Abstract
In immortal cells, the existence of a mechanism for the maintenance of telomere length is critical. In most cases this is achieved by the reactivation of telomerase, a cellular reverse transcriptase that prevents telomere shortening. Here we report that the telomerase gene (hTERT) promoter is up-regulated during transmission of human T-cell lymphotropic virus type-I (HTLV-I) to primary T cells in vitro and in ex vivo adult T-cell leukemia/lymphoma (ATLL) samples, but not asymptomatic carriers. Although Tax impaired induction of human telomerase reverse transcriptase (hTERT) mRNA in response to mitogenic stimulation, transduction of Tax into primary lymphocytes was sufficient to activate and maintain telomerase expression and telomere length when cultured in the absence of any exogenous stimulation. Transient transfection assays revealed that Tax stimulates the hTERT promoter through the nuclear factor κB (NF-κB) pathway. Consistently, Tax mutants inactive for NF-κB activation could not activate the hTERT or sustain telomere length in transduced primary lymphocytes. Analysis of the hTERT promoter occupancy in vivo using chromatin immunoprecipitation assays suggested that an increased binding of c-Myc and Sp1 is involved in the NF-κB–mediated activation of the hTERT promoter. This study establishes the role of Tax in regulation of telomerase expression, which may cooperate with other functions of Tax to promote HTLV-I–associated adult T-cell leukemia.
Introduction
The human T-cell leukemia virus type I (HTLV-I) infects and transforms human CD4 T cells in vitro and in vivo causing adult T-cell leukemia/lymphoma (ATLL).1 HTLV-I–mediated T-cell transformation presumably arises from a multistep oncogenic process in which the virus induces chronic T-cell proliferation resulting in accumulation of genetic defects and deregulated cell growth. While it is not yet fully understood how HTLV-I engenders ATLL, several lines of evidence have established that Tax plays a central role in the immortalization of HTLV-I–infected cells in vitro. Tax's effect on G1/S cell cycle checkpoints is decisive in stimulating cellular growth and in concert with an increased cellular proliferation; Tax represses the expression of the DNA β-polymerase, interferes with the host DNA repair machinery, and inactivates the mitotic arrest defective (MAD1) protein thereby increasing mutation rate and possibly genetic rearrangements in infected cells.2-9
The lack of proofreading and subsequent high error rates of reverse-transcriptase enzymes have been linked to the diversity observed in retroviruses. However, HTLV-I is unusual in that the observed genetic variability among isolates is extremely low.10 Therefore it has been postulated that HTLV-I provirus replication occurs mainly with the high-fidelity process of cellular DNA replication during cell division.10 ATLL disease progression is intimately linked to the proviral load, which in turn depends on long-term survival and proliferation of the infected cells. To efficiently achieve clonal expansion, HTLV-I–infected cells must acquire an extended life span while avoiding clearance by the host immune response. Increased longevity may be related to an increased expression of antiapoptotic Bcl-2 and Bcl-xL in HTLV-I–transformed T cells in vitro and of Bcl-xL in uncultured peripheral blood mononuclear cells (PBMCs) isolated from ATLL patients.11,12 Escape from immune defenses may result from a combinatorial effect of the regulatory proteins p30 and p12 that reduces viral expression and down-regulates major histocompatibility complex (MHC) expression, respectively.13,14
Yet to achieve clonal expansion, HTLV-I–infected cells must also acquire an increased capacity to replicate DNA. Because DNA polymerase is unable to replicate the very end of linear DNA, every replication cycle leads to a progressive shortening of the telomeric ends and limited proliferative capacity of normal cells termed “replicative senescence.” Activation of human telomerase, an RNA-dependent DNA polymerase that elongates telomeres through expression of its catalytic subunit hTERT (human telomerase reverse transcriptase), has been proposed as a mechanism for avoiding telomere shortening. Consistent with this model, most cancer cells have detectable telomerase activity, as opposed to normal somatic cells.15-17 In some cases, immortalized cells do not have any detectable telomerase activity in spite of long telomere length, suggesting the existence of an alternative mechanism referred to as ALT.18
Consistent with their transformed phenotype, several studies have reported that HTLV-I–infected cells present an elevated telomerase activity.19-21 It is, however, not possible to conclude as to whether HTLV-I plays a direct role in this process. A recent report suggests that HTLV-I Tax may repress transcription from the hTERT promoter and inhibit telomerase activity.22 Yet, such a function of Tax is difficult to reconcile with its capacity to immortalize T cells and prompts us to investigate the transcriptional regulation of hTERT in HTLV-I– and Tax-expressing human T cells. In this report we confirm the observation made by Gabet et al22 that Tax impairs hTERT mRNA expression when Tax-expressing cells are subject to mitogenic stimulation. However, in the absence of exogenous stimulation we found that Tax stimulates the hTERT promoter through the nuclear factor κB (NF-κB) pathway. We propose that in response to antigen stimulation, interference of Tax with the full induction of hTERT expression may result in a transient genetic instability during mitosis. Once the mitogenic effect has vanished, Tax-mediated activation of hTERT gene expression offers a long-term proliferative advantage to these cells that have acquired chromosomal abnormalities, increasing their chances to repeat this cycle. Successive repetition of transient proliferation and stabilization phase may be required for the development of adult T-cell leukemia.
Materials and methods
Plasmids
Expression and luciferase reporter constructs used in this study have been previously reported: Tax and Tax mutants M47, M22,23 and G148V23 were subcloned into the pHRCMV vector; hTERT luciferase reporter constructs P-1009 and P-33024,25 ; pHRCMV, VSV-G, and pDLN626 ; and I-κBαm and RelA were obtained from Dr W. C. Greene27,28 ; c-Rel and Rel B were obtained from Dr N. Rice.
Cell lines
The human 293T cell line was maintained in Dulbecco modified Eagle medium (DMEM) complete media supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT). HTLV-I–transformed T-cell lines MT-2 and HUT102 were cultivated in RPMI 1640 with 10% FBS. Primary peripheral blood mononuclear cells from healthy volunteers were purified by Ficoll-Hypaque gradient (Hyclone) and used without or after phytohemagglutinin (PHA) stimulation as indicated in the figure legends. Lethal irradiation of HTLV-I virus-producing MT-2 cell line was carried out at 100 Gy. Coculture was initiated with a ratio of 1:20 (MT-2/PBMCs) and maintained in RPMI medium with 10% FBS and 50 units of interleukin-2 (IL-2)/mL. RNAs were extracted from uncultured peripheral blood mononuclear cells (PBMCs) obtained after informed consent.
