Abstract
BLyS, recently shown to be critical for survival of normal B cells, has been found to be elevated in a number of immune disease models. A role for BLyS in the survival of malignant B cells has also been revealed and we therefore sought to identify a role for BLyS and its receptors in non-Hodgkin lymphoma (NHL). We found that tumor cells from all NHL histologic subtypes expressed one or more of 3 known receptors (BCMA, TACI, and BAFF-R) for BLyS; however, the pattern of expression was variable. We provide evidence that BLyS is expressed in tumors from patients with NHL and that BLyS levels increase as tumors transform to a more aggressive phenotype. Additionally, we provide evidence that serum BLyS levels are elevated in a subgroup of patients with NHL. In patients with de novo large B-cell lymphoma, a high BLyS level correlated with a poorer median overall survival, the presence of constitutional symptoms, and elevated values of lactic dehydrogenase. When BLyS levels were correlated with response to therapy in all patients, responding patients had a significantly lower BLyS level than those with progressive disease. In summary, we found that BLyS and its receptors represent a potentially important therapeutic target in B-cell lymphoma.
Introduction
B-cell non-Hodgkin lymphoma (NHL) is a serious and frequently fatal illness. Some of the genetic changes that lead to lymphoma have been discovered, but many of the other contributing mechanistic details underlying transformation events are not yet known. It is clear, however, that dysregulation of the balance between cell survival and programmed cell death is a central feature. In this regard, members of the tumor necrosis factor (TNF) superfamily have been shown to be key mediators in the formation and regulation of normal B-cell responses.1 BLyS, a TNF family member expressed by monocytes, macrophages, and dendritic cells, is critical for maintenance of normal B-cell development and homeostasis.2 BLyS shares significant homology with a proliferation-inducing ligand (APRIL), which stimulates tumor cell growth as well as proliferation of primary lymphocytes.3 Initial studies on the effects of BLyS on B-cell physiology suggested that it costimulated B-cell proliferation and immunoglobulin secretion.4 Subsequently, a role for BLyS in controlling cell survival was proposed because B cells isolated from BLyS transgenic mice have prolonged survival compared with wild-type B cells.5 Transgenic overexpression of BLyS in mice results in elevated numbers of mature B cells in the spleen and periphery and development of autoimmune-like manifestations reminiscent of systemic lupus erythematosus.5,6 In addition, as BLyS transgenic mice age, they develop a secondary pathology, Sjögren syndrome,7 which is associated with intense B-cell activity and a predisposition to development of B-cell NHLs.8,9 Furthermore, when BLyS transgenic mice are crossed with TNF–/– mice, 35% of the mice develop B-cell lymphomas.10 Taken together, these data suggest a possible role for dysregulated BLyS expression in the pathogenesis of B-cell malignancies.
Three receptors, B-cell maturation antigen (BCMA),11 transmembrane activator and CAML interactor (TACI),12 and BAFF-R,13 have been identified as receptors for BLyS. BCMA and BAFF-R are predominantly expressed on B lymphocytes, whereas TACI can be found on B cells and activated T cells. TACI and BCMA can also bind to APRIL, whereas BAFF-R is specific for BLyS. A key role for BAFF-R in BLyS binding has been suggested by studies demonstrating that A/WySnJ mice, which carry a mutation in BAFF-R, have a loss of follicular and marginal zone B cells in secondary lymphoid organs, a phenotype similar to BlyS-deficient mice.13-15 However, an important role for TACI in B-cell homeostasis has been implicated because TACI-deficient mice were found to have an accumulation of splenic B cells and TACI–/– B cells have increased immunoglobulin production.16-18 The accumulation of B cells in the TACI–/– mice resulted in the development of lymphoma, indicating a possible role for this receptor system in the dysregulated growth of lymphoma B cells.18 A role for BCMA in B-cell function remains to be fully elucidated, but growing evidence indicates that this receptor may be important in normal and malignant plasma cell biology.19-21
The ability of BLyS to modulate normal B-cell homeostasis has been well documented.22 However, it is less clear how BLyS influences the growth and survival of malignant B cells. In previous work we have demonstrated a role for BLyS in the survival and growth of B-cell chronic lymphocytic leukemia (B-CLL) and multiple myeloma cells.19,23 Briones et al24 have studied the pattern of BLyS binding in NHL and found that receptors for BLyS were expressed on all B-cell lymphomas tested, but differences in the level of expression were detected in different types of NHL. In addition, they found that patients with follicular and diffuse large cell NHL had increased levels of soluble BLyS in their serum, suggesting that it may be involved in the biology of these tumors.
This study was therefore conducted to determine expression of BLyS and its receptors, BCMA, TACI, and BAFF-R, in various subtypes of NHL. Our data suggest that BLyS is expressed in tumor specimens isolated from all subtypes of NHL examined. We also find that NHL B cells can bind BLyS and predominantly express BAFF-R, to a lesser extent TACI, and are deficient for BCMA expression. Additionally, we examined the serum BLyS levels in patients with newly diagnosed large cell lymphoma and compared these levels to those in healthy volunteers and those in patients with transformed lymphoma. We present evidence indicating that serum BLyS levels are elevated in patients with NHL and that BLyS levels correlated with patient and disease characteristics, response to therapy, and overall clinical outcome. Taken together, these findings suggest a role for BLyS and its receptors in malignant B-cell biology. Identification of targets to inhibit this interaction may therefore have therapeutic efficacy.
