Abstract
In tumor dormancy, tumor cells persist in the host over a long period of time but do not grow. We investigated in the DA1-3b mouse model of acute myeloid leukemia how leukemic cells could persist for months in spite of an effective antileukemic immune response. Mice were immunized with irradiated interleukin 12 (IL12)- or CD154-transduced DA1-3b cells, challenged with wild-type DA1-3b cells, and randomly killed during 1-year follow-up. Quantification of residual disease 1 year after challenge showed that persistent leukemic cells represented less than 0.02% of spleen cells in most animals. These residual cells were still able to kill naive hosts, even when isolated after 1 year of persistence. Persistent leukemic cells were more resistant to specific cytotoxic T-cell (CTL)-mediated killing and had enhanced B7-H1 and B7.1 expression proportional to the time they had persisted in the host. Blocking B7-H1 or B7.1/cytotoxic T-lymphocyte-associated antigen (CTLA-4) interaction enhanced CTL-mediated killing of the persistent cells, and blocking B7-H1, B7.1, or CTLA-4 in vivo prolonged survival of naive mice injected with persistent leukemic cells. Thus, escape of leukemic cells from tumor immunity via overexpression of B7-H1 or B7.1 might represent a new mechanism of tumor dormancy in acute leukemia. (Blood. 2004;104:2124-2133)
Introduction
Tumor dormancy is a condition in which tumor cells persist in the host for a long period of time but do not grow.1 It is observed in several solid tumor types, such as breast cancer, melanoma, and renal carcinoma, in which relapse can occur decades after diagnosis. Tumor dormancy is also a common phenomenon in hematologic malignancies. Low-grade lymphomas can remain in partial or complete remission for many years before disease progression. Residual lymphoma cells have been found in patients treated with anti-idiotype antibodies and who have been in continuous remission for at least 3 to 8 years.2 Late relapse can be observed in patients with acute leukemia, and minimal residual disease (MRD) is frequently detected during complete clinical remission. Malignant hematopoietic progenitors persist in chronic myelogenous leukemia (CML) patients with a complete cytogenetic response despite continuous treatment with imatinib mesylate, and BCR/ABL+ cells are detected in the bone marrow of patients many years after allogeneic bone marrow transplantation, offering examples of tumor dormancy in patients exposed to continuous treatment.3 Thus, understanding how malignant cells can persist in a dormant state may lead to new strategies to prevent relapse.
Little is known about the mechanisms involved in tumor dormancy in acute leukemia. In solid tumors, lack of angiogenesis can lead to tumor dormancy, but in most tumors dormant states are in the form of micrometastases where angiogenesis plays little role. In hematologic malignancies, the best characterized model of tumor dormancy is the B-cell lymphoma 1 (BCL1) mouse lymphoma.1,4-16 In this model, tumor dormancy can be induced by prior immunization with the BCL1-derived immunoglobulin (Ig) to generate an anti-idiotype (anti-Id) immune response, which causes partial cell cycle arrest and apoptosis. Dormant cells remain malignant, as adoptive transfer to naive hosts results in lymphoma. An important finding was that CD8+ T cells and interferon γ (IFN-γ), in collaboration with humoral immunity, had a role in maintaining tumor dormancy.5 These findings suggest that lymphoma cells can persist in the host when a balance is established between an immune response, at least partially mediated by T cells, and lymphoma growth. However, the conclusions obtained from the BCL1 model are difficult to extend to other hematologic malignancies because anti-Id linking to Id at the cell surface is specific to lymphoid cells.
We previously reported that in the DA1-3b/C3H mouse model of acute myeloid leukemia (AML), vaccination of mice with leukemia cells transduced with CD154, granulocyte-macrophage colony-stimulating factor (GM-CSF), B7.1, or interleukin 12 (IL12) led to significant protection and generated specific cytotoxic T cells (CTLs) against leukemic cells.17,18 In spite of these effective antileukemic responses, we detected MRD in a fraction of long-term surviving mice. We then decided to investigate how myeloid leukemic cells could persist for months in the host, in spite of an effective antileukemic immune response.
Materials and methods
Mouse AML model
Female C3H/HeJ mice, 8 to 10 weeks old, were obtained from IFFA CREDO (Lyon, France). The establishment of the leukemic DA1-3b BCR/ABL-expressing cell line has been described previously.18,19 Mice injected intraperitoneally with 104 to 106 DA1-3b cells developed systemic lethal leukemia in 3 to 4 weeks, with massive infiltration of bone marrow, spleen, and peripheral blood.
Transfection of DA1-3b cells
Mouse B7-H1 cDNA was kindly provided by Miyuki Azuma.20 The pGT-IL12, pGT-CD154, pGT-Zeo, pSelect-B7.1, pSelect-B7-H1, and pSelect-Zeo expression plasmids were purchased from Invivogen (Tou-louse, France). DA1-3b cells were electroporated at 1500 μF and 250 V and further selected with zeomycin (Invitrogen, Carlsbad, CA). Expressions of CD154, B7-1, and B7-H1 transgenes were analyzed by flow cytometry after incubation with a mouse CD16/CD32 (Fc block) and anti-CD154-phycoerythrin (anti-CD154-PE; clone MR1), anti-B7-1-allophycocyanin (anti-B7-1-APC), or anti-B7-H1-PE monoclonal antibody (BD Pharmingen, San Diego, CA). IL12 expression was assessed by an enzyme-linked immunosorbent assay (ELISA) IL12 kit, OptEIA mouse IL12 (p70; BD Pharmingen). Large batches of selected transduced bulk cultures were frozen until use.
Immunization of mice, quantification of residual disease, and isolation of live persistent leukemic cells
Groups of 30 C3H/Hej mice were injected intradermally with irradiated (100 Gy from a 137C source) CD154-transduced (DA1-3b/CD154) or IL12-transduced (DA1-3b/IL12) cells. DA1-3b cells transfected with the pGT-Zeo empty plasmid (DA1-3b/Zeo) or wild-type DA1-3b cells were used as controls. Cells were diluted in 200 μL of phosphate-buffered saline (PBS) before injection. Each mouse received 3 injections, a week apart, and immunity was challenged 7 days after the last injection by intraperitoneal injection of 104 leukemic DA1-3b cells. Five mice were randomly killed in each group on days 35, 45, 60, 90, and 365 after challenge. All organs were removed and RNA and genomic DNA were extracted. Polymerase chain reaction (PCR) was performed on ABI PRISM 7700 system using TaqMan Master Mix 2 × (Applied Biosystems, Foster City, CA). Data were normalized by levels of 18S ribosomal RNA for cDNA (PDAR 18S; Applied Biosystems) or by levels of the albumin gene for genomic DNA. Primers and probes were designed with the Primer Express software and purchased from Applied Biosystems (Table 1). Targets were quantified by comparison to the expression of BCR/ABL of the DA1-3b cell line.
