Abstract
Between 1990 and 1996, we conducted a randomized trial in adults with newly diagnosed acute myeloid leukemia (AML) in order to compare relapse-free interval (RFI) after double induction (arm B), timed-sequential induction (arm C), or control “3 + 7” induction (arm A). Patients achieving complete remission (CR) after induction ± salvage received the same consolidation chemotherapy, which included a dosage stratification according to patient's age (younger or older than 50 years). This long-term analysis was performed in 592 patients (arm A/B/C, 197/198/197 patients). Overall CR rate was 76% without differences between the 3 arms, even if a salvage course was less frequently needed in arm B. Treatment-related mortality, either during the induction or the postremission phase, was not significantly higher in arms B and C than in arm A. Among the 449 CR patients, 250 relapsed (arm A/B/C, 90/87/73 patients) without significant differences in RFI in arms B and C versus arm A (P = .39 and .15, by the Gray test). However, when analyzing the 345 patients younger than 50, RFI was significantly improved in younger patients receiving timed-sequential induction (P = .038 by the Gray test), while not in those receiving double induction. Event-free survival and overall survival were similar in the 3 randomization arms.
Introduction
Treatment of patients with acute myeloid leukemia (AML) consists of 2 phases, namely complete remission (CR) induction and postremission consolidation. During the last 15 years, many clinical trials have been designed to evaluate and compare different postremission treatments. Three main options have been compared in the younger patient population: allogeneic bone marrow transplantation from an HLA-identical sibling donor, autologous stem cell transplantation, and intensive chemotherapy, often including high-dose cytarabine (HDAC).1 On the other hand, fewer trials have studied the impact of modulation of induction chemotherapy on CR duration. As daunorubicin (DNR) and cytarabine (AraC) have been the 2 major drugs used for CR induction for more than 20 years, these trials have tested the use of mitoxantrone2 or idarubicin3-5 in place of DNR and the value of the addition of a third cytotoxic agent, such as etoposide.6 Otherwise, a few cooperative groups have investigated the impact of intensified induction regimens. Administration of HDAC as part of induction chemotherapy has been evaluated in a couple of controlled phase 3 studies.7,8 Reinforcement of the daily dosage of DNR used during the induction course also has been tested in older patients.9
Double induction and timed-sequential chemotherapy represent another way to intensify induction through the systematic administration of 2 sequential induction cycles, whatever the marrow status of the patient after the first cycle. In double induction, the second induction course is usually administered between days 20 and 22 of the first one.10 In timed-sequential chemotherapy, which is based on the early recruitment of leukemic blasts into the cell cycle after the first induction course,11-13 the second course is applied earlier, between days 8 and 10, in order to achieve a maximal kill of residual leukemic cells.14-16 When we initiated the present trial, no controlled phase 3 study evaluating these 2 strategies had been conducted in patients with newly diagnosed AML.
The present ALFA-9000 trial was conducted in France between 1990 and 1996 in adult patients 65 or younger with AML, with the aim to randomly compare double induction and timed-sequential induction to a standard, even if reinforced, induction arm comprising 7 days of standard-dose AraC and 3 days of DNR at the high dosage of 80 mg/m2/d. The same consolidation chemotherapy, which included another course of intensive timed-sequential chemotherapy, was administered to remitters in the 3 induction arms. The primary objective was relapse-free interval (RFI). In addition, the role of central nervous system (CNS) prophylactic therapy was randomly evaluated in a subset of patients at risk of CNS relapse. We report here the long-term analysis of this randomized trial. Approval for thy study was obtained from the Hôpital Saint Louis institutional review board.