Patients, materials, and methods
Cells and reagents
Lymph node biopsy mononuclear cells were isolated as previously described23 from healthy donors or patients with NHL, who provided written informed consent. ZymoGenetics (Seattle, WA) provided anti-TACI, anti-BCMA, and anti–BAFF-R biotinylated antibodies and biotinylated BLyS. RED670- and phycoerythrin (PE)–conjugated streptavidin were purchased from Gibco (Carlsbad, CA) and Caltag (Burlingame, CA), respectively. Mouse IgG control was purchased from PharMingen (San Diego, CA).
Flow cytometry
Cells (1 × 106) were washed in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and incubated with 0.1 μg biotinylated recombinant BLyS, or 0.5 μg biotinylated anti-BCMA, anti–BAFF-R, anti-TACI, or mouse immunoglobulin control for 30 minutes at 4° C. Cells were washed and incubated with fluorescein isothiocyanate (FITC)–conjugated anti-CD19 and streptavidin-PE for 30 minutes at 4° C, washed, and analyzed using FACScalibur and CellQuest (Becton Dickinson, San Jose, CA). Membrane-bound BLyS expression was determined with PE-conjugated anti-CD19 (Becton Dickinson) and FITC-conjugated anti-BLyS (Alexis Biotechnology, San Diego, CA) as described.
PCR analysis
For reverse transcription–polymerase chain reaction (RT-PCR), TRIzol (Invitrogen, Carlsbad, CA) was used to isolate total RNA from NHL patients' frozen specimens or NHL cell lines. RNA was converted into cDNA using the First-Strand cDNA Synthesis Kit (Amersham Pharmacia, Little Chalfont, England) according to the manufacturer's instructions. BLyS and β-actin cDNAs were detected by PCR amplification with HotStarTaq (Qiagen, Valencia, CA) in steps of 1 minute each at 94° C, 60° C, and 72° C for 35 cycles, using primers previously described as being specific for BLyS.4,23 Primers for β-actin were designed using the published cDNA nucleotide.23
Immunohistochemistry
Paraffin-embedded tumor specimens obtained from patients with lymphoma were cut into 4-μm sections and treated as previously described.19 The preparations were incubated with 10 μg/mL mouse rabbit-human BLyS (Chemicon, Temecula, CA) or isotype-matched mouse immunoglobulin control (Dako Cytomation, Carpinteria, CA) for 30 minutes at room temperature and rinsed twice with PBS. The preparations were then incubated for 30 minutes with biotinylated anti–mouse IgG (1:300; Dako Cytomation) and followed by an additional 30 minutes of incubation with horseradish peroxidase (HPR)–conjugated streptavidin (1:300, Dako Cytomation). BLyS was visualized using 3,3′-diaminobenzidine (Dako Cytomation) and counterstaining with hematoxylin. The slides were cover-slipped and mounted with Cytoseal 280 (Stephens Scientific, Kalamazoo, MI). All slides were observed with light microscopy (Olympus AX70, 200× aperture 0.46, 400× aperture 0.75, 600× aperture 0.80; Olympus America, Melville, NY) with images being captured with a SPOT RT camera and software (Diagnostic Instruments, Burlingame, CA).
Cell viability
Cells isolated from NHL tumors were washed and cultured in a 96-well plate (0.1 × 106 cells/well) in phenol red-free RPMI supplemented with 10% FCS and 0.5 μg/mL Flag-BLyS (Zymogenetics) at 37° C. After 48 hours of incubation, cells were assayed for viability with the LIVE/DEAD Viability/Cytotoxicity kit from Molecular Probes (Eugene, OR). Briefly, viable cells were detected by the addition of calcein am to each well to a final concentration of 2 μM and assessed by fluorescence using a CytoFluor Multi-well Plate Reader (Applied Biosystems, Foster City, CA).
Patient selection
Patients were eligible for this study if they had a tissue biopsy specimen that on pathologic review showed B-cell NHL (Table 1). Patients were aged 23 to 86 years, with a median age of 63 years.The patients were seen and their specimens obtained between January 1988 and June 1998 and all patients were untreated at the time the biopsy was taken. The biopsy specimens were reviewed and classified using the World Health Organization (WHO) classification. Staging was commonly performed with a complete blood count, serum chemistries, chest radiograph, computed tomography scan of the abdomen, and bilateral bone marrow aspiration and biopsy. The pretreatment prognostic factors, the response to therapy, and overall survival data were obtained by chart review or correspondence. Patients were followed up to September 2003 and all patients have 5 years or longer of follow-up. This study was approved by the Institutional Review Board of the Mayo Clinic/Mayo Foundation.