Telomeric repeat amplification protocol (TRAP) assays
TRAP assays were performed using the TRAPeze telomerase detection kit according to manufacturer's instructions (Intergen, Purchase, NY). Exponentially growing MT-2, MU04, or PBMCs were lysed in CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid) buffer and 125 ng (5 μL) was used per reaction. Results are representative of 2 independent experiments using different cellular extracts. Extension was performed for 30 minutes at 30° C; Taq polymerase was then added to each sample and polymerase chain reaction (PCR) amplification performed as follows: 30 cycles at 94° C for 30 seconds; 59° C for 30 seconds; and 72° C for 15 seconds. Telomeric products were separated on 8% Tris boric acid EDTA (TBE) gel and stained with SYBR Green.
Telomere length assay
Telomere length was estimated using the Telomere length assay (Roche Molecular, Indianapolis, IN) following the manufacturer's instructions. Briefly, high-molecular-weight genomic DNA was isolated from HTLV-I–transformed cells and short-term cultures of PBMCs and Tax-transduced primary T cells. Genomic DNAs including control DNA for high or low telomere lengths, provided by the manufacturer, were digested with HinfI and RsaI restriction enzymes for 3 hours at 37° C. Digested DNA was separated by agarose gel electrophoresis and transferred by capillarity onto Hybond+ nylon membrane (Amersham, Arlington Heights, IL). Membrane was incubated in blocking solution and hybridized with a telomere probe provided by the manufacturer, washed, and revealed using chemiluminescence (CDP Star; Roche Molecular).
Real-time polymerase chain reaction amplification
Total RNA (2 μg) was reverse transcribed using Superscript first-strand synthesis system for reverse transcription (RT)–PCR (Invitrogen Life Technologies, Frederick, MD). PCR reactions contained the first-strand cDNA corresponding to 25 ng RNA, TaqMan universal PCR master mix (Applied Biosystems, Foster City, CA), TaqMan predeveloped assay reagents for human TERT (FAM/MGB probe; Applied Biosystems, catalog no. 4319447F), and for human β-2-microglobulin (B2M) endogenous control (VIC/TAMRA probe; Applied Biosystems, catalog no. 4310886E). Real-time detection of PCR products was performed by ABI PRISM 7700 Sequence Detector (Applied Biosystems). Reactions were performed in triplicate for each sample. Based on Ct (cycle threshold) values from TERT and B2M detections, normalized TERT expression (TERT/B2M) was calculated using the ΔΔCt method according to the supplier's protocol (protocol no. 4310255B and User Bulletin no. 4303859B at Applied Biosystems).
Transfections, luciferase assays, and Western blots
Transfections were carried out using the Effectene reagent (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The amount of DNA transfected is as indicated in the figure legends. Transfection efficiencies and potential toxicity were normalized by cotransfection of a Renilla expression vector (RL-TK; Promega, Madison, WI). Assays were performed using the Dual Luciferase Reporter assay (Promega) and a Berthold Junior luminometer (Oak Ridge, TN). Results are representative of 2 independent sets of experiments. For lentivirus transduction, Tax and green fluorescent protein (GFP) coding sequences were cloned into the HIV-based lentivirus pHRCMV between BamHI and XhoI, and pseudotype viruses were produced as previously reported.29 Western blots were performed using 40 μg protein lysates. Gag protein–specific p24 antibody was given by Dr G. Franchini (National Cancer Institute [NCI]), Tax mouse monoclonal antibody was a gift from Dr J. N. Brady (NCI), rabbit antiserum directed against the HTLV-I envelope was given by Dr C. Pique (Hôpital St-Louis), and β-tubulin antibody was purchased from Roche Molecular. All secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunofluorescence
Cells were fixed with 3.5% paraformaldehyde at room temperature for 10 minutes, washed with phosphate-buffered saline (PBS), and permeabilized 3 minutes at room temperature with 0.5% Triton X-100 in PBS. Rabbit anti-Tax primary antibody was obtained from the National Institutes of Health (NIH) AIDS reagent program and used at 1:500 dilution for 2 hours at room temperature in PBS. Secondary antibodies and antirabbit tetramethylrhodamine isothiocyanate (TRITC) were diluted in PBS 1:200 and incubated for one hour at room temperature in the dark. Slides were mounted with slow fade (Molecular Probes, Eugene, OR) containing DAPI (4,6 diamidino-2-phenylindole) staining reagent.
Images were captured using a Nikon EFD3 microscope (Boyce Scientific, St Louis, MO) and Nikon camera with an Eplan 100× (160/0.17) objective. Imaging medium Slowfade was from Molecular Probes (Eugene, OR). Acquisition software, Image-ProExpress version IV, was from Media Cybernetics (Silver Spring, MD).
Chromatin immunoprecipitation (ChIP) assay
Cells were fixed with formaldehyde at a final concentration of 1% vol/vol for 10 minutes at room temperature, and glycine (final concentration, 0.125 M) was added to stop cross-linking. Pellets of the fixed cells were resuspended at 5 × 106 cells/mL in sodium dodecyl sulfate (SDS) lysis buffer (1% SDS/10 mM EDTA [ethylenediaminetetraacetic acid]/50 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 8.1/protease inhibitor cocktail; Roche Diagnostics, Mannheim, Germany) and sonicated on ice 6 times (30 seconds each) to obtain soluble chromatin (with average DNA size of ∼ 600 bp). Each ChIP reaction contained the chromatin preparation from 1 × 106 cells. Preclearing of the chromatin samples, immunoprecipitation, washing of the immune complex, and recovery of DNA were carried out using the ChIP assay kit (Upstate Biotechnology, Lake Placid, NY) according to the supplier's protocol. Following immunoprecipitation, the recovered DNA was used in PCR reaction to amplify the 159-bp region of the hTERT promoter with the Takara LA PCR kit version 2.1 (Takara Mirus Bio, Madison, WI) and the following primers: 5′-TGCCCCTTCACCTTCCAG-3′ and 5′-CAGCGCTGCCTGAAACTC-3′.30 The PCR reactions were processed through 32 cycles of 98° C for 10 seconds, 60° C for 10 seconds, and 72° C for 15 seconds. As a positive control locus for NF-κB p65 binding, the promoter region of the IL-8 gene was amplified with 5′-GGGCCATCAGTTGCAAATC-3′ and 5′-TTCCTTCCGGTGGTTTCTTC-3′.31 DNA samples recovered from chromatin samples before immunoprecipitation, which correspond to 1% or 10% of chromatin samples included in each immunoprecipitation reaction, were also PCR amplified as loading controls (input 1% or input 10%). The PCR products were run on a 10% polyacrylamide gel, stained with SYBR Green (BioWhittaker Molecular Applications, Rockland, ME), and detected using the Typhoon system (Molecular Dynamics, Sunnyvale, CA). For quantitative analysis of Sp1 and c-Myc proteins, the ImageQuant version 5.2 software (Molecular Dynamics) was used.