To isolate persisting leukemic cells, we cultured spleen cells from all mice killed on days 35, 45, 60, 90, 120, and 365 after challenge. Cells (106) were placed in 5 mL medium and were maintained in culture for at least 1 month or until detection of live DA1-3b cells. These leukemic cell lines were phenotyped immediately and then frozen for further analysis.
Flow cytometry analyses of leukemic cells and cell sorting
Monoclonal antibodies specific for the following mouse antigens and corresponding isotype control monoclonal antibodies (mAbs) were used: B7-H1-PE (clone MIH5), TRAIL-PE (tumor necrosis factor [TNF]-related apoptosis-inducing ligand-PE; clone N2B2), PD1-FITC (programmed death 1-fluorescein isothiocyanate; clone J43), B7-H2-PE (clone HK5.3), B7-dendritic cell (Dc)-PE (clone TY25), FAS-L-PE (clone MFL3; Clinisciences, Montrouge, France); B7.1-FITC (clone 1G10), B7.2-PE (clone GL1), CD8-FITC (clone 53-3.1), CD3-PE (clone 17A2), CD4-PE (clone H129.19), rat anti-DX5 (pan natural killer [NK])-FITC (clone DX5), CD28-PE (clone 37.51), CD152-PE (clone UC10-4F10-11), CD44-PE (clone IM7), CD25-FITC (clone 7D4), FAS-PE (clone Jo2), CD45R-PE (clone RA3-6B2), CD11a-PE (clone 2D7), CD40-FITC (clone 3/23), CD19-FITC (clone 1D3), H2Dd-FITC (clone 34-2-12), CD14-FITC (clone rmC5-3), CD122-FITC (clone TM-β1), CD11b-PE (clone M1/70), CD11c-FITC (clone HL3), H2k-FITC (clone AF3-12.1), I-Ad-FITC (clone AMS-32.1), I-Ak-PE (clone 11-5.2; BD Pharmingen). Cells were analyzed on an EPICS XL3-MCL (Beckman Coulter, Hialeah, FL).
To obtain DA1-3b cell lines transfected with B7.1 and/or B7-H1, and with different expression levels of the transgenes, cell subpopulations were purified by cell sorting on an EPICS ALTRA sorter (Beckman Coulter) using the function of mean fluorescent intensity (MFI) expression and either anti-B7.1 and/or anti-B7-H1 mAbs.
Cell cycle analysis
Cell cycle was analyzed by flow cytometry of DNA content. Cells were washed once with ice-cold PBS, resuspended in 100 μL of ice-cold PBS, mixed gently with 900 μL of cold ethanol, incubated on ice or in a -20°C freezer for at least 30 minutes, washed once with PBS, and then resuspended in 500 μL PBS. RNAse (100 μg/mL) was added and cells were incubated at room temperature for 60 minutes. Next, 500 μL of 0.1 mg/mL propidium iodide (PI) was added and cells were incubated at room temperature for 30 minutes, before flow cytometry.
CTL assay
Mice were injected subcutaneously with 106 irradiated DA1-3b/IL12 cells, DA1-3b/CD154 cells, or control DA1-3b/Zeo. Spleens were removed from these mice and from naive mice, and lymphocytes were isolated with the Miltenyi Biotec separation column with an anti-CD90 (THY1.2) mAb according the manufacturer's recommendations (Miltenyi Biotec, Bergisch Gladbach, Germany). CD90+ cells were cocultured with irradiated DA1-3b cells and 20 IU murine IL2/mL (Preprotech, Rocky Hill, NJ) in 5 mL/well in a 6-well tissue culture plate. For the CTL assay, CD8+ cells were isolated with the CD8a+ T-cell isolation kit (Miltenyi Biotec). CTL activity was measured after 15 days of coculture, using the Cytotox 96 nonradioactive assay (Promega, Madison, WI) with cultured cells from vaccinated mice as effectors and DA1-3b cells as targets mixed at various ratios. In certain experiments, CTL results obtained with the Cytotox assay were verified by using a flow cytometry technique (flow-cytometry CTL [FCC] assay), based on the measurement of CTL-induced caspase activation through detection of specific cleavage of the PhiPhiLux fluorogenic substrate (caspase-3 intracellular activity assay kit I; Calbiochem, San Diego, CA).23 Absence of NK cell-mediated cytotoxicity was verified with the YAC-1 cell line. Specificity of CTL lysis against DA1-3b cells was controlled with the EL4 cell line. The immunophenotype of effector cells was determined by flow cytometry using the following mAb antibodies: CD8-FITC (clone 53-3.1), CD3-PE (clone 17A2), CD4-PE (clone H129.19), Pan NK-FITC (clone DX5), CD28-PE (clone 37.51), CD152-PE (clone UC10-4F10-11), CD44-PE (clone IM7), CD25-FITC (clone 7D4), FAS-PE (clone Jo2), CD45R-PE (clone RA3-6B2), CD62L-PE (clone MEL-14; BD Pharmingen); PD1-FITC (clone J43), FAS-L-PE (clone MFL3), CCR7-PE (clone 2H4; Clinisciences). Levels of T-cell cytokines (IL2, IL10, TNF-α, and IFN-γ) were measured in 24-hour supernatants of the T-cell cultures that had been used for cytotoxic activities, by ELISA kits (BD Pharmingen), and by real-time PCR with the appropriate PDAR kit (Applied Biosystems).
For blocking experiments, 0.5 μg/106 cells of a blocking antibody was added to the effectors cells or the targets cells as appropriate, and cells were incubated for 30 minutes at 4°C. The following mAbs and corresponding isotype control mAbs were used24-28 : B7-H1 (clone MIH5), TRAIL (clone N2B2), FAS-L (clone MFL3), PD1 (clone J43; Clinisciences); B7.1 (clone 1G10), CTLA-4 (CD152; clone 9H10), CD28 (clone 37.51), CTLA-4-Ig (BD Pharmingen). To inhibit perforin-mediated cytotoxicity, effector cells were incubated for 2 hours in 100 nM concanamycin A (CMA; Sigma, St Louis, MO).