International Prognostic Index and other prognostic factors
Prognostic factors considered to be predictive of patient outcome were evaluated in all patients. The International Index of prognostic factors (IPI) was calculated for each patient based on stage, lactate dehydrogenase (LDH) level, performance status, number of extranodal sites, and age. The predicted outcome of the patient was determined using the IPI and also by the use of each variable in the IPI formula separately. Other prognostic factors including patient sex, presence of constitutional symptoms, and the site of extranodal involvement, were also examined. Each of these prognostic factors was analyzed to determine its effect on failure-free survival (FFS) and overall survival (OS).
BLyS ELISA
Enzyme-linked immunosorbent assay (ELISA) plates (Nunc Maxisorp; Nalge Nunc International, Rochester, NY) were coated with 100 μL of 3 μg/mL anti–human BLyS (monoclonal antibody [mAb] clone 148725; R&D Systems, Minneapolis, MN) overnight at 4° C. Wells were washed with PBS and blocked with 1% BSA in PBS. Patient serum samples were diluted 1:10 in PBS/1% BSA and 50 μL was added to each well in duplicate and incubated for 2 hours. Wells were washed and biotinylated goat anti–human BLyS (2 μg/mL; R&D Systems) was added to each well. Wells were again washed and HRP-conjugated streptavidin (Chemicon) was added to the wells for 1 hour, washed, and developed with Turbo TMB-ELISA (Pierce Chemical, Rockford, IL). The reaction was stopped by addition of 1 N H2SO4 and results were measured with a plate reader (Molecular Devices, Palo Alto, CA) and analyzed using Softmax software (version 2.34; Molecular Devices). BLyS serum levels were calculated from a standard curve generated with serially diluted recombinant human BLyS (Alexis Biochemicals) in 10% normal human sera.
Statistical analysis
Comparisons between patient groups were based on χ2 tests for nominal variables; the Wilcoxon rank-sum test or the Kruskal-Wallis test was used for continuous variables. OS and FFS were measured from the date of study entry or date of achievement of complete response (CR), respectively, until death from any cause (survival, FFS) or relapse (FFS). Patients alive at last follow-up evaluation were censored for analysis of OS, whereas patients still at risk of relapse were censored for FFS. The FFS and OS of all patients was estimated using the Kaplan-Meier method. The univariate associations between individual clinical features and survival were determined with the 2-tailed log-rank test. Features independently associated with survival were identified in multivariate analyses by the Cox proportional-hazards regression model. All patients had complete data sets and could be included in the Cox model.
Results
Expression of BLyS receptors in NHL
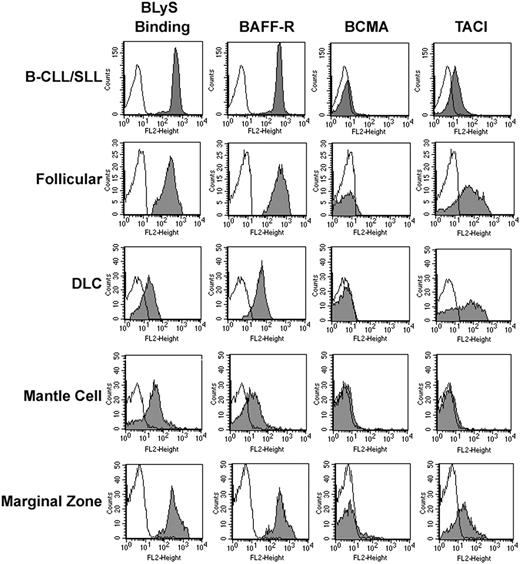
Previous studies of NHL B cells indicate that they express BLyS receptors. However, specific expression of BCMA, BAFF-R, and TACI was not described. Therefore, we wished to gain a better understanding of the BLyS receptor profile on NHL B cells. We used flow cytometric analyses to determine cell surface expression of TACI, BCMA, and BAFF-R on various subtypes of human NHL cells (Figure 1). The NHL samples examined were diagnosed with either small lymphocytic lymphoma (B-CLL/SLL), follicular lymphoma, diffuse large cell (DLC) lymphoma, mantle cell lymphoma, or marginal zone lymphoma according to the WHO classification. Mononuclear cells from patient tumor biopsies were costained with anti-CD19, to differentiate B cells, and antibodies to BAFF-R, BCMA, and TACI. Soluble biotinylated BLyS was used to determine total BLyS binding (Figure 1). Similar to memory and naive B cells,19 NHL B cells express BAFF-R and express low to no BCMA. The presence of BAFF-R and lack of BCMA was consistently found; however, TACI expression was variable on all histologic subtypes examined. Regardless of the BLyS receptor profile, all samples tested had the ability to bind soluble BLyS.
Expression of BCMA, TACI, and BAFF-R on NHL cells. Tumor cells from patients with B-CLL/SLL (n = 40), follicular (n = 7), DLC (n = 7), mantle cell (n = 7), or marginal zone NHL (n = 8) were stained with biotin-conjugated anti-TACI, anti–BAFF-R, anti-BCMA, or BLyS for 30 minutes on ice, washed, and incubated with PE-streptavidin and CD19-FITC (gray histograms). Histograms correspond to CD19+ cells from a representative example of each histologic subtype. Isotype and fluorochrome controls were done for each sample (open histograms).