Results
In vitro transmission of HTLV-I to primary T cells activates the hTERT gene promoter
Reactivation of the hTERT promoter is frequent in most tumor cells, while in some cases (10%), telomerase-negative tumor cells have acquired an alternative pathway (ALT) responsible for continuous proliferation and maintenance of the transformed phenotype. Real-time PCR analyses indicated that in HTLV-I–transformed cell lines MT-2 and HUT102, hTERT mRNA expression can be detected (Figure 1A and data not shown). However, because MT-2 and HUT102 cell lines have been transformed in vitro it raises the possibility that genetic alterations rather than the presence of HTLV-I are responsible for hTERT mRNA expression in these cell lines. To assess whether HTLV-I is capable of activating expression of the endogenous hTERT gene, we initiated a coculture of lethally irradiated MT-2 virus-producing cells with primary PBMCs from an HTLV-I–negative donor. Relative amounts of hTERT mRNA were investigated by real-time PCR at different time points. In these experiments, irradiated MT-2 cells died in the second week. Because hTERT mRNA can readily be detected in the coculture but not in PBMC controls after 5 weeks, this result indicates that transmission of HTLV-I to the primary PBMCs activates and maintains hTERT gene expression (Figure 1A).
HTLV-I–associated up-regulation of hTERT mRNA expression. (A) Expression of hTERT mRNA was investigated by real-time RT-PCR at time 0 and at 3, 5, 7, and 12 weeks after coculture of PHA-stimulated PBMCs with HTLV-I–transformed lethally irradiated MT-2 cells (100 Gy). Human β-2-microglobulin was used as endogenous control. Reactions were in triplicate for each sample. Experimental variations are indicated by error bars. The expression level in nonactivated PBMCs was defined as 1.0 and the expression levels were expressed as fold induction relative to the PBMCs. Expression of hTERT mRNA in MT-2 is shown for comparison. (B) Western blot analysis of viral protein expression in MT-2–derived cocultured MU04 cell line and MT-2 control. (C) Telomeric amplification protocol (TRAP) assay was performed as described in “Materials and methods” using 125 ng protein lysate in CHAPS buffer. IC indicates internal 36-bp control. Quantification of telomerase activity, telomerase generated product (TGP) was calculated according to the standard formula32 : TGP (units) = {[(X – X0)/C]/[R – X0/CR]} ×100. X indicates telomerase amplification signal of sample; X0, background; C, internal control signal of sample; R, telomerase amplification signal of TSR8 positive control (1 μL = 0.1 amole); and CR, internal control signal of TSR8. It is normal that the intensity of the internal control (IC) is very low in the TSR8 reaction, however, this was previously demonstrated not to affect quantification as linear range of this assay is 0.001 amole to 10 amole.32 In our assay, 0.1 amole TSR8 was used and corresponds to 100% of TGP.
HTLV-I–associated up-regulation of hTERT mRNA expression. (A) Expression of hTERT mRNA was investigated by real-time RT-PCR at time 0 and at 3, 5, 7, and 12 weeks after coculture of PHA-stimulated PBMCs with HTLV-I–transformed lethally irradiated MT-2 cells (100 Gy). Human β-2-microglobulin was used as endogenous control. Reactions were in triplicate for each sample. Experimental variations are indicated by error bars. The expression level in nonactivated PBMCs was defined as 1.0 and the expression levels were expressed as fold induction relative to the PBMCs. Expression of hTERT mRNA in MT-2 is shown for comparison. (B) Western blot analysis of viral protein expression in MT-2–derived cocultured MU04 cell line and MT-2 control. (C) Telomeric amplification protocol (TRAP) assay was performed as described in “Materials and methods” using 125 ng protein lysate in CHAPS buffer. IC indicates internal 36-bp control. Quantification of telomerase activity, telomerase generated product (TGP) was calculated according to the standard formula32 : TGP (units) = {[(X – X0)/C]/[R – X0/CR]} ×100. X indicates telomerase amplification signal of sample; X0, background; C, internal control signal of sample; R, telomerase amplification signal of TSR8 positive control (1 μL = 0.1 amole); and CR, internal control signal of TSR8. It is normal that the intensity of the internal control (IC) is very low in the TSR8 reaction, however, this was previously demonstrated not to affect quantification as linear range of this assay is 0.001 amole to 10 amole.32 In our assay, 0.1 amole TSR8 was used and corresponds to 100% of TGP.
The decreased hTERT mRNA observed around 2 months is in agreement with previous data also showing a decrease in telomerase activity by TRAP assay at 60 days in HTLV-I coculture assay.33 This coincides with a crisis period, in which many HTLV-I–infected cells died and a selected population outgrows to become immortalized; in fact, at 12 weeks after coculture hTERT mRNA is still expressed in MU04 cells. Western blot analyses confirmed the expression of HTLV-I viral genes in the MU04-derived cell line (Figure 1B).
Levels of hTERT mRNA were slightly lower in MU04 when compared with MT-2 (Figure 1A), despite higher levels of Tax expression in MT-2 (Figure 1B). Finally, telomerase activity in MT-2 and MU04 was measured by the telomeric amplification protocol (TRAP) assay, and consistent with hTERT mRNA expression levels, MT-2 showed higher telomerase activity (Figure 1C).