For activating experiments, 5 μg/106 cells of antibody was added to the effectors cells or the targets cells as appropriate, and cells were incubated for 1 hour at 37°C. The following mAbs and corresponding isotype control mAbs were used24-28 : CTLA-4 (CD152; clone 9H10) and CD28 (clone 37.51; BD Pharmingen).
In vivo blocking of B7-H1 or B7.1
Groups of 10 mice were injected intraperitoneally with 105 live DA1-3b cells, and 2 days later they were injected intraperitoneally with 500 μg of anti-B7-H1 (clone MIH5), anti-B7.1 (clone 1G10), anti-B7.1 plus anti-B7-H1, or anti-CTLA-4 (clone 9H10) mAb, or the isotype control mAb (Rat IgG2a, λ; Clinisciences), according to previously published papers.24-26,29
Statistical analysis
Statistical analysis was performed using the Sigma Stat 3.0 software (SPSS Science, Chicago, IL).
Results
Quantification of residual disease
When C3H/Hej mice were vaccinated with DA1-3b leukemic cells that had been transduced with IL12 or CD154 and then irradiated, they became significantly protected against subsequent challenge with DA1-3b cells and there was significant long-term survival, as previously reported.17,18 Compared with our previous experiments, intradermal rather than subcutaneous injection enhanced the vaccine's effects and led to a more pronounced survival difference between the DA1-3b/IL12 group and the DA1-3b/CD154 group (Figure 1A).17 Thus, this model led to a large number of long-term surviving mice in each group. The difference in efficiency between the DA1-3b/IL12 and DA1-3b/CD154 cell vaccines and the occasional long-term survival of mice vaccinated with DA1-3b cells transduced with empty plasmid (DA1-3b/Zeo) allowed us to study the persistence of minimal residual disease under various antileukemic immune responses.
Quantification of minimal residual leukemic disease in the DA1-3b/C3H mouse model. (A) Survival curves. C3H/Hej mice were vaccinated with irradiated DA1-3b leukemic cells that had been transduced with IL12 (DA1-3b/IL12), CD154 (DA1-3b/CD154), or control empty plasmid (DA1-3b/Zeo), and the mice were subsequently challenged with 104 wild-type DA1-3b cells. (B-D) Number of residual leukemic cells in the spleen of mice treated as described in panel A at selected times after challenge. Horizontal mark represents the median.
Quantification of minimal residual leukemic disease in the DA1-3b/C3H mouse model. (A) Survival curves. C3H/Hej mice were vaccinated with irradiated DA1-3b leukemic cells that had been transduced with IL12 (DA1-3b/IL12), CD154 (DA1-3b/CD154), or control empty plasmid (DA1-3b/Zeo), and the mice were subsequently challenged with 104 wild-type DA1-3b cells. (B-D) Number of residual leukemic cells in the spleen of mice treated as described in panel A at selected times after challenge. Horizontal mark represents the median.
One year after challenge by intraperitoneal injection with 104 wild-type DA1-3b cells, the total number of leukemic cells in spleen varied widely (Figure 1B-D). However, at day 120, the median numbers of cells were only 470 (range, 3-2700), 401 (range, 5-7700), and 130 (range, 78-177) in the DA1-3b/Zeo, DA1-3b/CD154, and DA1-3b/IL12 groups, respectively. After 1 year of follow-up, all surviving mice were killed. MRD was detected in all groups but was less common in mice vaccinated with DA1-3b/IL12 (χ2, P = .001; Table 2). Persistent leukemic cells represented 0.001% to 1% of spleen cells but most animals had less than 0.02%. MRD was also detected in the liver but at lower levels (Table 3). All other organs appeared free of MRD. Levels of BCR/ABL mRNA (quantified by real-time PCR) were correlated with the number of DA1-3b cells (evaluated by real-time PCR performed on DNA; data not shown).
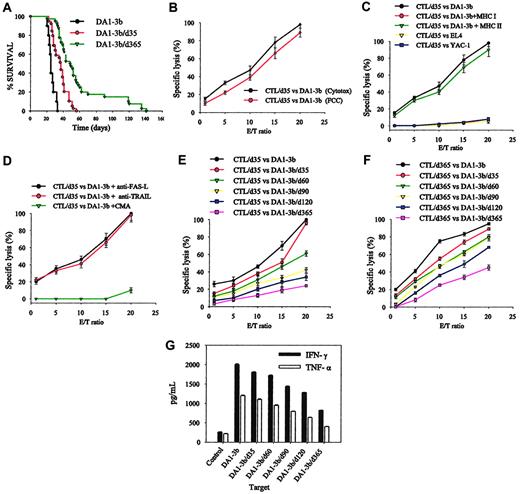
To analyze the characteristics of persisting leukemic cells, we cultured 106 spleen cells per mouse and after 2 or 3 weeks obtained cell lines from mice killed at day 35 (cell line DA1-3b/d35), day 60 (DA1-3b/d60), day 90 (DA1-3b/d90), day 120 (DA1-3b/d120), and day 365 (DA1-3b/d365). Specifically, we were able to obtain 9 cell lines from mice alive at 1 year (Table 2). These MRD-derived cells were morphologically indistinguishable from the original wild-type DA1-3b cells and they expressed BCR/ABL mRNA at the same level. When 105 persisting MRD-derived cells were injected intraperitoneally into naive hosts, they were still able to kill them. DA1-3b/d365 cells were slightly but significantly (P = .001, log-rank test) less tumorigenic than DA1-3b/d35 cells and DA1-3b cells (Figure 2A), probably due to slight differences in percentage of cells in S phase (Table 4).