Patients and methods
Patients
Patients 15 to 65 years old with a morphologically proven diagnosis of AML according to the French-American-British (FAB) classification17-19 and not previously treated for their disease were eligible for the study in the absence of severe heart, liver, or renal failure. Bone marrow morphology was not centrally reviewed. Patients with a secondary AML defined on the basis of history of previous exposure to chemotherapy or radiotherapy or antecedent hematologic disorders including myelodysplastic and Philadelphia chromosome–negative myeloproliferative syndromes also were eligible. Patients with AML-M3 in the FAB classification were not eligible. Informed consent was obtained before each patient entered the trial. Cytogenetic studies on pretreatment bone marrow or nonstimulated blood samples were performed using standard G banding with trypsin-Giemsa staining. Cytogenetic abnormalities were grouped according to standard criteria20 and classified according to the Southwest Oncology Group/Eastern Cooperative Oncology Group (SWOG/ECOG) classification.21
Treatments
All patients were randomized, using a centralized phone procedure, between 1 of the 3 induction arms: (1) reinforced standard “3 + 7” induction (arm A); (2) double induction (arm B); or (3) timed-sequential induction (arm C). Treatments are summarized in Table 1. Arm A consisted of DNR at 80 mg/m2/d for 3 days and AraC at 200 mg/m2/d by continuous infusion for 7 days. Patients allocated to the double induction regimen (arm B) first received the same “3 + 7” course as in arm A, followed by a second course with mitoxantrone (MTZ) at 12 mg/m2/d on days 20 and 21 and AraC at 500 mg/m2 over 1-hour infusion every 12 hours on days 20 through 22. Patients allocated to the timed-sequential induction regimen (arm C) received DNR at 80 mg/m2/d and AraC at 500 mg/m2/d by continuous infusion on days 1 to 3, followed by an early second course with MTZ at 12 mg/m2/d on days 8 and 9 and AraC at 500 mg/m2 over 1 hour every 12 hours on days 8 to 10. Of note, whereas treatment schemes were different in arms B and C, patients received the same cumulative doses of DNR, MTZ, and AraC. In the 3 arms, bone marrow response was evaluated at day 20. Patients treated in arm A with resistant disease at day 20 may receive the salvage course at that time. Patients treated in arm B received the second course of induction whatever their marrow status at day 20, and the salvage therapy may be offered later to those who failed after this second induction course. In patients treated in arm C with resistant disease at day 20, the salvage course was delayed until day 28 to avoid excessive toxicity due to repeated administration of intermediate and high doses of AraC. The salvage course was similar among the randomization arms, comprising amsacrine at 100 mg/m2/d for 3 days and HDAC at 3 g/m2 over 1-hour infusion every 12 hours for 6 days. All patients who achieved CR received then the same 2 consolidation courses, except those eligible for allogeneic bone marrow transplantation (BMT) in first CR from an HLA-identical sibling donor. The first consolidation consisted of amsacrine at 90 mg/m2 for 1 day and AraC at 60 mg/m2 by subcutaneous injection twice a day for 5 days and was administered in outpatients. The second consolidation course consisted of an etoposide-mitoxantrone-AraC (EMA) timed-sequential regimen22,23 that comprised MTZ at 12 mg/m2/d for 3 days, AraC at 500 mg/m2/d by continuous infusion from days 1 to 3 and days 8 to 10 and etoposide at 200 mg/m2/d by continuous infusion from days 8 to 10 (Figure 1). This second consolidation course required hospitalization. In order to limit toxicity, half daily doses of AraC and etoposide were administered to patients 50 years or older. Patients with AML-M4, -M4eo, and -M5 in the FAB classification, as well as patients with a white blood cell (WBC) count higher than 100 × 109/L at diagnosis were randomly allocated to receive or not a CNS prophylactic treatment with 6 intrathecal (IT) administration of 15 mg methotrexate, 30 mg AraC, and 40 mg methylprednisolone, and a 18-Gy cranial irradiation. Cranial irradiation was performed after the end of both consolidation courses.
Response criteria
Response to induction or induction/salvage chemotherapy was evaluated just before the onset of the first consolidation course. Standard National Cancer Institute (NCI) criteria were used to define CR, including a normocellular bone marrow with fewer than 5% blast cells, peripheral blood counts showing at least 1 × 109/L neutrophils and 100 × 109/L platelets, and the disappearance of all clinical signs of leukemia.24 Patients alive after induction or induction and salvage but not reaching these CR criteria were considered as patients with resistant disease. Induction deaths were defined as deaths occurring between the onset of induction chemotherapy and evaluation of response to induction or induction/salvage chemotherapy. Induction treatment–related deaths were defined as induction deaths occurring either before the day 20 marrow evaluation or later in patients without resistant marrow disease at day 20. Postremission treatment–related deaths were defined as deaths in first CR occurring either during or after receiving the first consolidation course or during the second EMA consolidation course or from adverse events that occurred during the aplastic phase following EMA consolidation.