Tax differentially modulates hTERT mRNA expression in primary T cells in response to T-cell stimulation
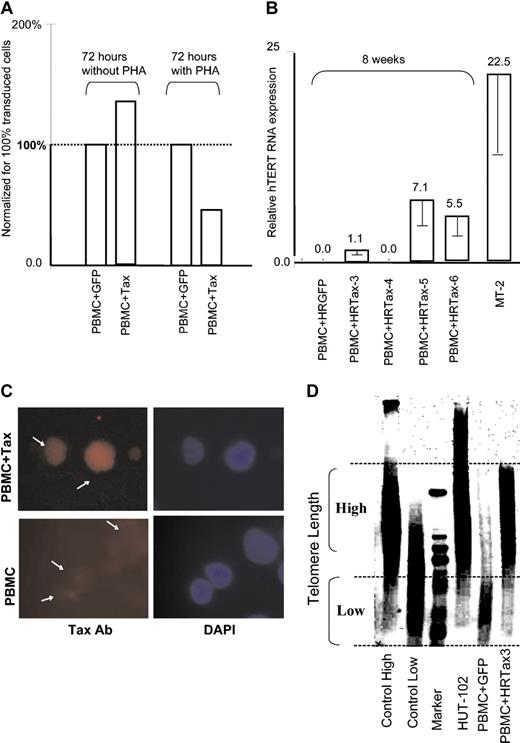
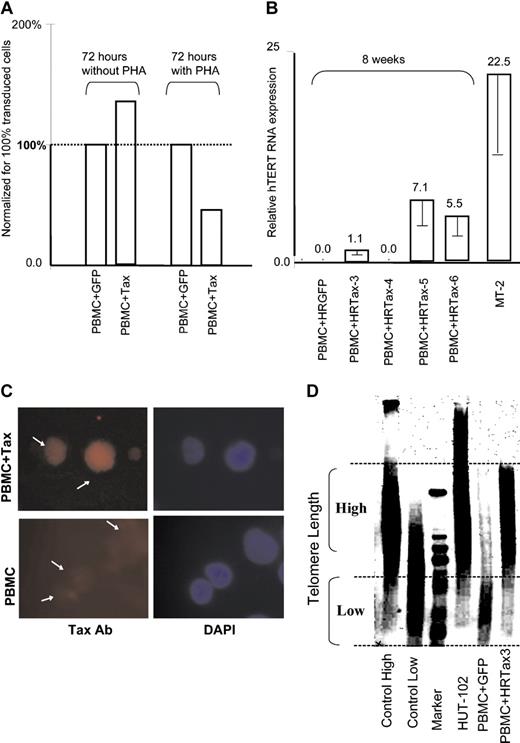
We next investigated the ability of Tax to modulate expression of the telomerase gene in human primary T cells, the natural target of the virus. The tax coding sequence was cloned into the HRCMV lentivirus vector, and pseudotype virus particles were produced to transduce the tax gene into primary T cells. Transduced T cells were then cultivated in the presence or absence of PHA in 20% serum media with IL-2 for 72 hours. In these experiments, our transduction efficiency estimated by observation of GFP-transduced PBMCs was approximately 40%. Total RNAs were extracted and analyzed by real-time PCR for expression of hTERT mRNA, and results were normalized to 100% transduced cells to compensate for nontransduced cells. Consistent with the recent report from Gabet et al,22 we found that in the presence of PHA, hTERT mRNA was decreased about 50% in PBMCs transduced by Tax (Figure 2A). However, in the absence of PHA stimulation, hTERT mRNA was increased by 25% in the presence of Tax (Figure 2A).
Expression of hTERT mRNA in Tax-expressing T cells. (A) Quantitative real-time RT-PCR of hTERT mRNA expression in primary T cells transduced by Tax at 72 hours in the presence or absence of PHA. Results were normalized to represent 100% of transduced cells. (B) Quantitative real-time RT-PCR of hTERT mRNA expression in primary T cells transduced by Tax after 8 weeks of culture in IL-2–supplemented media without stimulation; expression of hTERT in MT-2 cells is given for reference. Experimental variations are indicated by error bars. As shown in Figure 1A, the expression level in nonactivated cultured PBMCs was defined as 1.0. (C) Immunofluorescence of Tax expression in transduced T cells using a mouse monoclonal antibody (Ab); untransduced PBMCs are shown as negative control (bottom panel) and DAPI stain (right). Experimental variations are indicated by error bars. (D) Southern blot analysis of telomere length in HTLV-I–transformed HUT-102 cell line, Tax-transduced PBMCs, and control GFP-transduced PBMCs after 10 weeks of culture in RPMI 1640, 20% FBS, and IL-2. Positive and negative controls were provided by the kit (Intergen).
Expression of hTERT mRNA in Tax-expressing T cells. (A) Quantitative real-time RT-PCR of hTERT mRNA expression in primary T cells transduced by Tax at 72 hours in the presence or absence of PHA. Results were normalized to represent 100% of transduced cells. (B) Quantitative real-time RT-PCR of hTERT mRNA expression in primary T cells transduced by Tax after 8 weeks of culture in IL-2–supplemented media without stimulation; expression of hTERT in MT-2 cells is given for reference. Experimental variations are indicated by error bars. As shown in Figure 1A, the expression level in nonactivated cultured PBMCs was defined as 1.0. (C) Immunofluorescence of Tax expression in transduced T cells using a mouse monoclonal antibody (Ab); untransduced PBMCs are shown as negative control (bottom panel) and DAPI stain (right). Experimental variations are indicated by error bars. (D) Southern blot analysis of telomere length in HTLV-I–transformed HUT-102 cell line, Tax-transduced PBMCs, and control GFP-transduced PBMCs after 10 weeks of culture in RPMI 1640, 20% FBS, and IL-2. Positive and negative controls were provided by the kit (Intergen).
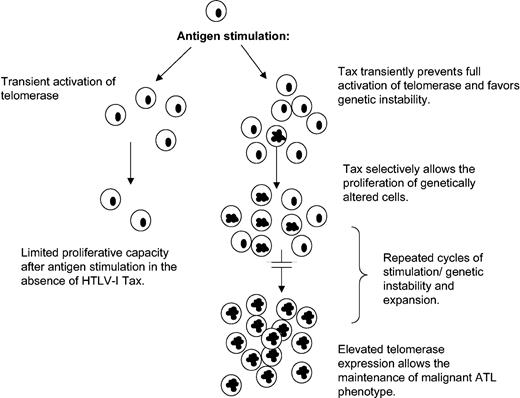
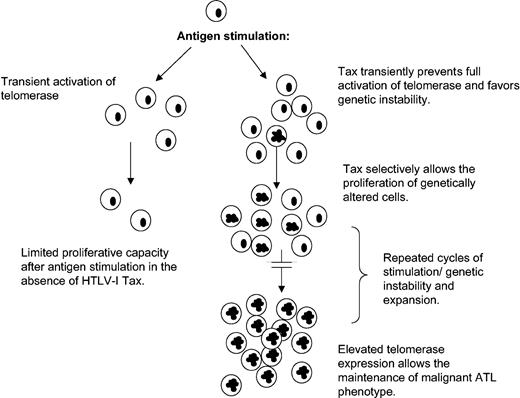
This paradoxical effect of Tax on hTERT regulation may give HTLV-I a proliferative advantage by transiently favoring genetic instability (Figure 6; see “Discussion”).