Tumorigenicity of persistent leukemic cells and their sensitivity to CTL-mediated killing. (A) Survival curves of naive mice injected intraperitoneally with the leukemic cell line (DA1-3B) or with leukemic cells that had persisted in other animals for 1 month (DA1-3b/d35) or 1 year (DA1-3b/d365). (B) Sensitivity of DA1-3b cells to CTL-mediated killing measured by lactate dehydrogenase (LDH) release (Cytotox) or by detecting caspase activation (FCC) using flow cytometry. E/T indicates effector-target ratio. (C) Sensitivity to CTL-mediated killing of DA1-3b cells or control YAC-1 or EL4 cells and inhibition of lysis with anti-MHC I or II. (D) CTL-mediated killing of DA1-3b cells treated with CMA, anti-FAS-L, or anti-TRAIL. (E) CTLs were isolated 1 month after challenge from mice vaccinated with irradiated DA1-3b/IL12 cells and tested for their ability to kill leukemic cells that had persisted in other animals for 1 month (DA1-3b/d35), 2 months (DA1-3b/d60), 3 months (DA1-3b/d90), 4 months (DA1-3b/d120), and 1 year (DA1-3b/d365). (F) The procedure described for panel E was followed except that the CTLs were isolated from mice 12 months after challenge (CTL/d365). (G) CTLs were isolated from mice 1 month after challenge, incubated at a 20:1 ratio with leukemic cells that persisted in other animals for up to 1 year, and production of IFN-γ and TNF-α was measured. All experiments were performed in quadruplicate and repeated at least 3 times. Error bars indicate standard deviation.
Tumorigenicity of persistent leukemic cells and their sensitivity to CTL-mediated killing. (A) Survival curves of naive mice injected intraperitoneally with the leukemic cell line (DA1-3B) or with leukemic cells that had persisted in other animals for 1 month (DA1-3b/d35) or 1 year (DA1-3b/d365). (B) Sensitivity of DA1-3b cells to CTL-mediated killing measured by lactate dehydrogenase (LDH) release (Cytotox) or by detecting caspase activation (FCC) using flow cytometry. E/T indicates effector-target ratio. (C) Sensitivity to CTL-mediated killing of DA1-3b cells or control YAC-1 or EL4 cells and inhibition of lysis with anti-MHC I or II. (D) CTL-mediated killing of DA1-3b cells treated with CMA, anti-FAS-L, or anti-TRAIL. (E) CTLs were isolated 1 month after challenge from mice vaccinated with irradiated DA1-3b/IL12 cells and tested for their ability to kill leukemic cells that had persisted in other animals for 1 month (DA1-3b/d35), 2 months (DA1-3b/d60), 3 months (DA1-3b/d90), 4 months (DA1-3b/d120), and 1 year (DA1-3b/d365). (F) The procedure described for panel E was followed except that the CTLs were isolated from mice 12 months after challenge (CTL/d365). (G) CTLs were isolated from mice 1 month after challenge, incubated at a 20:1 ratio with leukemic cells that persisted in other animals for up to 1 year, and production of IFN-γ and TNF-α was measured. All experiments were performed in quadruplicate and repeated at least 3 times. Error bars indicate standard deviation.
As MRD leukemic cells persisted in the host in spite of an efficient antileukemic immune response, we investigated whether they had become less sensitive to CTL-mediated lysis. DA1-3b/IL12, DA1-3b/CD154, and DA1-3b/Zeo vaccinated mice were all able to generate CD8+ major histocompatibility complex class I (MHC I)-restricted CTL with specific activity against DA1-3b cells. CTLs obtained from DA1-3b/IL12 vaccinated mice were more efficient, showed minimal variability between experiments, and therefore were used for all further experiments (Figure 2B-C). As lysis of target cells was nearly 100%, results obtained with the Cytotox assay were compared with those obtained by a second assay, the FCC test (Figure 2B). Both methods yielded similar results. Specific CTLs were also detected in vaccinated mice that had been in complete remission for 1 year (Figure 2F). The CTLs were positive for CD3, CD8, CD45R, CD62L, CCR7, FAS-L, FAS, CD44, and CD25, suggesting a central memory T-cell phenotype (data not shown). Pan-NK staining was not detected. The cells also expressed costimulatory receptor CD28, CTLA-4, and PD1. Addition to the CTLs of CMA, but not blocking antibody against FAS-L and TRAIL, almost totally inhibited CTL-mediated killing, suggesting that the cytotoxic effect was mostly mediated by the perforin/granzyme pathway (Figure 2D). The sensitivity of MRD-derived cells to CTL-mediated killing declined with the length of time that the MRD-derived cells had persisted in the host (Figure 2E). This decrease was observed when CTLs were isolated at day 35 (CTL/d35; Figure 2E) or at 1 year (CTL/d365; Figure 2F). Production of IFN-γ and TNF-α by CTLs also significantly decreased when target cells were MRD-derived cells (Figure 2G).
Immunophenotypic analyses of MRD leukemic-derived cells
Flow cytometry with a large panel of antigens, including MHC I and II molecules, showed that the MRD-derived cells isolated at days 35, 45, 60, 90, 120, and 365 had the same phenotype as wild-type DA1-3b cells (data not shown). Several molecules involved in costimulation of T cells or regulation of T-cell activity (FAS-L, CD86, TRAIL, B7-H2, and B7-DC) were not expressed in DA1-3b or MRD-derived cells. However, MRD-derived cells showed enhanced expression of B7-H1 and to a lesser degree B7.1, and enhancement appeared proportional to time that the MRD had persisted in the host (Figure 3A). These findings were also observed at the level of transcription (Figure 3B-C).
Quantification of B7.1 and B7-H1 in persistent leukemic cells. (A) Time course of B7.1 and B7H-1 expression by persistent leukemic cells measured by flow cytometry. Thin lines indicate isotype control. (B-C) Real-time PCR quantification of (B) B7.1 mRNA and (C) B7-H1 mRNA in persistent leukemic cells taken from naive mice or mice that had been vaccinated with DA1-3b cells transduced with various plasmids. Error bars indicate standard deviation.
Quantification of B7.1 and B7-H1 in persistent leukemic cells. (A) Time course of B7.1 and B7H-1 expression by persistent leukemic cells measured by flow cytometry. Thin lines indicate isotype control. (B-C) Real-time PCR quantification of (B) B7.1 mRNA and (C) B7-H1 mRNA in persistent leukemic cells taken from naive mice or mice that had been vaccinated with DA1-3b cells transduced with various plasmids. Error bars indicate standard deviation.