Expression of BCMA, TACI, and BAFF-R on NHL cells. Tumor cells from patients with B-CLL/SLL (n = 40), follicular (n = 7), DLC (n = 7), mantle cell (n = 7), or marginal zone NHL (n = 8) were stained with biotin-conjugated anti-TACI, anti–BAFF-R, anti-BCMA, or BLyS for 30 minutes on ice, washed, and incubated with PE-streptavidin and CD19-FITC (gray histograms). Histograms correspond to CD19+ cells from a representative example of each histologic subtype. Isotype and fluorochrome controls were done for each sample (open histograms).
BLyS promotes survival of NHL B cells
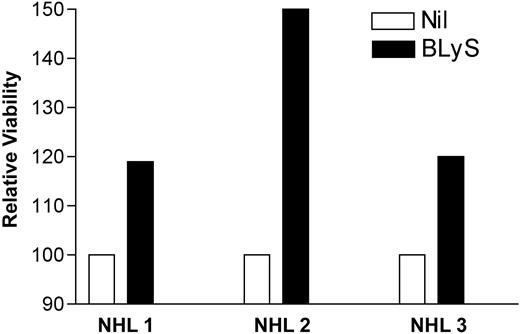
Initially, it was proposed that BLyS functioned to enhance B-cell proliferation as well as immunoglobulin secretion.4,25 However, recent literature now suggests that the predominant function of BLyS is maintenance of the normal B-cell population and attenuation of B-cell apoptosis.26 Recent work by our group,19,23 as well as others, indicates a role for BLyS in the survival of multiple myeloma27 and CLL28 B cells as well. Because NHL B cells express the receptors for BLyS, we next wanted to determine the influence of BLyS on these cells. NHL B cells were cultured in the presence of BLyS for 48 hours and cell survival was assessed with the cell viability dye calcein am (Figure 2). Consistent with previously published work, our data clearly indicate that BLyS reduces the level of apoptosis and enhances survival of malignant B cells.19,23,27,28
BLyS promotes survival of NHL. Cell isolated from NHL tumors were washed and cultured in triplicate in a 96-well plate (0.1 × 106 cells/well) in phenol red-free RPMI supplemented with 10% FCS alone or with the addition of 0.5 μg/mL Flag-BLyS at 37° C. After 48 hours of incubation, cells were assayed for viability with the LIVE/DEAD Viability/Cytotoxicity kit as described in “Patients, materials, and methods.” In all cases the fluorescence of a media-alone control was subtracted for the experimental value. Cell viability in the presence of BLyS was normalized to the media-alone control and is shown as relative viability.
BLyS promotes survival of NHL. Cell isolated from NHL tumors were washed and cultured in triplicate in a 96-well plate (0.1 × 106 cells/well) in phenol red-free RPMI supplemented with 10% FCS alone or with the addition of 0.5 μg/mL Flag-BLyS at 37° C. After 48 hours of incubation, cells were assayed for viability with the LIVE/DEAD Viability/Cytotoxicity kit as described in “Patients, materials, and methods.” In all cases the fluorescence of a media-alone control was subtracted for the experimental value. Cell viability in the presence of BLyS was normalized to the media-alone control and is shown as relative viability.
Expression of BLyS in NHL
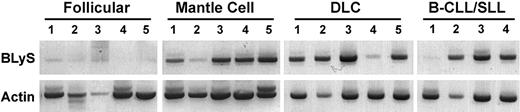
BLyS is predominantly expressed by activated monocytes, dendritic cells, and neutrophils.29,30 However, a growing body of literature indicates that BLyS may be produced by stromal cells, including radioresistant splenic stromal cells31 and nurselike cells.32 The ability of BLyS to support B-cell survival and homeostasis in regions rich in B cells raises the possibility that BLyS expression within a tumor microenvironment may attenuate apoptosis and support dysregulated growth of malignant B cells. Therefore, we wished to determine whether BLyS was expressed in tumor specimens from patients with NHL. To examine this possibility, we used RT-PCR to detect BLyS mRNA in whole NHL tumor biopsies (Figure 3). Of interest, BLyS mRNA was detected in 14 of 19 tumors (Figure 3). When characterized by NHL histologic subtype, we find that BLyS was detected in 5 of 5 mantle cell lymphomas, 5 of 5 DLC lymphomas, 3 of 4 B-CLL/SLLs, and 1 of 5 follicular lymphomas.
Expression of BLyS RNA in NHL tumors. Expression of BLyS mRNA was analyzed by RT-PCR in 5 follicular, 5 mantle cell, 5 DLC, and 4 B-CLL/SLL NHL samples. Normal human monocytes were used as a positive control (data not shown).