Model for HTLV-I–induced chromosomal instability and leukemogenesis.
When Tax-transduced T cells were maintained longer (8 weeks) in culture, in the presence of IL-2, hTERT mRNA expression was detected in 3 of 4 PBMC cultures but not in 2 GFP controls, further suggesting a role of Tax in hTERT expression (Figure 2B). The fact that no hTERT mRNA could be detected in HRTax4 cells after 8 weeks in culture may suggest that this cell line has acquired an alternative pathway (ALT) or may simply not become immortalized. These possibilities remain to be investigated. After 10 weeks in culture, telomere length was evaluated in Tax-transduced T cells and GFP-transduced control cells. Tax expression was confirmed by indirect immunofluorescence (Figure 2C). Genomic DNA was extracted and analyzed by Southern blot using the Telomere length assay. In agreement with the real-time RT-PCR, Tax-expressing T cells sustained long telomeres as opposed to their GFP-transduced PBMC counterparts in which telomeres were significantly shorter (Figure 2D). This finding is underscored by the fact that Tax-expressing T cells grew faster than their GFP-transduced counter-parts and therefore had replicated their DNA more often. As expected, long telomeres were also detected in the HTLV-I–transformed cell line HUT 102 (Figure 2D).
HTLV-I Tax increases transcription of the hTERT promoter through activation of the NF-κB pathway
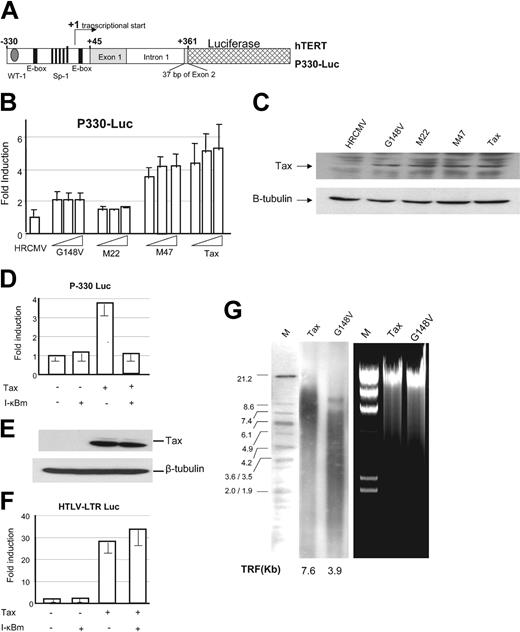
Initial studies reported that the hTERT proximal core promoter essential for its transcriptional regulation was located between nucleotides –188 and +78.34 In a separate study, the core promoter region of hTERT was found to extend from –330 to +361. We obtained 2 hTERT promoter luciferase constructs spanning the region –330 to +361, and previously described as P-330 and P-1009, respectively.24,25 These reporter vectors encompass the first exon, the first intron plus 37 bases of exon 2 of the hTERT gene (Figure 3A).
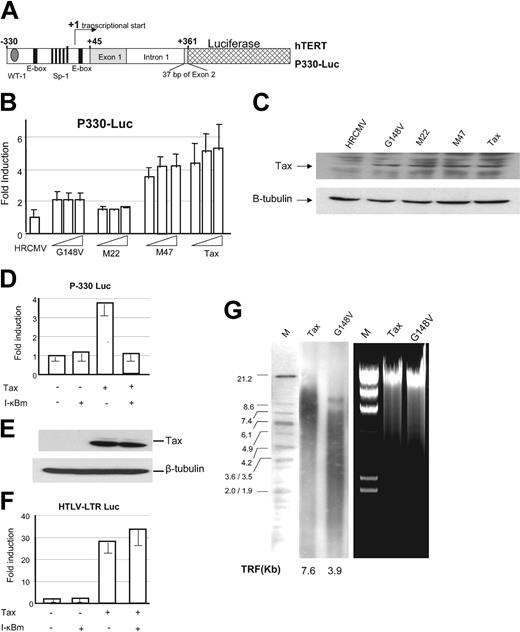
NF-κB–mediated activation of the hTERT promoter. (A) Schematic representation of the hTERT luciferase reporter construct P-330. (B) P-330 (1 μg) was transfected into 293 T cells in the absence (HRCMV, empty vector) or in the presence of increasing amounts of cytomegalovirus (CMV) Tax or CMV Tax mutants M22, G148V, and M47 (0.1, 0.3, and 0.6 μg) and TK-Renilla (60 ng) using the Effectene Reagent (Qiagen). Dual luciferase assay was performed 36 hours later. Normalized results using Renilla values are representative of 2 independent experiments. Experimental variations are indicated by error bars. (C) Western blot analysis for relative expression of Tax and Tax mutants in transfected 293 T cells. (D) P-330 (1 μg) was transfected into 293T cells in the absence or presence of Tax (0.25 μg) with or without I-κBα mutant (50 ng), and luciferase assays were performed as described in “Materials and methods.” (E) Tax expression, detected by Western blot, was not affected by I-κBα mutant expression. (F) HTLV-I–LTR luciferase (1 μg) was transfected into 293T cells in the absence or presence of Tax (0.25 μg) with or without I-κBα mutant (50 ng), and luciferase assays were performed as described in “Materials and methods.” Experimental variations are indicated by error bars. (G) Southern blot analysis of telomere length in Tax-transduced PBMCs and G148V-transduced PBMCs after 10 weeks of culture in RPMI 1640, 20% FBS, and IL-2. Ethidium bromide gel showing high–molecular weight DNA used in telomere length analysis. TRF indicates telomere restriction length fragments.