Blocking experiments
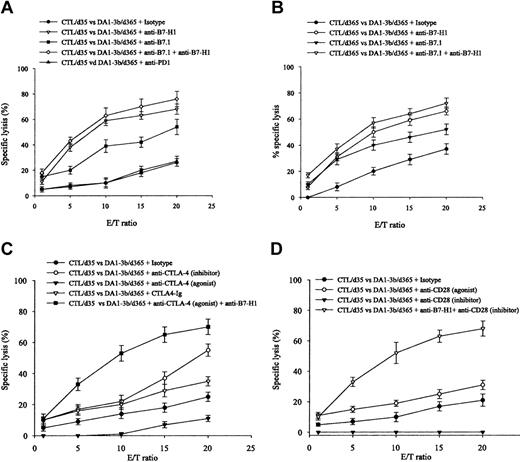
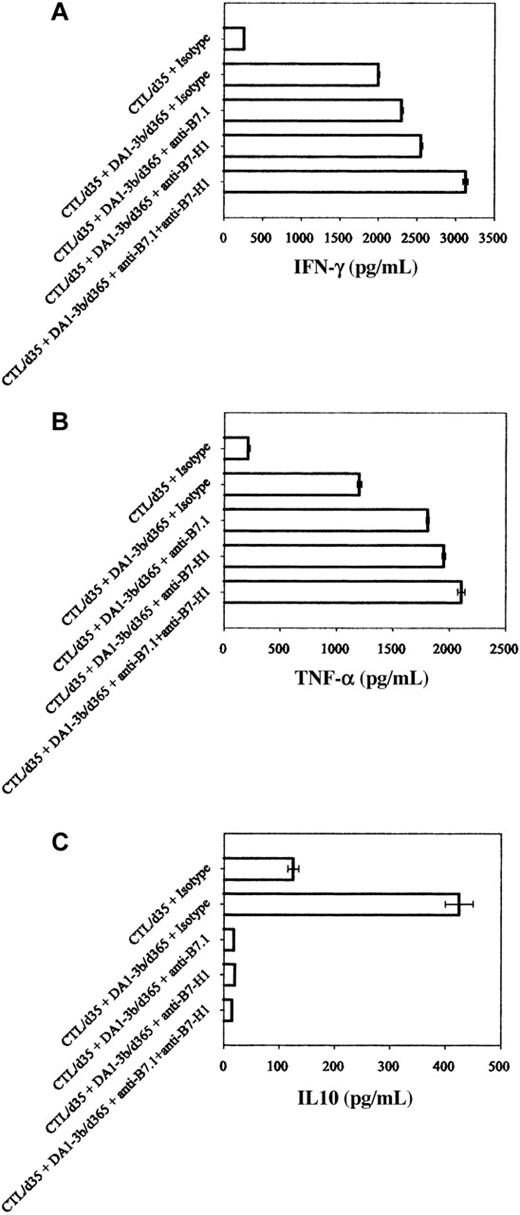
To analyze the effect of enhanced B7.1 and B7-H1 expression, we performed CTL experiments with selective blocking of B7.1 and B7-H1 costimulatory pathways. Blocking enhanced CTL-mediated killing of DA1-3b/d365 cells (Figure 4A). These results were observed with effectors isolated from mice at 1 month (CTL/d35; Figure 4A) or 1 year (CTL/d365; Figure 4B). Blocking B7.1 using CTLA-4-Ig induced a similar effect to inhibition of CTLA-4 but blocking B7-H1 reversed the effect of stimulation of CTLA-4 (Figure 4C). Inhibition of CD28 reduced CTL-mediated killing (Figure 4D). However, simultaneous blocking of B7-H1 and CD28 enhanced CTL-mediated killing. Blocking PD1, currently the only known receptor of B7-H1, did not enhance killing (Figure 4A).30 Cytokine profiles from the supernatant showed that blocking B7-H1 and B7.1 enhanced IFN-γ and TNF-α expression and inhibited IL10 expression (Figure 5). Similar results were observed when cytokine mRNAs were quantified by real-time PCR (data not shown).
CTL-mediated killing of DA1-3b/d365 persistent leukemic cells and in vitro blocking experiments. (A) Incubation of CTL/d35 and DA1-3b/d365 cells with blocking antibodies against B7-H1, B7.1, B7-H1+B7.1, PD1, or control isotype. (B) Incubation of CTL/d365 and DA1-3b/d365 cells with the same antibodies. (C) Incubation of CTL/d35 and DA1-3b/d365 cells with blocking or activating antibodies against CTLA-4, soluble CTLA-4-Ig, anti-B7-H1 plus activating antibodies against CTLA-4, or control isotype. (D) Incubation of CTL/d35 and DA1-3b/d365 cells with blocking or activating antibodies against CD28, anti-B7-H1 plus blocking antibody against CD28, or with a control isotype. All experiments were performed in quadruplicate and repeated at least 3 times. Error bars indicate standard deviation.
CTL-mediated killing of DA1-3b/d365 persistent leukemic cells and in vitro blocking experiments. (A) Incubation of CTL/d35 and DA1-3b/d365 cells with blocking antibodies against B7-H1, B7.1, B7-H1+B7.1, PD1, or control isotype. (B) Incubation of CTL/d365 and DA1-3b/d365 cells with the same antibodies. (C) Incubation of CTL/d35 and DA1-3b/d365 cells with blocking or activating antibodies against CTLA-4, soluble CTLA-4-Ig, anti-B7-H1 plus activating antibodies against CTLA-4, or control isotype. (D) Incubation of CTL/d35 and DA1-3b/d365 cells with blocking or activating antibodies against CD28, anti-B7-H1 plus blocking antibody against CD28, or with a control isotype. All experiments were performed in quadruplicate and repeated at least 3 times. Error bars indicate standard deviation.
Cytokines produced by CTL in presence of DA1-3b/d365 cells. CTLs were obtained from mice on day 35 after inoculation; DA1-3b/d365 cells were obtained from mice on day 365 after inoculation. Quantification by ELISA of (A) IFN-γ, (B) TNF-α, and (C) IL10, with blocking antibodies against B7-H1 or B7.1. Error bars indicate standard deviation.
Cytokines produced by CTL in presence of DA1-3b/d365 cells. CTLs were obtained from mice on day 35 after inoculation; DA1-3b/d365 cells were obtained from mice on day 365 after inoculation. Quantification by ELISA of (A) IFN-γ, (B) TNF-α, and (C) IL10, with blocking antibodies against B7-H1 or B7.1. Error bars indicate standard deviation.