Expression of BLyS RNA in NHL tumors. Expression of BLyS mRNA was analyzed by RT-PCR in 5 follicular, 5 mantle cell, 5 DLC, and 4 B-CLL/SLL NHL samples. Normal human monocytes were used as a positive control (data not shown).
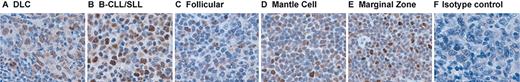
To confirm our RT-PCR results we next examined NHL tumor specimens for BLyS protein by immunohistochemistry. BLyS was detected in all NHL specimens studied (Figure 4A-E). Variable levels of BLyS were detected within the histologic subtypes, with the lowest levels seen in follicular lymphoma. The corresponding immunoglobulin control is shown in Figure 4F. The antibody used for these studies is specific for the soluble and membrane-bound forms of BLyS; therefore, we were unable to determine if the BLyS present in the NHL specimens was soluble BLyS bound to B cells or surface-expressed BLyS. In an attempt to gain a better understanding of the source of BlyS, we stained identical slides with antibodies directed against the membrane-bound BLyS. We found no positive staining with this reagent, suggesting that the BLyS detected in the NHL specimens is soluble BLyS bound to malignant B cells (data not shown). Additionally, we analyzed NHL cells for BLyS expression by flow cytometry. Cells isolated from NHL specimens (n = 14) were costained with anti-CD19 and anti-BLyS (membrane specific) and we detected minimal to no surface expression of BLyS. The change in mean fluorescent intensity (ΔMFI) of anti-BLyS staining compared with a matched isotype control was 1.26 (1.0 being no expression), whereas our positive control cell line, HL60, had a ΔMFI of 3.47 (data not shown). Our results contradict those recently published by He et al,33 whose data suggest that NHL B cells have the ability to express both the membrane-bound and soluble forms of BLyS. The discrepancy in the data may be due to experimental differences or patient variability and will hopefully be resolved with ongoing investigations. Regardless, our results, in addition to that of He et al,33 indicate a potential role for BLyS and its receptors in NHL.
Expression of BLyS in NHL tumors. Immunohistochemical analysis of BLyS expression in (A) DLC, (B) B-CLL/SLL, (C) follicular, (D) mantle cell, and (E) marginal zone NHL specimens (n = 5 for each histologic subtype) was performed with the anti-BLyS mAb from Chemicon as described in “Patients, materials, and methods.” An isotype control was done for each specimen and a representative example is shown in panel F. Original magnification × 400.
Expression of BLyS in NHL tumors. Immunohistochemical analysis of BLyS expression in (A) DLC, (B) B-CLL/SLL, (C) follicular, (D) mantle cell, and (E) marginal zone NHL specimens (n = 5 for each histologic subtype) was performed with the anti-BLyS mAb from Chemicon as described in “Patients, materials, and methods.” An isotype control was done for each specimen and a representative example is shown in panel F. Original magnification × 400.
BLyS levels correspond with disease severity
The lack of significant BLyS mRNA detected in follicular lymphoma (Figure 3) in conjunction with the low level of staining seen in tumor specimens (Figure 4C) suggests that BLyS levels may correlate with disease severity, that is, indolent versus aggressive. Patients with follicular lymphoma are at risk of relapse over time and they frequently undergo transformation to the more aggressive DLC lymphoma. Acquisition of genetic insults usually accompanies transformation; however, the mechanisms that underlie the process have not been fully elucidated. Because of the differences seen in BLyS expression between the histologic subtypes, we next examined BLyS levels in biopsy specimens obtained from the same patient at the time of diagnosis of large cell transformation (Figure 5B) and compared them to biopsies obtained before transformation (Figure 5A). BLyS expression in the areas of lymphoma involvement increased significantly after large cell transformation. These data suggest that BLyS expression may correlate with disease severity.
Increased BLyS expression with transformation from follicular to large cell NHL. Immunohistochemical analysis of BLyS expression in biopsy specimens from a patient with follicular lymphoma before (Ai, original magnification × 200; Aii, original magnification × 600) and after (Bi, original magnification × 200; Bii, original magnification × 600) transformation from follicular to large B-cell lymphoma.
Increased BLyS expression with transformation from follicular to large cell NHL. Immunohistochemical analysis of BLyS expression in biopsy specimens from a patient with follicular lymphoma before (Ai, original magnification × 200; Aii, original magnification × 600) and after (Bi, original magnification × 200; Bii, original magnification × 600) transformation from follicular to large B-cell lymphoma.
Serum BLyS levels are elevated in patients with aggressive NHL
Elevated serum BLyS levels have been found in a number of disease models including Sjörgren syndrome,34 rheumatic diseases,35 and multiple myeloma.27 Additionally, serum BLyS levels have been studied in patients with follicular NHL and were found to be elevated.24 To expand on this data, we next examined serum BLyS levels in patients with indolent or DLC lymphoma (Figure 6A). Stored serum specimens from patients with DLC (de novo or transformed) or indolent lymphoma were obtained from the Mayo Clinic lymphoma serum bank. Healthy volunteers served as controls. The median serum BLyS level in healthy controls was 17.95 ng/mL (mean, 18.24 ± 4.27; range, 7.62-32.41 ng/mL; n = 6) compared with 15.75 ng/mL (mean, 17.29 ± 3.96; range, 4.63-45.14 ng/mL; n = 11) for patients with indolent lymphoma and 26.84 ng/mL (mean, 33.52 ± 4.18; range, undetectable to 161 ng/mL; n = 56) for DLC lymphomas (Figure 6A). The median serum BLyS level among the 3 groups was not significantly different; however, a subset of patients with DLC lymphoma had elevated serum BLyS levels.