NF-κB–mediated activation of the hTERT promoter. (A) Schematic representation of the hTERT luciferase reporter construct P-330. (B) P-330 (1 μg) was transfected into 293 T cells in the absence (HRCMV, empty vector) or in the presence of increasing amounts of cytomegalovirus (CMV) Tax or CMV Tax mutants M22, G148V, and M47 (0.1, 0.3, and 0.6 μg) and TK-Renilla (60 ng) using the Effectene Reagent (Qiagen). Dual luciferase assay was performed 36 hours later. Normalized results using Renilla values are representative of 2 independent experiments. Experimental variations are indicated by error bars. (C) Western blot analysis for relative expression of Tax and Tax mutants in transfected 293 T cells. (D) P-330 (1 μg) was transfected into 293T cells in the absence or presence of Tax (0.25 μg) with or without I-κBα mutant (50 ng), and luciferase assays were performed as described in “Materials and methods.” (E) Tax expression, detected by Western blot, was not affected by I-κBα mutant expression. (F) HTLV-I–LTR luciferase (1 μg) was transfected into 293T cells in the absence or presence of Tax (0.25 μg) with or without I-κBα mutant (50 ng), and luciferase assays were performed as described in “Materials and methods.” Experimental variations are indicated by error bars. (G) Southern blot analysis of telomere length in Tax-transduced PBMCs and G148V-transduced PBMCs after 10 weeks of culture in RPMI 1640, 20% FBS, and IL-2. Ethidium bromide gel showing high–molecular weight DNA used in telomere length analysis. TRF indicates telomere restriction length fragments.
Consistent with previous studies, decreased basal transcription from the hTERT promoter was observed when downstream sequences of the transcription start site were present (not shown). When hTERT luciferase vector P-330 was tested in transient transfection assays, we found that Tax stimulated transcription (Figure 3B,D). To further investigate the mechanism by which Tax stimulated transcription from the hTERT core proximal promoter, we used several Tax mutants previously characterized and selectively inactivated for their ability to activate transcription through the NF-κB M22, G148V, or the CREB pathway, M47, respectively.23,27 P-330 hTERT promoter luciferase reporter construct was transfected into 293T cells along with a dose increase of Tax or Tax mutants M22, G148V or M47, and TK-Renilla. Results from these experiments suggested that Tax-mediated NF-κB activation was essential for hTERT promoter activation since Tax mutants M22 and G148V were not able to activate the P-330 construct compared with wild-type Tax or M47 (Figure 3B), although Tax and Tax mutants expressed to similar levels (Figure 3C).
To further confirm these findings, Tax was expressed in the presence or in the absence of a nondegradable form of I-κBα that potently and specifically inhibits Tax-mediated NF-κB activation. In the presence of the nondegradable form of I-κBα, Tax was unable to activate the hTERT proximal promoter P-330 thereby indicating that Tax-mediated NF-κB activation is required for the transcriptional activation of the P-330 hTERT proximal promoter (Figure 3D). In these experiments I-κBαm did not alter Tax expression levels (Figure 3E) or Tax transcriptional activity on the CREB/ATF pathway (Figure 3F). Consistent with these data, telomere length was significantly shorter in G148V than wild-type Tax-transduced lymphocytes after 10 weeks of continuous culture in RPMI and IL-2 (Figure 3G).
Finally, we tested different NF-κB family members RelA, Rel B, and c-Rel for their ability to stimulate transcription of the P-330 in the absence of Tax and found that RelA/p65 had a significant effect, while Rel B and c-Rel were less efficient (not shown).
hTERT promoter occupancy in vivo: increased binding of c-Myc and Sp1 in HTLV- and Tax-expressing but not G148V-expressing T cells
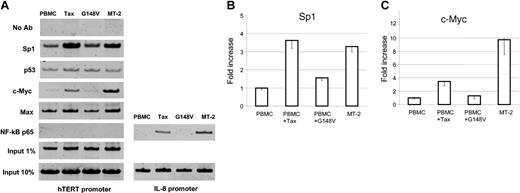
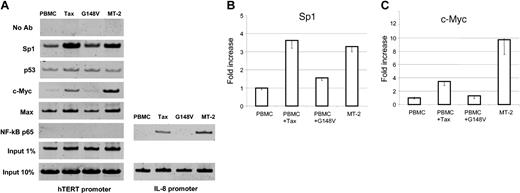
Although the use of promoter reporter assays constitutes a valuable approach to identify potential cis-acting regulatory elements, study of the natural promoter in the chromatin context is essential to reveal active sites accessible to transcription factors. We have found that Tax-mediated NF-κB activation stimulates transcription of hTERT reporter construct P-330 despite the absence of NF-κB consensus binding site in the hTERT promoter region. Importantly, previous studies have shown that Tax-mediated NF-κB activation stimulates c-Myc– and Sp1-dependent transcription.35-37 Because these are 2 well-known transcription factors that can activate the hTERT promoter,38-41 we investigated in vivo hTERT promoter occupancy by chromatin immunoprecipitation (ChIP) assays. These experiments revealed an increased binding of both c-Myc and Sp1 to the hTERT promoter in HTLV-I– and Tax-expressing cells when compared with PBMCs or G148V-transduced PBMCs. We also found that p53 and Max binding to the hTERT promoter was not significantly affected in HTLV-I– or Tax-expressing cells. These results suggest that c-Myc/Max complex might replace Mad1/Max repressor complex at the hTERT promoter in those cells and warrant further investigations. This possibility is consistent with a recent report that a switch from Mad1/Max to c-Myc/Max occurs during cellular transformation.39 In agreement with the absence of a NF-κB consensus binding sequence at the proximal hTERT promoter region and the failure to detect the NF-κB binding in an electrophoretic mobility shift assay,42 we did not observe a direct binding of NF-κB p65 to the hTERT promoter in vivo (Figure 4A), while its presence on the IL-8 promoter was evident in HTLV-I– and Tax-expressing cells but not in PBMCs or G148V-transduced PBMCs (Figure 4A).
hTERT promoter occupancy in vivo by chromatin immunoprecipitation assay. Control PBMCs, wild-type Tax-transduced PBMCs, and mutant Tax (G148V)–transduced PBMCs were cultured in RPMI 1640 supplemented with 20% FBS and 50 U/mL IL-2 for 8 weeks and used as described in “Materials and methods.” The HTLV-I–transformed cell line MT-2 was included. The following antibodies (all from Upstate Biotechnology) were used for immunoprecipitation: anti-Sp1 (sc-59), anti-p53 (sc-126), anti–c-Myc (sc-764), anti-Max (sc-197), and anti–NF-κB p65 (sc-109). Samples with no antibody (No Ab) as negative controls and samples corresponding to 1% or 10% input as loading controls were also PCR amplified. PCR products were run on a 10% polyacrylamide gel and stained with SYBR Green I. (A) The representative results of in vivo protein binding to the hTERT promoter. For NF-κB p65, PCR products from the IL-8 promoter region as a positive control are also shown. (B) Quantitative analysis of the binding of Sp1 (B) and c-Myc (C) proteins to the hTERT promoter. The PCR products from 1% input samples were used to normalize the Sp1 and c-Myc binding in each cell line. The value of PBMCs is defined as 1.0 and those of the other 3 cell lines are expressed relatively. The data (average ± SD) are from 3 independent experiments.