Statistical analysis
The main end point was RFI, which was calculated from the date of the first CR to the date of the first relapse. Complete remission rate, tolerance, event-free-survival (EFS), and overall survival defined secondary end points. Overall survival was measured from the date of randomization. Event-free survival was measured from the date of randomization, with CR achievement failures, deaths in first CR, and relapses included as events. Toxicity and adverse events during the period of induction and consolidation phases were classified in the 3 arms according to the World Health Organization (WHO) criteria.25 Statistical analysis was performed on an intention-to-treat basis, with 2 comparisons of each experimental arm (B and C) to the control arm (A). Failure time data were analyzed at the reference date of June 1, 2001. Failure time data except for RFI were estimated by the Kaplan-Meier method,26 then compared by the log-rank test.27 Proportional hazards assumptions were graphically checked. By contrast, in estimating RFI, we took into account competing risks deaths and allogeneic bone marrow transplantations in first CR using the cumulative incidence curves, then compared by the Gray test while the Fine and Gray model was used to estimate specific hazard ratio (SHR).28,29 Complete response rates and the percentages of patients with severe infectious event (grades 3 and 4) were compared using the Fisher exact test. Type I error was fixed at the 5% level. All tests were 2-tailed. Statistical analysis was performed on SAS 8.2 (SAS, Cary, NC) and S-plus 2000 (MathSoft, Seattle, WA) software packages.
Results
Patient population and characteristics
The trial began in March 1990 and ended in February 1996, with 601 patients included from 15 institutions. Of note, 5 institutions entered 80% of the patients. Nine patients were excluded: 2 had acute lymphoblastic leukemia, 1 had acute promyelocytic leukemia, 1 had megaloblastic anemia, 2 had myelodysplastic syndrome, 2 refused therapy, and the remaining one died before onset of therapy. Present results deal thus with 592 randomized patients (arm A, 197 patients; arm B, 198 patients; arm C, 197 patients). Median follow-up is 118 months. Pretreatment characteristics are presented in Table 2. There were no main imbalances between the 3 randomization arms. Overall, the median age was 46 years, and 247 patients (42%) were age 50 years or older. Most patients had de novo AML (542 patients, 92%). Cytogenetic analysis was performed in 405 patients (68%).
Compliance to the planned therapy
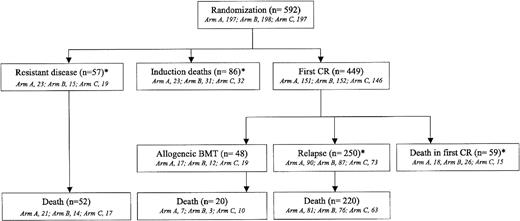
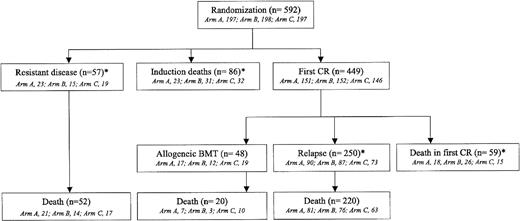
All patients from arm A and arm C received the planned induction therapy. In arm B (double induction), the second induction cycle was administered in 159 (82%) of the 194 patients alive at day 20, while 35 of these patients never received it. This second induction cycle actually started on day 20 in 141 patients and was delayed in 18 patients (between day 22 and day 67). Among the 449 patients achieving CR, 416 (93%) received the first consolidation course and 33 did not (9, 15, and 9 patients, in arm A, B, and C, respectively; P = .83) due to either early death in CR (2 patients), severe infectious (12 patients) or noninfectious (5 patients) toxicity, planned allogeneic BMT (7 patients), early relapse (3 patients), refusal (1 patient), or protocol violation (3 patients). Of the 416 patients who received the first consolidation, 305 (73%) received the second consolidation course and 111 did not (29, 36, and 46, in arm A, B, and C, respectively; P = .045) due to early relapse (29 patients), planned allogeneic BMT (45 patients), severe infectious or noninfectious toxicity (28 patients), refusal (6 patients), or death (3 patients). Overall, 51.5% of the patients randomized received the entire planned chemotherapy. As 48 of the 449 CR patients received an allogeneic BMT in first CR (arm A, 17 patients; arm B, 12 patients; arm C, 19 patients), the feasibility of the planned postremission chemotherapy in the 401 remaining CR patients was thus 76%. Of note, median time intervals between randomization, first, and second consolidations were not different between the 3 randomization arms (not shown).
