Comment on Ma et al, page 2549
Ma and colleagues describe an intermediate state of integrin activation induced by P-selectin binding to PSGL-1 on neutrophils, which may be integral to how endothelial cell and platelet P-selectin (as well as soluble P-selectin shed from them) activate leukocytes.
P-selectin, a member of the selectin family, is localized in the membranes of platelet alpha granules, as well as endothelial cell Weibel-Palade bodies, and translocates to the cell surface when these cells are activated.1 P-selectin can be shed from activated cells and circulates as soluble P-selectin in the plasma. The prominent role of cellular P-selectin in leukocyte rolling and extravasation, as well as platelet-leukocyte interactions, is well established.2 However, recent studies show that high levels of soluble P-selectin in blood result in a procoagulant state3 and that P-selectin immunoglobulin (Ig) chimeras generate tissue factor–positive microparticles that correct hemostasis in a mouse model of hemophilia A.4 This clearly indicates that P-selectin is more than just an adhesion molecule. A principal ligand for P-selectin is P-selectin glycoprotein ligand 1 (PSGL-1), which is expressed on a variety of leukocytes.5 Recent studies suggest that P-selectin binding to PSGL-1 promotes responses typically dependent on integrin activation, for example, tyrosine phosphorylation and activation of mitogen-activated protein (MAP) kinases. Nevertheless, whether PSGL-1 ligation by P-selectin is sufficient to activate integrins on leukocytes is a question that remains controversial.
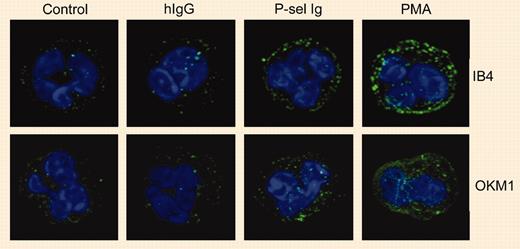
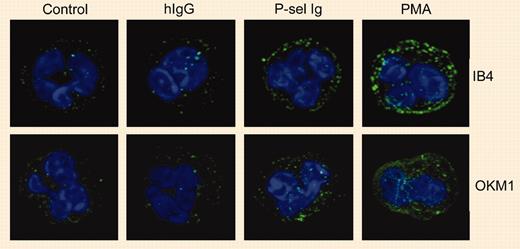
By studying the effects of P-selectin Ig chimeras on the activation and expression of αMβ2 integrin on human neutrophils, Ma and colleagues found that PSGL-1 ligation resulted in an intermediate state of cell activation. The characteristics of this intermediate state of neutrophil activation were moderate clustering of integrin αMβ2, no increase in the binding of antibodies specific for the activation epitope on the αM subunit, and no increase in the surface expression of αMβ2. On the other hand, full activation (for example, as demonstrated by phorbol myristate acetate [PMA]) induces extensive changes in all of these parameters. P-selectin Ig chimeras definitely perform induction of integrin activation, but this induction results in an intermediate state of integrin activation rather than fully activated integrins. This is clearly demonstrated by findings that show that P-selectin Ig-stimulated neutrophils readily bind to immobilized fibrinogen but do not bind soluble fibrinogen.FIG1
P-selectin triggers αMβ2 clustering. See the complete figure in the article beginning on page 2549.
P-selectin triggers αMβ2 clustering. See the complete figure in the article beginning on page 2549.
These discoveries by Ma and colleagues further fortify the notion that binding of P-selectin to PSGL-1 on a leukocyte induces activation signals in the leukocyte. Nevertheless, the molecular details of this signaling remain unknown. Another important question remaining is which other extracellular stimuli are able to act cooperatively with the signaling induced by P-selectin/PSGL-1. Platelet-activating factor (PAF) and interleukin 8 (IL-8) are probably important to achieve full activation of integrins required for firm adhesion, as suggested here by the results of Ma et al and by others. It will also be important to establish what kind of stimuli can act cooperatively with P-selectin to mediate leukocyte granule exocytosis and increased microvesiculation. ▪