hTERT promoter occupancy in vivo by chromatin immunoprecipitation assay. Control PBMCs, wild-type Tax-transduced PBMCs, and mutant Tax (G148V)–transduced PBMCs were cultured in RPMI 1640 supplemented with 20% FBS and 50 U/mL IL-2 for 8 weeks and used as described in “Materials and methods.” The HTLV-I–transformed cell line MT-2 was included. The following antibodies (all from Upstate Biotechnology) were used for immunoprecipitation: anti-Sp1 (sc-59), anti-p53 (sc-126), anti–c-Myc (sc-764), anti-Max (sc-197), and anti–NF-κB p65 (sc-109). Samples with no antibody (No Ab) as negative controls and samples corresponding to 1% or 10% input as loading controls were also PCR amplified. PCR products were run on a 10% polyacrylamide gel and stained with SYBR Green I. (A) The representative results of in vivo protein binding to the hTERT promoter. For NF-κB p65, PCR products from the IL-8 promoter region as a positive control are also shown. (B) Quantitative analysis of the binding of Sp1 (B) and c-Myc (C) proteins to the hTERT promoter. The PCR products from 1% input samples were used to normalize the Sp1 and c-Myc binding in each cell line. The value of PBMCs is defined as 1.0 and those of the other 3 cell lines are expressed relatively. The data (average ± SD) are from 3 independent experiments.
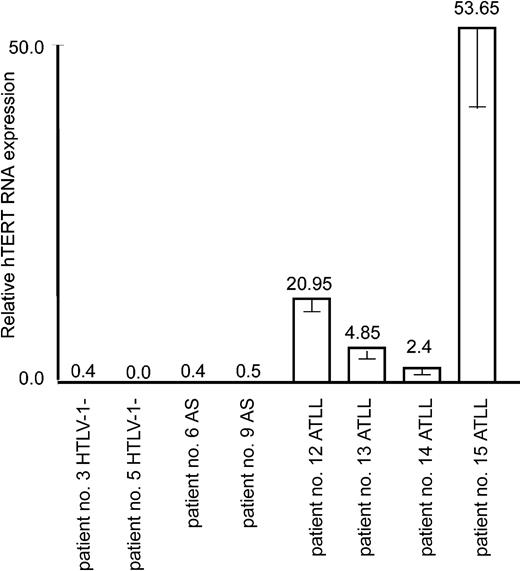
hTERT transcription is up-regulated in ATLL but not in HTLV-I–infected asymptomatic patient samples
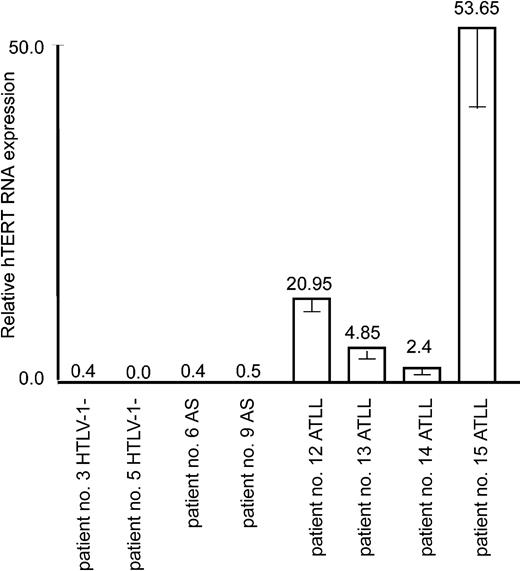
It is well established that leukemicATLL cells derive from a monoclonal expansion of infected T cells and that ATLL patients present a very high proviral load. Despite the fact that Tax is often not detectable in ATLL cells in vivo and perhaps no longer required for the maintenance and proliferative and invasive nature of ATLL cells in vivo, a “Tax-expressing phenotype” has usually been acquired through diverse genetic and epigenetic alterations. For instance, the NF-κB pathway is constitutively activated,43 p53 is stabilized in absence of genetic mutation,44 and antiapoptotic Bcl-xL is overexpressed.12 Consistent with leukemic potential, real-time RT-PCR revealed an increased expression of hTERT mRNA in all ATLL patient samples but not in those of HTLV-I–infected asymptomatic carriers or noninfected individuals (Figure 5).
Up-regulation of the hTERT expression in in vivo ATLL patient samples. Real-time PCR measure of hTERT mRNA expression in 2 donors negative for HTLV-I, 2 asymptomatic carriers (AS), and 4 ATLL patients was performed as described in “Materials and methods.” The expression level in nonactivated cultured PBMCs (shown in Figure 1A) was defined as 1.0. Experimental variations are indicated by error bars.
Up-regulation of the hTERT expression in in vivo ATLL patient samples. Real-time PCR measure of hTERT mRNA expression in 2 donors negative for HTLV-I, 2 asymptomatic carriers (AS), and 4 ATLL patients was performed as described in “Materials and methods.” The expression level in nonactivated cultured PBMCs (shown in Figure 1A) was defined as 1.0. Experimental variations are indicated by error bars.