Serum BLyS levels in NHL are elevated and correlate with overall patient survival. (A) Serum BLyS levels were analyzed by ELISA in specimens obtained from healthy individuals (n = 6) or patients with indolent (n = 11) and DLC lymphoma (n = 56). Serum concentrations in patients were compared to those in healthy controls. The solid line represents the median value for the group. (B) Kaplan-Meier analysis was done to compare the survival of patients with low BLyS levels (< 40 ng/mL) at diagnosis to those with high levels (> 40 ng/mL). Patients with high levels had a significantly worse outcome (P = .05).
Serum BLyS levels in NHL are elevated and correlate with overall patient survival. (A) Serum BLyS levels were analyzed by ELISA in specimens obtained from healthy individuals (n = 6) or patients with indolent (n = 11) and DLC lymphoma (n = 56). Serum concentrations in patients were compared to those in healthy controls. The solid line represents the median value for the group. (B) Kaplan-Meier analysis was done to compare the survival of patients with low BLyS levels (< 40 ng/mL) at diagnosis to those with high levels (> 40 ng/mL). Patients with high levels had a significantly worse outcome (P = .05).
BLyS levels correlate with the clinical outcome of patients with large cell lymphoma
Because BLyS serum levels were elevated in a subgroup of patients, we next determined whether the serum BLyS level at the time of diagnosis correlated with patient or disease parameters and subsequent clinical outcome. Using the serum BLyS level determined by ELISA from a specimen obtained at diagnosis, patients were separated into 2 groups based on serum BLyS levels. Using 40 ng/mL as a cutpoint (mean value for healthy controls plus 2 SDs), we compared the overall survival (OS) of those with a high pretreatment BLyS level (n = 20) to those with a low pretreatment level (n = 47). Patients with a high pretreatment BLyS level had a significantly inferior OS (Figure 6B). Fifteen (75%) of 20 patients with high serum BLyS died compared with 24 (51%) of 47 patients with low serum BLyS, and the median OS was 39 months compared with 78 months (P = .05). Patients with high serum BLyS had a lower complete response (CR) rate than those with low BLyS levels (65% versus 83%) and a shorter median failure-free survival (FFS) (52 versus 89 months). Due to smaller numbers in these groups, these differences did not achieve statistical significance (P = .12 and P = .14, respectively). In summary, the pretreatment BLyS level correlated significantly with overall patient outcome.
Elevated pretreatment BLyS levels were also found to correlate with other clinical features. When the patients with de novo large cell lymphoma were considered, the serum BLyS levels obtained at diagnosis correlated with the presence of constitutional symptoms (fever, sweating, or weight loss) and an elevated serum LDH at diagnosis. Only 5 (11%) of 47 patients in the group with low BLyS levels had constitutional symptoms, whereas 9 (45%) of 20 in the high BLyS group had constitutional symptoms. For those patients with constitutional symptoms, the median BLyS level was 60.5 ng/mL (range, undetectable to 161 ng/mL) compared with a median BLyS of 13.1 ng/mL (range, undetectable to 103 ng/mL) in those without B symptoms (P = .002).
Similarly, serum BLyS levels at diagnosis significantly correlated with the serum LDH levels at diagnosis (P = .03). Twelve (60%) of the 20 patients with an elevated BLyS level before treatment also had an elevated LDH level, whereas 13 (45%) of 47 patients with low BLyS had an elevated LDH level. In those patients with a high LDH level, the median serum BLyS level was 38.9 ng/mL (range, undetectable to 161 ng/mL), whereas in those patients with a normal LDH value the median BLyS level was 13 ng/mL (range, undetectable to 144 ng/mL). Serum BLyS levels did not correlate with the age of the patient (P = .28), sex (P = .73), stage of disease (P = .6), or the IPI (P = .56).
Elevated BLyS levels correlate with transformation to an aggressive lymphoma phenotype and response to therapy
To determine if BLyS serum levels correlated with transformation of indolent lymphoma to large cell lymphoma, we analyzed a cohort of patients from whom we obtained serum specimens before transformation and at the time of diagnosis of transformation (Figure 7A). The mean serum BLyS levels for patients before transformation (at the time the histology showed indolent lymphoma) was 16.2 ± 3.85 ng/mL (range, undetectable to 45.1 ng/mL; n = 12). This value increased significantly (P = .03) at the time of large cell transformation to 32.4 ± 8.97 ng/mL (range, 1.12-88.38 ng/mL; n = 33; Figure 7A). The increase in BLyS expression was similarly seen in tissue sections obtained from 5 of the patients (Figure 5 and data not shown).