Overall treatment results
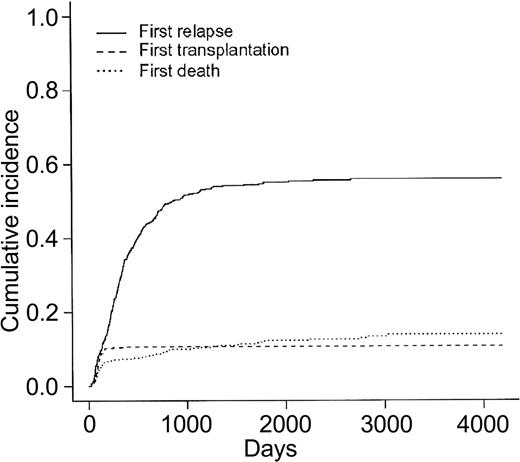
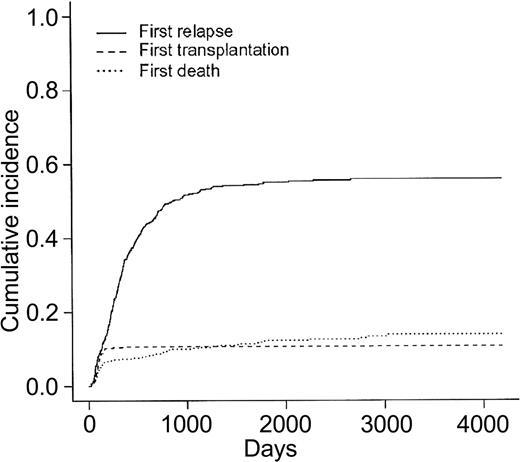
Overall patient outcome is summarized in Figure 1. The overall CR rate was 449 (76%) of 592 patients. Among the 401 CR patients who did not receive allogeneic BMT in first CR, 250 relapsed and 59 died in first CR. Cumulative incidence of death in first CR, allogeneic BMT in first CR, and relapse as first events are indicated in Figure 2 (these 3 events being considered as competing events). At 2 years, cumulative incidence of relapse and death in first CR were estimated at 47% (95% CI, 42.4%-51.6%) and 9% (95% CI, 6.4%-11.6%), respectively. At 5 years, cumulative incidence of relapse and death in first CR were estimated at 55% (95% CI, 50.4%-59.6%) and 12% (95% CI, 9%-15%), respectively. Eventfree survival was estimated at 20.5% (95% CI, 17%-24%) and 19.4% (95% CI, 16%-22.8%), at 2 and 5 years, respectively. Overall survival was estimated at 45.4% (95% CI, 41.6%-49.6%) and 29.8% (95% CI, 26.3%-33.7%), at 2 and 5 years, respectively.
Cumulative incidences of relapse, death in first CR, and allogeneic BMT in first CR as first events. N = 449 CR patients.
Cumulative incidences of relapse, death in first CR, and allogeneic BMT in first CR as first events. N = 449 CR patients.
In the 405 patients with available karyotype, cytogenetics was identified as strongly predictive for CR achievement, but interestingly not for RFI once the CR was achieved (Table 3). Similar results were observed in the subgroup of 242 patients younger than 50 years with available karyotype (not shown).
Induction results according to randomization arm
No difference in CR rate was observed between the 3 randomization arms (Table 4). Of note, fewer patients needed a salvage course to reach CR in arm B (6%) than in arm A (20%) or C (13%) (P < .001), and there was a significant difference in time to reach CR among the 3 arms (median time estimated at 30, 44.5, and 35 days, in arms A, B, and C, respectively). There was a total of 86 induction deaths (14.5%), including 23 deaths in arm A patients (12%), 31 deaths in arm B patients (16%; P = .30, as compared to arm A), and 32 deaths in arm C patients (16%; P = .25, as compared to arm A) (Table 4). Among these 86 deaths, 28 deaths occurred before the day 20 marrow evaluation (6, 12, and 10, in arms A, B, and C, respectively), while 58 occurred later (17, 19, and 22, in arms A, B, and C, respectively). In this later group, 26 patients had persistent marrow blasts at day 20 (8, 7, and 11 patients, in arms A, B, and C, respectively), 10 of them (5, 1, and 4, in arms A, B, and C, respectively) dying during or after the administration of a salvage course. The overall induction treatment–related death rate was thus 10%, without significant differences among randomization arms (8% in arm A; 12% in arm B, P = .18 as compared to arm A; and 11% in arm C, P = .38 compared with arm A). Median duration of neutropenia (< 0.5 × 109 neutrophils/L) was 25 days in arm A, 37 days in arm B, and 30 days in arm C (P < .0001). Severe infectious events occurred in 46, 54, and 49 patients from the 3 arms, respectively (P = .67). Median duration of hospitalization was longer in arm B (45 days) than in arm C (37 days) and arm A (31 days) (P < .0001).