Effects of B7-H1 and B7.1 transfectants with different expression levels on different CD8+ T-cell subsets
We transfected DA1-3b cells with B7-H1 (DA1-3b/B7-H1), B7.1 (DA1-3b/B7.1), or both genes (DA1-3b/B7-H1+B7.1). Stable transfectants were sorted into 4 different expression levels (termed Very low, Low, High, or Very high) of each gene and combination of genes. CTL experiments were performed using as targets DA1-3b/B7.1 cells that did not express B7-H1, and these showed a dose-dependent inhibitory effect of B7.1 on CTL-mediated lysis (Figure 6A). This inhibition appeared when expression of B7.1 rose above a threshold. Similar experiments were performed using as targets DA1-3b/B7-H1 cells that did not expressed B7.1, and these also showed a dose-dependent inhibitory effect of B7-H1 on CTL-mediated lysis and a threshold of B7-H1 expression (Figure 6B). All inhibitory effects were at least partially reversible with blocking antibodies. CTL experiments performed with double transfectants DA1-3b/B7-H1+B7.1 showed that the B7-H1's inhibitory effect was more pronounced for low expression of B7.1 (Figure 6C). Dose-dependent effects were also seen with DA1-3b/d365 cells sorted for different expression levels of B7-H1 and B7.1 (data not shown).
CTL-mediated killing of DA1-3b cells transfected with B7.1 and/or B7-H1. (A) Sensitivity to CTL-mediated killing of DA1-3b cells transfected with B7.1 and cell sorted for very low (VL), low (L), high (H), or very high (VH) expression. (B) Sensitivity of DA1-3b cells transfected with B7-H1 and cell sorted as described above. (C) Sensitivity of DA1-3b cells transfected with both B7.1 and B7-H1 and cell sorted for very low, low, high, or very high expression level of B7.1, B7-H1, or both genes. (D) Cytotoxic activity against DA1-3b, DA1-3b/d35, and DA1/d365 cells of naive (CD8+ CD62L- CD45R- CD44- CD69-), effector (CD8+ CD62L- CD45R- CD44+ CD69+), effector memory (CD8+ CD62Llow CCR7- CD45R+ CD44+ CD69+), and central memory (CD8+ CD62Lhigh CCR7+ CD45R+ CD44+ CD69+) T cells with or without blocking antibodies against B7-H1 and/or B7.1. All experiments were performed at 1:1, 5:1, 10:1, 15:1, and 20:1 E/T ratios; only results obtained at 20:1 E/T ratio are presented. (E) The procedure described for panel D was followed but with DA1-3b cells transfected with both B7.1 and B7-H1 as targets. (F) Quantification by ELISA of IL2 produced by CD8 T-cell subsets in presence of DA1-3b, DA1-3b/d35, DA1-3b/d365, and DA1-3b cells transfected with both B7.1 and B7-H1. Error bars indicate standard deviation.
CTL-mediated killing of DA1-3b cells transfected with B7.1 and/or B7-H1. (A) Sensitivity to CTL-mediated killing of DA1-3b cells transfected with B7.1 and cell sorted for very low (VL), low (L), high (H), or very high (VH) expression. (B) Sensitivity of DA1-3b cells transfected with B7-H1 and cell sorted as described above. (C) Sensitivity of DA1-3b cells transfected with both B7.1 and B7-H1 and cell sorted for very low, low, high, or very high expression level of B7.1, B7-H1, or both genes. (D) Cytotoxic activity against DA1-3b, DA1-3b/d35, and DA1/d365 cells of naive (CD8+ CD62L- CD45R- CD44- CD69-), effector (CD8+ CD62L- CD45R- CD44+ CD69+), effector memory (CD8+ CD62Llow CCR7- CD45R+ CD44+ CD69+), and central memory (CD8+ CD62Lhigh CCR7+ CD45R+ CD44+ CD69+) T cells with or without blocking antibodies against B7-H1 and/or B7.1. All experiments were performed at 1:1, 5:1, 10:1, 15:1, and 20:1 E/T ratios; only results obtained at 20:1 E/T ratio are presented. (E) The procedure described for panel D was followed but with DA1-3b cells transfected with both B7.1 and B7-H1 as targets. (F) Quantification by ELISA of IL2 produced by CD8 T-cell subsets in presence of DA1-3b, DA1-3b/d35, DA1-3b/d365, and DA1-3b cells transfected with both B7.1 and B7-H1. Error bars indicate standard deviation.
As the CTL experiments in Figures 2B-G, 4, and 6A-C were performed with CD8+ T cells showing a central memory phenotype, we investigated if other subsets might have a different cytotoxic activity toward MRD-derived cells. We sorted from vaccinated mice naive T cells (CD8+ CD62L- CD45R- CD44- CD69-), effector T cells (CD8+ CD62L- CD45R- CD44+ CD69+), effector memory T cells (CD8+ CD62Llow CCR7- CD45R+ CD44+ CD69+), and central memory T cells (CD8+ CD62Lhigh CCR7+ CD45R+ CD44+ CD69+).21,22 MRD-derived cells showed decreased sensitivity to CTL-mediated killing with all these subsets (Figure 6D). However, both effector memory and central memory CD8+ T cells were the most effective at killing DA1-3b and MRD-derived cells. In all subsets, blocking B7-H1 and/or B7.1 inhibited CTL-mediated killing. Same results were obtained with DA1-3b cells transfected with B7-H1 and/or B7.1 (Figure 6E). Production of IFN-γ and TNF-α decreased in all subsets of CD8 T cells when target cells were MRD-derived cells or DA1-3b cells transfected with B7-H1 and/or B7.1 (data not shown). Production of IL2 also decreased in all subsets of CD8 T cells when target cells were MRD-derived cells (Figure 6F). However, DA1-3b cells transfected with B7-H1 and/or B7.1 did not affect IL2 production by central memory CD8 T cells.
In vivo blocking of B7-H1 and B7.1
We injected anti-B7-H1, anti-B7.1, or isotype control mAbs into naive mice and then challenged them with 105 DA1-3b/d365 cells. Mice injected with anti-B7-H1, anti-B7.1, or anti-CTLA-4 survived for longer than control-treated mice (P < 10-3, P < 10-4, and P < 10-4, respectively, log-rank test; Figure 7). Blocking both B7-H1 and B7.1 resulted in an additive effect.
Effect of in vivo blocking of B7-H1 and B7.1 on overall survival. (A) Survival curve of naive mice (10/group) injected intraperitoneally with 105 DA1-3b/d365 cells with either blocking antibody against B7-H1 or a control isotype. (B) Survival curve of naive mice injected intraperitoneally with 105 DA1-3b/d365 cells with either blocking antibody against B7.1 or a control isotype. (C) Survival of naive mice injected intraperitoneally with 105 DA1-3b/d365 cells with either blocking antibody against CTLA-4 or a control isotype. (D) Survival of naive mice injected intraperitoneally with 105 DA1-3b/d365 cells with either blocking antibodies against B7-H1 and B7.1 or a control isotype.