Serum BLyS levels correlate with transformation and response to therapy. Serum BLyS levels were analyzed by ELISA in specimens obtained from patients with NHL before (pretransformation; n = 12) and after (n = 33) transformation to DLC lymphoma (transformed) (A). (B) Serum BLyS levels were analyzed in patients with transformed DLC lymphoma (transformed; n = 33) who did (responder; n = 11) or did not (nonresponder; n = 7) respond clinically to subsequent therapy. (C) Serum BLyS levels were analyzed in newly diagnosed patients with de novo large cell lymphoma (diagnosis; n = 67) who did (responder; n = 12) or did not (nonresponder; n = 14) respond clinically to subsequent therapy (C). The results are presented as the mean ± SE.
Serum BLyS levels correlate with transformation and response to therapy. Serum BLyS levels were analyzed by ELISA in specimens obtained from patients with NHL before (pretransformation; n = 12) and after (n = 33) transformation to DLC lymphoma (transformed) (A). (B) Serum BLyS levels were analyzed in patients with transformed DLC lymphoma (transformed; n = 33) who did (responder; n = 11) or did not (nonresponder; n = 7) respond clinically to subsequent therapy. (C) Serum BLyS levels were analyzed in newly diagnosed patients with de novo large cell lymphoma (diagnosis; n = 67) who did (responder; n = 12) or did not (nonresponder; n = 14) respond clinically to subsequent therapy (C). The results are presented as the mean ± SE.
We next wanted to determine if BLyS serum levels correlated with response to therapy. Patients with transformed lymphoma were broken into 2 groups, those who responded to therapy and those who did not (Figure 7B). For patients with transformed disease, the serum BLyS level decreased from a mean of 32.4 ± 8.97 ng/mL (range, undetectable to 45.1 ng/mL; n = 33) at transformation to a mean of 16.7 ± 5.24 ng/mL (range, 1.12-4.17-42.0 ng/mL; n = 11) for responding patients (partial or complete response), but increased to a mean of 41.2 ± 7.73 ng/mL (range, 11.5-65.9 ng/mL; n = 7) for those with no clinical response to therapy (P = .03; n = 7). These data suggest that serum BLyS levels correlate with response to therapy. However, pretreatment serum BLyS levels did not significantly correlate with the likelihood of achieving a CR.
In a similar fashion, we next correlated serum BLyS levels of patients with de novo large cell lymphoma with response to therapy (Figure 7C). The mean serum BLyS level at diagnosis was 33.5 ± 4.18 ng/mL (range, undetectable to 161 ng/mL; n = 67) and this decreased to a mean of 16.2 ± 5.24 ng/mL (range, 4.17-42.0 ng/mL; n = 12) for those with a clinical response (partial or complete response) to therapy. The serum BLyS levels increased to a mean of 68.4 ± 9.84 ng/mL (range, 18.06-102.0; n = 14) for patients who did not respond or who had progression of their disease. These differences were statistically significant (P = .03). In summary, we found that BLyS serum levels correlated with both transformation of indolent to DLC lymphoma and with response to therapy.
Discussion
In this study, we examined the BLyS binding and receptor profile of various histologic subtypes of NHL cells. Our results indicate that malignant B cells from patients with NHL predominantly express BAFF-R and TACI, whereas BCMA was undetectable. Additionally, we found that BLyS is expressed in NHL tumors and that BLyS levels increased as tumors transform to a more aggressive phenotype. Finally, we present evidence that serum BLyS levels are elevated in patients with aggressive NHL and serum BLyS levels correlate with response to therapy and OS.
The BLyS-binding profile and expression of BAFF-R on NHL cells was expected because normal mature B cells express this receptor.19 Undetectable levels of BCMA were also expected because this receptor is predominately expressed on normal and malignant plasma cells.19-21 TACI was expressed on 5 of 6 histologic subtypes, being undetectable in mantle cells. Lack of TACI has also been found in a number of B-CLL specimens and NHL cell lines (data not shown). In addition to the freshly isolated NHL samples shown in Figure 1, we also studied the BLyS binding and receptor profile on 50 additional frozen NHL specimens by flow cytometry. In a majority of cases, BLyS binding and receptor levels were extremely low to undetectable when compared to fresh specimens (data not shown). Therefore, we found that studies on surface expression of this family of receptors were best examined on freshly isolated NHL cells. Studies are currently underway to examine the BLyS binding and receptor profile of a larger cohort of all histologic subtypes of NHL in freshly isolated tumor specimens.