Death in first CR according to randomization arm
Cumulative incidence of death in first CR was similar in the 3 randomization arms. Among the 59 patients who did not receive transplants and who died in first CR (arm A, 18 patients; arm B, 26 patients; arm C, 15 patients), 28 deaths were classified as treatment related. In the 401 CR patients who did not receive transplants, the postremission treatment–related death rate was thus 7%. Actually, 5 of these 28 patients died during or after receiving the first consolidation course (2 from arm B and 3 from arm C), and the 23 remaining ones died during the second EMA consolidation course or from adverse events that occurred during the aplastic phase following EMA consolidation (9 from arm A, 8 from arm B, and 6 from arm C, including 13 patients 50 years or older). These differences in postremission treatment–related mortality between the 3 induction arms were not statistically significant. Among the 48 patients who received allogeneic BMT in first CR, the overall graft-related mortality was 21%, without apparent difference between the 3 induction arms (3, 1, and 6 deaths in patients from arm A, B, and C, respectively; P = .29).
RFI, EFS, and overall survival according to randomization arm
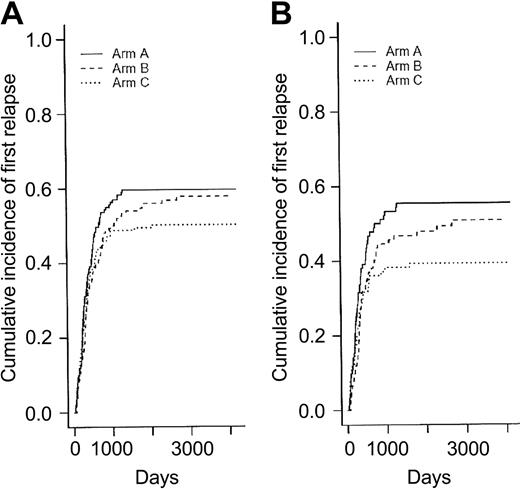
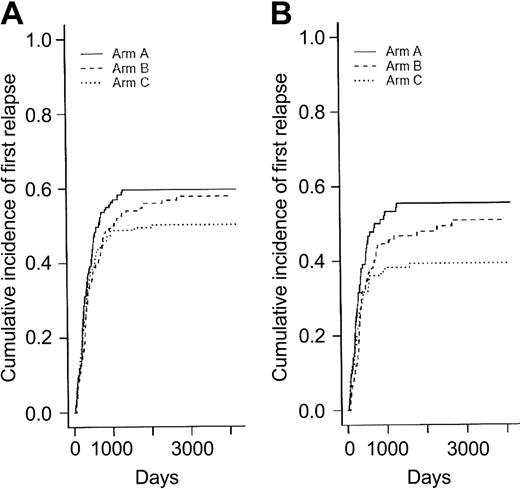
Among the 250 CR patients who experienced a relapse as first event, 90 patients belonged to the arm A, 87 to the arm B, and 73 to the arm C. Differences in RFI were not significant among the 3 randomization arms (P = .39 and .15 when arms B and C were compared to arm A, respectively, using the Gray test) (Figure 3A). Consequently, there was no more difference between randomization arms either in EFS (Figure 4A) or overall survival (Table 4). In eligible patients, the randomization for CNS prophylaxis or not did not affect RFI.
Relapse-free interval (cumulative incidence of relapse as first event) according to the 3 randomization arms. Panel A shows relapse-free interval in the 449 CR patients (P = .39 and .15 when arms B and C were compared to arm A, respectively, using the Gray test) (Table 3); and panel B, in the 282 CR patients younger than 50 (P = .26 and .038 when arms B and C were compared to arm A, respectively, using the Gray test) (Table 5).