Effect of in vivo blocking of B7-H1 and B7.1 on overall survival. (A) Survival curve of naive mice (10/group) injected intraperitoneally with 105 DA1-3b/d365 cells with either blocking antibody against B7-H1 or a control isotype. (B) Survival curve of naive mice injected intraperitoneally with 105 DA1-3b/d365 cells with either blocking antibody against B7.1 or a control isotype. (C) Survival of naive mice injected intraperitoneally with 105 DA1-3b/d365 cells with either blocking antibody against CTLA-4 or a control isotype. (D) Survival of naive mice injected intraperitoneally with 105 DA1-3b/d365 cells with either blocking antibodies against B7-H1 and B7.1 or a control isotype.
Discussion
We observed in this report that in mice vaccinated with leukemic cells transduced with IL12, CD154, or control empty plasmid and subsequently challenged with live leukemic cells, MRD can persist for up to 1 year. Although the number of persistent cells varied greatly, most animals that were MRD+ after 1 year had fewer than 2000 leukemic cells in the spleen, a few hundred in the liver, and none in other organs. Even after only 4 months survival, the tumor mass was very small. Thus, it seems likely that MRD remains stable for months in the host.
Long-term persistence of tumor cells has been reported in other mouse tumor models but most of those were lymphomas or solid tumors.1,31-34 The very low number of dormant leukemic cells found in our experiments contrasts with the high numbers reported in the BCL1 lymphoma model, where tumor dormancy was induced by vaccination against the tumor's Ig idiotype.7 In the L5178 lymphoma model, dormant cells persisted in the bone marrow of mice that had been vaccinated with irradiated or live tumor cells, and after 3 months they constituted 0.001% to 0.01% of bone marrow cells.34 These findings suggest there is a balance between the antileukemic immune response and MRD growth. The large variation in the number of persistent dormant cells among tumor models might be related to different mechanisms of tumor dormancy. Indeed, in the BCL1 lymphoma model, the anti-Id immune response was responsible for partial cell cycle arrest and apoptosis of dormant cells.6,11
The percentage of mice with MRD was significantly lower in the group vaccinated with IL12-transduced leukemic cells than in mice vaccinated with DA1-3b/CD154 or with DA1-3b/Zeo (controls). However, in all 3 groups, the mice with MRD had similar numbers of leukemic cells. Thus, it seems likely that the early immune rejection of myeloid leukemic cells and later establishment of a tumor dormant state are distinct events. When injected into naive hosts, all MRD-derived cell lines were still able to kill the animals, with a modest decrease of tumorigenicity related to a slightly lower percentage of cells in S phase. These findings indicate that persistence is an active phenomenon resulting from an interaction between tumor cells and the host.
CD8a+ CTLs able to kill specifically DA1-3b cells were also isolated. These CTLs killed with the same efficiency whether isolated 1 month or 1 year after vaccination. However, the sensitivity of MRD-derived cells to lysis and their ability to stimulate CTLs to secrete IFN-γ and TNF-α was inversely proportional to time spent in the host.
We also analyzed, on MRD-derived cells taken from hosts after various times of persistence, expression of molecules known to modulate T-cell activation. B7-H1 and B7.1 expression increased proportionally with the time the MRD cells had persisted in the host. B7-H1 (also known as PD-L1) is a B7 family member and ligand for PD1, a member of the CD28 family.35,36 B7-H1 can interact with receptors on CTLs and promote CTL death, and thus increased expression of B7-H1 might protect MRD from elimination. However, other mechanisms are probably involved in T-cell inhibition by B7-H1. In a model of antitumor immunity, T-cell apoptosis did not seem to be involved in B7-H1-mediated inhibition of T cells.37 Abundant expression of B7-H1 has been reported in human carcinomas.25,29,35,37,38 In mice, 2 independent reports have shown that growth of the P815 tumor cell line was significantly enhanced when it had been transfected with B7-H1.29,39 Similar results were also observed when mice were injected with myeloma cell lines naturally expressing high levels of B7-H1.39 Thus, B7-H1 overexpression appears as a possible mechanism for tumors to escape from a host immune response. In our experiments, the leukemic cells injected into the host expressed B7-H1 weakly, but MRD cells from vaccinated mice expressed B7-H1 more strongly, suggesting that B7-H1 might be an important molecule for tumor dormancy. Blocking B7-H1 in vitro increased CTL-mediated killing of MRD-derived cells and enhanced the production of IFN-γ and TNF-α by effector T cells. Production of IL10 decreased after B7-H1 blockade. Enhanced IFN-γ and decreased IL10 production have also been reported after B7-H1 blockade in myeloid dendritic cells.37,40 CTL experiments performed with DA1-3b leukemic cells transfected with B7-H1 and sorted for different expression levels showed that the inhibitory effect of B7-H1 was dose dependent. These data indicate that the progressively increasing B7-H1 expression observed in dormant tumor cells might increasingly inhibit CTLs in vivo. We also observed that blocking PD1, the receptor of B7-H1, had no effect on CTLs. Several studies have provided evidence for a second receptor for B7-H1, which might mediate the inhibitory effect on effector T cells.29,41 However, this receptor remains uncharacterized. Blocking B7-H1 in vivo prolonged the survival of mice injected with MRD-derived cells. Thus, if B7-H1 can help tumor cells escape the host immune system, at least in part via inhibition of CTL-mediated killing, it could contribute to establishing tumor dormancy.
