rats with EPO-induced erythrocytosis, the increase in endothelial NO production may be due to mechanisms independent of increased hematocrit that involve direct response of endothelium to EPO.29,45
We now provide evidence that EPO can have a direct action on endothelium that increases NO bioavailability by up-regulation of eNOS. This response to EPO, demonstrated here in several types of human endothelial cells, provides the basis for an alternate explanation for the elevation of eNOS activity and NO production during EPO treatment.
Previous short-term studies in cultured endothelial cells indicated that EPO had little or no effect on increasing eNOS activity.26,27 In fact, incubation of human coronary artery endothelial cells with EPO for 24 hours inhibited NO production and eNOS expression, while stimulating cell proliferation.25 We found instead a more complex situation related to oxygen tension. Firstly, at normoxia, EPO treatment decreased eNOS expression in HUVECs and had little or no effect on HUAECs and TrHBMECs for up to 48 hours in culture, analogous to previous results showing a lack of eNOS induction with EPO treatment. The basal eNOS levels were comparable in HUVECs and HUAECs, and decreased when cells were cultured at reduced oxygen tension, dropping by 2- to 4-fold at 2% O2. Secondly, in contrast to these observations at normoxia, at reduced oxygen tension EPO induced eNOS expression by 2- to 3-fold compared with hypoxia alone in HUVECs and HUAECs, with the greatest induction observed in HUVECs at 5% O2. With hypoxia, EPO also induced eNOS expression in TrHBMECs. Increased responsiveness to EPO at low oxygen tension could be explained in part by induction of EPOR. The fact that EPO induced eNOS at 5% to 2% O2 but not at normoxia suggests that the resulting vasodilatation may be physiologically meaningful.
In addition to increasing eNOS expression, EPO directly increased NO production in cultures of HUVECs, HUAECs, and TrHBMECs at low oxygen tension. Nitrite, a stable end product of NO oxidation, was significantly increased as early as 30 minutes after treatment with EPO, with the greatest effect at 2% O2, and was still detected after 24 hours of incubation. L-NAME, an inhibitor of NOS activity, diminished the effect of EPO, indicating that the induced eNOS was enzymatically active and was responsible for the increased NO production in EPO-treated endothelial cells. The rapid increase in eNOS activity following EPO stimulation is concomitant with an increase in eNOS phosphorylation at serine 1177 that increases eNOS activity and a possible increase in total eNOS protein. EPO induced NO dose dependently at low concentrations of EPO but above a maximal effect at 5 U/mL had less or no effect. NO activates soluble guanosine cyclase to produce the second messenger cGMP. Our results indicate that EPO stimulates cGMP production. cGMP triggers downstream events, activating 2 specific cGMP-dependent protein kinases (protein kinase G I [PKG I] and PKG II). In turn, PKG I can mediate vasodilatation and inhibit platelet aggregation.46,47 Addition of L-NAME diminishes the cGMP response, providing additional evidence that EPO stimulation of eNOS in endothelial cells is an important and necessary pathway for NO and subsequently cGMP production.
In vivo, transgenic mice overexpressing EPO exhibit marked increase in endothelial eNOS levels, NO production, and plasma nitrite levels.29 L-NAME administration to these mice results in systemic vasoconstriction, hypertension, and death, showing that NO compensates to offset elevated peripheral resistance resulting from high hematocrit (80%) in these mice. The concomitant induction by EPO of eNOS and NO would provide protection from increased NO destruction or decreased NO bioavailability due to NO binding by the increased hemoglobin mass, particularly by the small but more potently NO binding fraction of free hemoglobin in the plasma.48 Alternatively, it has been proposed that NO hemoglobin in adducts or nitrite reduction by hemoglobin may also contribute to the pool of available NO,49,50 but these effects are most significant at superphysiologic or pharmacologic concentrations of NO or nitrite ions.