Relapse-free interval (cumulative incidence of relapse as first event) according to the 3 randomization arms. Panel A shows relapse-free interval in the 449 CR patients (P = .39 and .15 when arms B and C were compared to arm A, respectively, using the Gray test) (Table 3); and panel B, in the 282 CR patients younger than 50 (P = .26 and .038 when arms B and C were compared to arm A, respectively, using the Gray test) (Table 5).
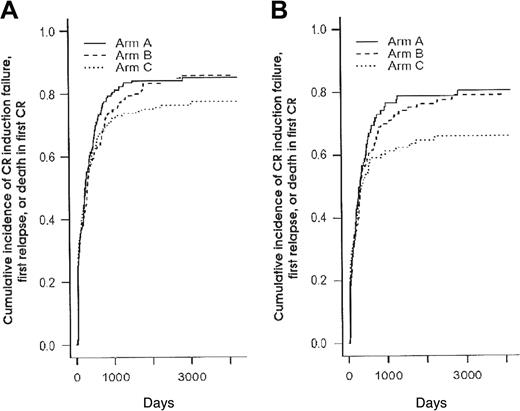
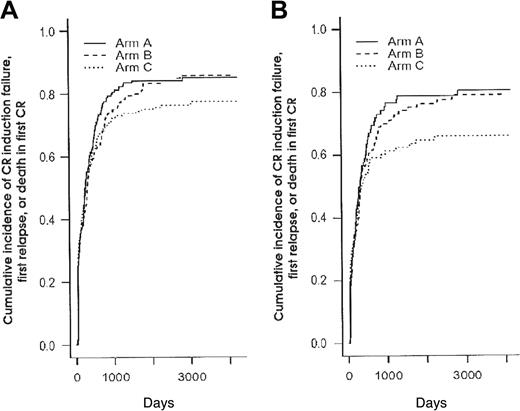
Cumulative incidence of CR induction failure, first relapse, or death in first CR (1-EFS) according to the 3 randomization arms. Panel A shows cumulative incidence in the 592 patients (P = .45 and .21 when arms B and C were compared to arm A, respectively, using the log-rank test) (Table 3); panel B, in the 345 patients younger than 50 years (P = .60 and .14 when arms B and C were compared to arm A, respectively, using the log-rank test) (Table 5).
Cumulative incidence of CR induction failure, first relapse, or death in first CR (1-EFS) according to the 3 randomization arms. Panel A shows cumulative incidence in the 592 patients (P = .45 and .21 when arms B and C were compared to arm A, respectively, using the log-rank test) (Table 3); panel B, in the 345 patients younger than 50 years (P = .60 and .14 when arms B and C were compared to arm A, respectively, using the log-rank test) (Table 5).
Age subgroup analyses
Given the fact that postremission chemotherapy was stratified among age subgroups (younger than 50 years or not) and the observation that intensified chemotherapy approaches in AML usually benefit only patients younger than 50 years, we looked for interactions between treatment effect and age. Table 5 shows the main outcome results according to the 3 randomization arms for the subgroup of 345 patients younger than 50 years. Baseline characteristics of these 345 patients were well balanced among the 3 randomization arms (not shown). Again, no difference in CR rate was observed between the 3 randomization arms (overall CR rate, 82%). Among the 136 young CR patients who experienced a relapse as first event, 52 patients belonged to the arm A, 46 to the arm B, and only 38 to the arm C. As a result, RFI was significantly improved in younger patients from the arm C compared with the control arm A (P = .038, using the Gray test) (Figure 3B). This did not translate, however, to a longer EFS (Figure 4B) or overall survival. Conversely, no differences, either in CR rate, RFI, cumulative incidence of death in first CR, EFS, or overall survival were observed in patients 50 years or older (not shown).