The first report that described B7-H1 inhibition of CTL activity indicated that B7-H1 was also effective when tumor cells expressed B7.1 (CD80).29 In our experiments on MRD-derived cells, B7.1 expression increased progressively with the length of time the MRD had persisted in the host, only when the host had an antileukemic immune response. In vitro blocking of B7.1 enhanced CD8+ CTL-mediated killing of MRD-derived cells and production of IFN-γ and TNF-α. This effect was mediated via CTLA-4 (CD152), as blocking CTLA-4 on effector T cells enhanced lysis of MRD-derived cells, whereas blocking of CD28 did not. Results obtained with MRD-derived cells were confirmed with DA1-3b leukemic cells transfected with B7.1 and sorted for different levels of B7.1 expression. We observed a dose-dependent CTL inhibitory effect of B7.1. Thus, results obtained with B7.1 resemble those obtained with B7-H1. The costimulatory functions of B7.1 have been extensively investigated in studies of proliferation or cytokine production by naive CD4+ T lymphocytes during T-cell priming by antigen-presenting cells. Recent reports suggest that B7.1 can also induce peripheral tolerance, depending on its basal level of expression on antigen-presenting cells.42 Less is known about the role of B7.1/CD28/CTLA-4 interactions on CD8+ effector T cells. In human CD8+ CTLs, blocking CTLA-4 or B7.1 increases lysis of targets.43 In a model of graft-versus-host disease, blocking B7.1 in vivo potentiated CD8+ T-cell activation and CTL function.44 In several tumor models, blocking CTLA-4 in vivo led to suppression or rejection.45 We observed in our experiments that blocking B7.1 or CTLA-4 in vivo enhanced the survival of mice injected with MRD-derived cells. Thus, B7.1 might contribute to the long-term persistence of leukemic cells by inhibiting CD8+ CTL-mediated killing.
Both B7-H1 and B7.1 were overexpressed in MRD-derived cells. To investigate the relative role of each molecule in CD8+ CTL inhibition, we transfected the DA1-3b cell line with B7-H1, B7.1, or both genes and sorted cell populations into subsets with different expression levels of each gene. As mentioned earlier in this section, cells expressing only B7.1 or only B7-H1 showed a dose-dependent inhibitory effect. These results suggest that B7.1-mediated inhibition of CD8+ CTLs does not require B7-H1 coexpression. Analysis of leukemic cells cotransfected with B7.1 and B7-H1 showed that inhibition was maximal when B7.1 and B7-H1 were coexpressed at the highest level. However, in cells with both transgenes, the B7-H1 contribution was more pronounced for lower expression of B7.1. Thus, in this model, B7-H1 and B7.1 might act independently to inhibit CTL activity against tumor dormant cells, but the overall effect is likely to result from the relative expression of B7-H1 and B7.1. Dong et al29 reported that B7-H1 transfection in the P815/B7.1+ immunogenic tumor cell line promoted tumor growth in vivo but had no effect on P815 cells that did not express B7.1. However, Iwai et al39 reported, in the same P815 cell line, that transfection with only B7-H1 inhibited CTL-specific activity and enhanced tumor growth and invasiveness in vivo via interaction with the PD1 receptor. Our data indicate that the B7-H1 inhibitory effect is not mediated via the PD1 receptor on CTLs and that the B7.1 effect is mediated via CTLA-4. These variations among tumor models are difficult to explain. Recent reports in nontumoral pathogenic models have reported an activating role for B7-H1. In a mouse model of diabetes, B7-H1 promotes autoimmune T-cell responses.46 In a model of colitis, blocking B7-H1 suppressed chronic intestinal inflammation.26 Thus, B7-H1 appears to have a dual activity. Inhibition has been reported mainly in tumor models, whereas activation of T cell has been reported in autoimmune disease models. CTL activity is regulated by various mechanisms, and lysis or protection of the target cell results from a subtle balance between inhibiting and activating signals. Tumor cells might deliver several types of inhibitory signals to CD8+ T cells, and integration of signals might favor an inhibitory response toward costimulatory molecules not necessarily observed under physiologic conditions. This hypothesis is probably more relevant in our model, where overexpression of B7-H1 and B7.1 was observed in dormant tumor cells that had survived months of exposure to the host's immune antileukemic response. Another hypothesis could be that different CD8+ T cell subsets might have different sensitivities toward B7-H1 and B7.1. In our model, both central memory and effector memory CD8+ T cells mediated the highest cytotoxic activity against leukemic cells. All CD8+ T-cell subsets showed decreased cytotoxic activity against MRD-derived cells, and this effect was mediated at least partially by B7-H1 and B7.1. However, IL2 production by central memory T cells was reduced by MRD-derived cells but not by B7-H1 and B7.1 transfectants. These data suggest that dormant tumor cells might induce in these central memory cells additional immunosuppressive effects than lysis inhibition, which are likely to be mediated by other pathways than B7.1 and B7-H1.
We observed that blocking B7-H1, B7.1, or CTLA-4 in vivo improved overall survival. Although our in vitro experiments demonstrate that B7.1 and B7-H1 can inhibit CD8+ T cells, injection of blocking antibodies in mice perhaps inhibited other cell types as well. CD4+ T cells play a major role in tolerance toward tumor cells.47,48 Stimulation of CD4+ T cells by B7-H1 promotes a T-helper 2 (Th2)-biased response.49,50 Whether dormant tumor cells might stimulate CD4+ T cells via B7-H1 remains to be investigated. Antigen-presenting cells can also express B7-H1 and Curiel et al37 have shown that in an ovarian cancer, myeloid dendritic cells from lymph nodes draining the tumor overexpressed B7-H1. Thus, dendritic cells might also participate in tumor dormancy in our animal model.
Long-term persistence of tumor cells in patients is a major reason why anticancer treatment fails. In myeloid malignancies, the persistence of MRD, in spite of intensive chemotherapy, limits progress. In a mouse model, we observed long-term persistence of a few myeloid leukemic cells, which overexpressed costimulatory molecules that may protect them from the host's immune response. These results have several implications. New treatments could include soluble inhibitors to block the molecular mechanisms involved in leukemia persistence. In a model system, B7-H1 blocking improves T-cell immunotherapy of squamous cell carcinoma.25 Finally, as leukemia cells persisted in our model in spite of an active immune response induced by a vaccine, leukemic cells might also persist in patients treated by new vaccine strategies. Thus, effective immunotherapy would require a combined strategy design to reject leukemic cells and to overcome mechanisms of tumor dormancy.
Prepublished online as Blood First Edition Paper, June 10, 2004; DOI 10.1182/blood-2004-01-0064.
Supported by the Ligue Contre le Cancer (Comité du Nord and Comité du Pas de Calais), the Association de Recherche sur le Cancer, the Groupement des Entreprises Francaises Dans la Lutte Contre le Cancer (GEFLUC), the Fondation contre la Leucémie, the Institut de Recherche sur le Cancer de Lille, and the CHU of Lille, France. A.S. is a recipient of a grant from the Region Nord-Pas-de-Calais/CHU de Lille.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Philippe Marchetti and Thierry Idziorek for precious help, and Miyuki Azuma for providing the B7-H1 cDNA.