The variable expression pattern of TACI seen in our studies has potential significance because this receptor has been implicated as a negative regulator of B-cell activation. TACI-deficient mice have an accumulation of B cells, develop autoimmune disease, and are predisposed to development of B-cell tumors.16-18 These data implicate a role for TACI in B-cell homeostasis and it may therefore be possible that dysregulated expression of TACI is involved in the progressive accumulation of malignant B cells in NHL. The precise mechanisms that control expression of all 3 BLyS receptors are currently unclear, but studies indicate that the developmental stage, cytokine milieu, and the microenvironment play a key role in regulation of receptor expression.20,21
Although it is clear that BLyS expression is required for normal B-cell development and homeostasis, the exact source of BLyS in these scenarios remains to be fully elucidated. Reports indicate that numerous cell types can express BLyS, including stromal cells, dendritic cells, neutrophils, and monocytes.29-31,36 Additionally, growing evidence indicates that BLyS can be expressed by malignant B cells and may therefore provide tumor cells with an autocrine survival mechanism.19,23,27,28,33 Immunohistochemical (Figure 4) and PCR (Figure 3) analyses of NHL tumors indicate that BLyS is expressed in NHL tumors. However, the source of BLyS is still unclear. Our antibody staining and immunopheno-typing studies (Figure 4 and data not shown) suggest that BLyS is not expressed by malignant NHL B cells and that it is likely from another cell type within the tumor microenvironment. Our data contradict those recently published by He et al,33 but are in line with previously published data indicating that malignant B cells do not express BLyS.29 Ongoing analyses are currently being done to determine the source of BLyS within the NHL tumors. However, regardless of the source, BLyS is present and bound to malignant B cells within the tumor; therefore, disruption of BLyS binding to its receptors on malignant B cells may be a potential target for clinical therapy.
Our data clearly indicate that BLyS is present in NHL tumors to varying extents. Low levels of BLyS RNA (Figure 3) and protein (Figure 4) were consistently seen in the follicular lymphoma tumors and higher levels were detected in DLC tumors. Accordingly, we stained tumor specimens obtained from patients before transformation and compared them to biopsies taken at the time of large cell transformation (Figure 5B). BLyS staining was consistently more intense on biopsies taken at the time of transformation. The mechanisms that underlie the increase in BLyS detected in the DLC tumors remain unclear. Both follicular and DLC B cells express BAFF-R and TACI and bind BLyS efficiently, indicating that increased receptor density is likely not the cause. One possible scenario is that as B-cell numbers increase during transformation, BLyS levels increase accordingly to maintain the B-cell population. How BLyS levels are regulated throughout normal B-cell homeostasis and during immune reactions is currently unclear. As these mechanisms are deciphered they may lend insight into how BLyS expression is regulated in the tumor microenvironment.
Increasing evidence indicates that elevated serum BLyS levels correlate with pathogenesis of various B cell–related disorders including B-cell malignancies.24,27,34,35,37 Recent work by Batten et al10 highlights the role of elevated BLyS levels in development of B-cell lymphomas, suggesting a potentially important role for this protein in the pathogenesis of B-cell malignancies. In this study we have found that a subset of patients with DLC lymphoma have elevated serum BLyS levels (Figure 6A). Unlike Briones et al,24 we did not find that patients with indolent lymphoma had increased levels of serum BLyS. Rather, we found that some of the patients with DLC lymphoma had significantly elevated serum BLyS levels. The high BLyS levels correlated with the presence of constitutional symptoms and elevated LDH values. Of note, BLyS levels did not correlate with the patient's age, sex, or stage of disease. Additionally, we note that the BLyS levels detected in our healthy control group were higher than those identified by other groups,34,35 and we believe this is likely due to the reagents used in our ELISA. For patients with transformed lymphoma, serum BLyS levels increased significantly at the time of transformation from an indolent lymphoma (Figure 7A).
The pretreatment BLyS levels in patients with DLC lymphoma were predictive of clinical outcome. Those patients with a high serum BLyS level at diagnosis had a significantly worse median OS when compared to those with a low level (Figure 6B). Patients with high serum BLyS also had a lower CR rate than those with low BLyS levels and a shorter median FFS. However, these differences did not achieve statistical significance. Additionally, when analyzed with other factors significant for OS by the Cox model, the pretreatment serum BLyS level was not found to be a significant independent predictor of OS. When serum BLyS levels were correlated with response to therapy (Figure 7B-C), responding patients had a significantly lower BLyS level than those with progressive disease. Pretreatment serum BLyS levels, however, did not significantly correlate with the likelihood of achieving a CR, but serum BLyS levels obtained while patients were receiving therapy correlated significantly with the clinical response to treatment.
In conclusion, our data indicate that NHL cells bind BLyS, express BAFF-R, and have variable expression of TACI. We found that BLyS is expressed in tumors from patients with NHL and that BLyS levels can increase as tumors transform to a more aggressive phenotype. We also found that serum BLyS levels are elevated in a subset of patients with DLC lymphoma. Additionally, we present evidence that serum BLyS levels in patients with NHL correlate with transformation to a more aggressive phenotype, response to therapy, disease activity, and OS. In summary, we believe that BLyS represents an important target in B-cell lymphoma and strategies to inhibit BLyS could therefore translate into important therapeutic modalities.
Prepublished online as Blood First Edition Paper, July 13, 2004; DOI 10.1182/blood-2004-02-0762.
Supported in part by grants CA92104 and CA97274 from the National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.