As expected, there was a significant imbalance in cytogenetics between both age subgroups (46 versus 10, 122 versus 93, 44 versus 36, and 30 versus 24 patients younger than 50 versus 50 years or older in the favorable, intermediate, unfavorable, and unknown significance subgroups, respectively; P = .002 by the Fisher exact test). However, no interaction was noted between cytogenetics and treatment effect on RFI, as measured by estimated coefficients in the Fine and Gray model with standard deviations (P = .42). Despite this result and the lack of prognostic impact of cytogenetics on RFI previously mentioned (Table 3), a multivariate analysis incorporating cytogenetics, treatment effect (arm C versus arm A), and age as covariates for RFI was performed in the subsample of 160 patients younger than 50 years with available karyotype and randomized in arm A or C. Using Fine and Gray model for the subdistribution hazard of relapse in presence of competing risks, P values were 0.13, 0.23, and 0.36 for treatment arm, age, and cytogenetics, respectively, when cytogenetics was considered as a 4-class variable. These P values became .09, .26, and .20, respectively, when cytogenetics was considered as a 2-class variable (unfavorable versus other). Of note, reanalyzing the unadjusted effect of treatment (arm C versus arm A) in this smaller subset of 160 patients did not allow to reach the statistical significance in favor of arm C (P = .14, by the Gray test), probably mainly because of the limited sample size, limiting the interpretation of these multivariate results.
NS prophylactic therapy
One hundred forty patients were randomized to receive (72 patients) or not receive (68 patients) the CNS prophylaxis, including 43 with AML-M4, 19 with AML-M4eo, 48 with AMLM5, and 30 with high count non-M4/5 AML. Of note, there was no difference in the distribution of induction arms across the 2 CNS prophylaxis randomization groups. Overall, 12 patients experienced a CNS relapse, including 5 patients not considered for the CNS prophylaxis randomization. Among the remaining 7 patients who had CNS relapse, 6 were from the no-prophylaxis group and 1 from the prophylaxis group (P = .058). Four of these 7 patients had AML-M4eo in the FAB classification.
Discussion
In the present randomized study we investigated the effects of 2 different intensified induction treatments as compared to a control “3 + 7” induction treatment in adult patients with AML. However, it must be stressed that patients from the control group received higher doses of chemotherapy than in a standard “3 + 7” regimen. First, we used higher DNR doses (ie, 80 mg/m2/d in place of the 45-60 mg/m2/d used by most cooperative AML groups). Some studies already have demonstrated a certain advantage associated with the use of high-dose DNR during the induction phase. Historical survey of noncomparative SWOG studies showed a higher CR rate using DNR at a dosage of 70 mg/m2/d as compared to 45 mg/m2/d.30 In elderly patients with AML, a comparison of 30 to 60 mg/m2/d DNR showed an improved survival in patients treated with higher dosage.9 Secondly, a more than usual intensive salvage course containing HDAC was early offered to patients with resistant AML after the first phase of induction.
This “3 + 7” regimen was compared to 2 different strategies of intensified induction. Double induction therapy has been initially reported by the German AML Cooperative Group in a randomized study in which all included adult patients first received a TAD course consisting of 6-thioguanine, standard-dose AraC, and daunorubicin followed by either a second TAD or a HAM course containing HDAC and mitoxantrone starting at day 21 of the first TAD.10 In this study, all patients thus received a double induction regimen, either standard or reinforced with HDAC for the second cycle. Timed-sequential therapy was initially designed and used by Burke and colleagues11-14 and further adapted by Mitus and colleagues15 and Wood and colleagues.16 We previously have reported the good results associated with the EMA timed-sequential regimen when used as salvage treatment in patients with refractory/relapsing AML or in combination with all-trans retinoic acid in those with relapsing acute promyelocytic leukemia.22,23,31 We also have demonstrated in patients with previously treated AML that the addition of granulocyte-macrophage colony-stimulating factor (GM-CSF) to EMA chemotherapy actually enhances the in vivo recruitment of quiescent leukemic blasts into the cell cycle, resulting in a trend to a longer time to progression in a randomized study.32,33 In the present study, both double induction and timed-sequential induction were prospectively randomly compared to the control “3 + 7” induction.
Intensified induction strategies were not associated with a significantly higher treatment-related mortality. Even if the salvage course was more frequently needed in patients from the control arm than in those from intensified arms, there were no differences in either induction death or treatment-related induction death rates among the 3 induction arms. In patients achieving a CR, postremission treatment–related mortality was not significantly higher after intensified induction than after “3 + 7” induction, either in patients who were allocated to receive postremission consolidation chemotherapy or in those who received allogeneic BMT in first CR. Approximately 75% of the patients allocated to receive postremission chemotherapy actually received the entire planned treatment. This proportion compares favorably with studies testing autologous stem cell transplantation in first CR.