In HUVECs and HUAECs, induction of EPOR was observed when EPO was administered at decreased oxygen tensions. This provides an explanation in part for the increased capacity for EPO to induce eNOS and NO bioavailability in these cells in these conditions. Although EPOR is modestly induced by hypoxia alone in microvascular TrHBMECs, EPO as well as hypoxia is required to induce EPOR in HUVECs and HUAECs suggesting that, unlike EPO, EPOR is not a HIF-1-dependent gene. Furthermore, the response of TrHBMECs to EPO at reduced oxygen tension implies that EPO may also induce eNOS and NO production in the bone marrow.
We observed a dose-dependent induction of NO by EPO that peaked at 5 U/mL and was absent at higher concentrations. While the normal range for plasma EPO is 0.004 to 0.026 U/mL, during EPO therapy in anemic patients with chronic renal failure and reduced endogenous EPO, the plasma level ranges from 0.15 U/mL to 4.4 U/mL, approaching the level of EPO at which we observed maximal in vitro response in NO production. In transgenic mice, which chronically overexpress EPO, where plasma levels are 0.26 U/mL, marked endothelial elevations in eNOS activity and NO production are clearly evident.
Increased plasma levels of VEGF detected in patients treated with recombinant human EPO have led to the speculation that VEGF may contribute to endothelial effects of EPO.51 However, we did not observe an induction of VEGF expression by EPO (data not shown), indicating that the EPO effect on cell proliferation and NO production observed in these cells is unlikely to be mediated by VEGF production. Furthermore, we did not observe induction of NO production by VEGF alone in TrHBMECs or HUVECs, at any oxygen tension examined, confirming that the increase of NO induced by EPO is not mediated by VEGF.
Recent DNA microarray analyses of endothelial cell gene expression suggest distinct, diverse expression profiles of cultured endothelial cells derived from different tissues and different vascular beds.52 Large vessel endothelial cells had different patterns from microvascular endothelial cells, and arterial endothelial cells had different patterns from venous endothelial cells suggesting that specific gene expression profiles in endothelium were important in their physiologic and pathologic responses.52 HUVECs, representative of venous endothelial cells, exhibited the greatest EPO responsiveness in induction of EPOR and eNOS, and NO production, at low oxygen tension compared with arterial endothelial cells (HUAECs) and microvascular endothelial cells (TrHBMECs). These relative changes suggest that in vivo, veins may be more responsive than arteries to EPO and hypoxia. Recent studies in vivo using the hamster chamber window model to monitor microcirculation changes showed that as an adaptation to hypoxia erythropoiesis and hematocrits increased, indicative of increased EPO levels, as well as vasodilatation, consistent with increased production of NO.53 Macroscopic observation revealed increased venular but not arterial diameter with no significant difference in blood pressure.
New approaches to the treatment of cardiovascular diseases suggest protective effects of EPO in the ischemic heart. Induction of NO by EPO in hypoxic conditions provides one explanation for the proposed EPO protection in myocardial infarction following coronary artery ligation in rats,54 although EPO may also stimulate neovascularization or myocyte survival10 and reduce associated apoptosis detectable as early as 24 hours. EPO induction of NO may rapidly increase vasodilatation and facilitate effective collateral circulation. The up-regulation of EPOR in human endothelial cells in vitro with their consequent increased sensitivity to EPO at low oxygen supports the view that EPO stimulation of endothelial cells in vivo may involve a 2-step process. Endothelial responses may require an increased exposure to EPO (by hypoxic induction, direct administration of EPO, or other means) combined with up-regulation of EPOR in selected endothelial beds. Without induction of EPOR, only a minimal EPO response would be expected. With increased availability of EPOR, EPO induction of eNOS would be up-regulated and subsequent increased endothelial NO production would permit the vasculature to improve NO bioavailability and blood flow.55
Prepublished online as Blood First Edition Paper, June 17, 2004; DOI 10.1182/blood-2004-02-0744.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.