Long-term outcome was similar in the 3 arms for the entire group of randomized patients. However, as a dosage stratification was planned for consolidation chemotherapy for patients older than 50 years, we performed a subgroup analysis according to 2 age subgroups (younger and older than 50 years). In patients younger than 50 years, the cumulative incidence of relapse as first event was estimated at 55.9% at 5 years for patients in arm A versus 47.9% and 39.3% for patients in arms B and C, respectively, and the difference between the timed-sequential induction arm and the control “3 + 7” arm reached the statistical significance level. As cumulative dosages of DNR, MTZ, and AraC were similar in both arms B and C, the difference in administration schedules may be critical. This also has been observed in the pediatric study from the Children's Cancer Group, which showed a better outcome when the second part of induction chemotherapy was administered at day 10 rather than at day 14 or later.16,34
As in many studies evaluating intensified regimens, the benefit of timed-sequential induction was observed only in younger patients. In the Australian study testing the addition of 75 mg/m2/d etoposide for 7 days to a standard “3 + 7” regimen, a significantly longer overall survival was observed only in patients younger than 55 years.6 In the Cancer and Leukemia Group B (CALGB) study evaluating the role of HDAC as consolidation treatment, a benefit was reported only for patients younger than 60.35 Finally, in the study published by Bishop and colleagues on the value of HDAC in induction, younger patients fared better than older patients with intensive arm.7
Given these results, the ongoing ALFA-9802 study was initiated in 1998 in younger adults with AML (younger than 50). In this study, patients were randomized at baseline to receive either a timed-sequential induction regimen similar to the arm C of the ALFA-9000 study either with or without GM-CSF administered from day 1 to day 10 of the induction chemotherapy. Patients reaching the first CR after induction or induction/salvage and not eligible for allogeneic BMT in first CR were then randomized to compare the ALFA-9000 to the CALGB postremission chemotherapy, which includes 4 cycles of HDAC followed by 4 additional DNR/AraC courses.
Finally, we report here that CNS prophylaxis, including IT chemotherapy and cranial irradiation, could reduce the incidence of CNS relapses in patients with AML-M4, AML-M5, or high-count AML. This effect is particularly marked in AML-M4eo patients and leads us to recommend CNS prophylaxis in these patients. However, it must be stressed that this result was observed in patients not receiving HDAC. The only study having randomly evaluated the role of prophylactic IT therapy was conducted by the SWOG in a population of 289 patients with AML of all FAB subtypes after CR achievement. No difference in CNS disease incidence was observed between patients receiving IT therapy or not.36
Appendix
Participating investigators from the ALFA were S. Castaigne, M. T. Daniel, L. Degos, H. Dombret, E. Gluckman, J. M. Micléa (Hôpital Saint-Louis, Paris); E. Archimbault, C. Charrin, D. Fière, X. Thomas (Hôpital Edouard Herriot, Lyon); F. Bauters, P. Fenaux, J. P. Jouet, C. Preudhomme (Centre Hospitalier-Universitaire [CHU], Lille); H. Tilly, C. Bastard, A. Stamatoullas-Bastard (Centre Henri Becquerel, Rouen); G. Auzanneau, T. de Revel, G. Nedellec (Hôpital du Val de Grâce, Paris); D. Bordessoule (CHU, Limoges); R. Leblay, B. Grosbois (Hôpital Sud, Rennes); G. Tertian (Hôpital Antoine Béclère, Clamart); J. M. Zini, E. Dupuy (Hôpital Lariboisière, Paris); M. Janvier, F. Turpin (Centre René Huguenin, Saint-Cloud); M. Renoux, F. Bauduer (Centre Hospitalier de la Côte Basque, Bayonne); J. Jaubert (Hôpital Saint-Anne, Toulon); B. Dupriez, P. Morel (Centre Hospitalier, Lens); M. Simon (Centre Hospitalier, Valenciennes); M. Legros (Centre Jean Perrin, Clermont-Ferrand); and M. Schoenwald (Hôpital La Source, Orléans).
Prepublished online as Blood First Edition Paper, May 13, 2004; DOI 10.1182/blood-2003-10-3561.
Eric Archimbaud died on March 25, 1998.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.