Discussion
In this study we demonstrate that the hTERT transcription is activated by infection of human primary T cells with HTLV-I. In addition, transduction of primary T cells by Tax-expressing lentiviral vector allows the maintenance of telomerase expression and telomere length as opposed to transduction of GFP or a Tax mutant that cannot activate the NF-κB pathway. In that sense, Tax should be considered as a positive regulator of the telomerase gene. This conclusion differs from the recent report by Gabet et al22 that Tax may act as a transcriptional repressor of the hTERT promoter. We think that this discrepancy may be due to different experimental settings and differences in the hTERT reporter constructs used. In the former study, the effect of Tax on hTERT activity in T cells was performed in the presence of PHA, known to mimic antigen stimulation and CD3 (T-cell receptor [TCR]) signaling. In agreement with Gabet et al,22 when the effect of Tax was studied in the presence of PHA, a decreased expression of the hTERT was detected; however, this was not the case if PHA was omitted. Previous studies have shown that antigen-specific induction of telomerase occurs in vitro through CD3 and CD28 costimulatory signals45 and that telomerase is highly inducible in peripheral lymphocytes following activation through the CD3 or after stimulation with phorbol myristate acetate (PMA)/ionomycin.46 Along those lines, it is important to note that HTLV-I–infected T cells have profound dysfunctions in CD3 responses with down-regulation of TCR, CD45, and Syk/zeta-associated protein 70 (Zap-70) kinase expression.47 Importantly, Tax expression itself is sufficient to down-regulate Zap-70 and Lck.47,48 Thus, through its interference with the CD3 signaling pathway, Tax may lower the effect of PHA stimulation on the hTERT gene activation, thereby leading to a reduced activation of hTERT in stimulated T cells. Our data do not exclude the role of the NF-κB pathway in telomerase activation through posttranslational modification and/or nuclear transport of the hTERT protein,49 which would rather cooperate with the transcriptional activation of the hTERT gene described in this study. It should also be noted that both HTLV-I–positive and –negative leukemia cell lines express hTERT mRNA and that we found no quantitative relationship between the hTERT and Tax expression levels in HTLV-I–expressing cell lines (Figure 1A-B and data not shown). Thus, the NF-κB–mediated activation of the hTERT transcription by Tax represents an important pathway for telomerase activation in leukemogenesis, which likely involves multiple cellular and viral mechanisms.
We propose a model (Figure 6) in which during active T-cell proliferation, as observed in response to antigen or PHA stimulation, interference of Tax with the full hTERT induction may result in a transient genetic instability state. Once the mitogenic effect is passed, Tax-mediated activation of hTERT through NF-κB stabilizes telomeres and promotes long-term proliferation of cells that have acquired chromosomal abnormalities. Several cycles of transient active proliferation and stabilization phase may be required for the development of adult T-cell leukemia. Consistent with this model, chronic stimulation with Strongyloides stercoralis has been linked to higher incidence of ATL development.50
Using promoter reporter assays, we found that Tax can stimulate the hTERT promoter through the NF-κB pathway. Because we did not find any NF-κB consensus binding sites in the hTERT core promoter and p65 was not detected on the hTERT promoter, we think that the effect of NF-κB may be indirect. Several studies have shown that Tax-mediated NF-κB activation potently stimulates expression of c-Myc,37,51,52 a strong transactivator of the hTERT promoter.53-55 Indeed, our results using chromatin immunoprecipitation assays (ChIP) revealed an increased binding of c-Myc to the hTERT promoter in HTLV-I– and Tax-expressing cells but not in cells transduced with a Tax mutant unable to activate the NF-κB pathway. Results from our ChIP assays further indicated that Tax-mediated transcriptional activation of the hTERT promoter may also occur through an increased binding of Sp1, a transactivator of the hTERT promoter.34 Consistent with this hypothesis, several reports have demonstrated that Tax stimulates transcription of various cellular promoters through Sp1,35,36 that Sp1 binding sites can be positively regulated by NF-κB factors,56,57 and that c-Myc and Sp1 cooperatively function in activating the hTERT promoter.58,59 In agreement with the study by Gabet et al,22 when an hTERT promoter luciferase construct that covers from –255 to +40 (pBT-255)60 was used, we found Tax to have a repressive effect that was dependent on the downstream E-box (not shown). These findings illustrate the complexity of hTERT regulation by Tax and suggest that in addition to NF-κB stimulation, acquired mutation and/or inactivation of E-box–mediated repression may further increase telomerase activity in HTLV-I–transformed cells. In fact, E-box–mediated repressive mechanisms are often inactivated in immortal, endogenous telomerase positive cells.60 Genetic and epigenetic changes involved in these additional mechanisms remain to be investigated in HTLV-I–infected cell lines and patient samples.
HTLV-I Tax adversely affects DNA repair4-6 and targets the mitotic arrest defect protein MAD1.7 In addition, Tax aberrantly targets and activates the anaphase promoting complex.9 Consequently, HTLV-I–transformed cells present genomic instability, chromosomal aberrations, and mutation in tumor suppressor genes. Mutation in the tumor suppressor p53 gene is found in approximately 30% of ATLL cases,61 and loss of heterozygosity is associated with increased risk of disease progression in ATLL.62 Wild-type p53 is a negative regulator of hTERT promoter that forms complexes with Sp1 and prevents Sp1-mediated hTERT promoter transcription.63 Surprisingly, mutated p53 still interacts with Sp1 but the resultant mutant p53-Sp1 complexes stimulate gene transcription.64 Whether this is also the case for the hTERT promoter remains to be demonstrated; nonetheless, in addition to stimulating NF-κB, inactivation of p53 and/or selective pressure for HTLV-I–infected cells carrying mutated p53 may play a role in the telomerase reactivation process. In fact, Tax mutants that do not activate the NF-κB pathway cannot antagonize p53 functions and do not immortalize human T cells when expressed in the context of an infectious molecular clone.65 Our results support the current view that Tax-mediated NF-κB activation is critical for initiation and maintenance of HTLV-I–mediated cellular transformation and is a promising target for future therapeutic treatment of ATLL.66-72 Finally, observations made from patient samples suggest that drugs designed to inhibit telomerase expression and/or activity may be effective for the treatment of ATLL, as complete remission has been described in acute ATLL patients after receiving prolonged azidothymidine (AZT)/interferon (IFN) treatment.73,74
Prepublished online as Blood First Edition Paper, June 29, 2004; DOI 10.1182/blood-2003-12-4251.
Supported by a Lied Basic Science Research Grant from the Kansas University Medical Center Research Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. HTLV-I–associated up-regulation of hTERT mRNA expression. (A) Expression of hTERT mRNA was investigated by real-time RT-PCR at time 0 and at 3, 5, 7, and 12 weeks after coculture of PHA-stimulated PBMCs with HTLV-I–transformed lethally irradiated MT-2 cells (100 Gy). Human β-2-microglobulin was used as endogenous control. Reactions were in triplicate for each sample. Experimental variations are indicated by error bars. The expression level in nonactivated PBMCs was defined as 1.0 and the expression levels were expressed as fold induction relative to the PBMCs. Expression of hTERT mRNA in MT-2 is shown for comparison. (B) Western blot analysis of viral protein expression in MT-2–derived cocultured MU04 cell line and MT-2 control. (C) Telomeric amplification protocol (TRAP) assay was performed as described in “Materials and methods” using 125 ng protein lysate in CHAPS buffer. IC indicates internal 36-bp control. Quantification of telomerase activity, telomerase generated product (TGP) was calculated according to the standard formula32: TGP (units) = {[(X – X0)/C]/[R – X0/CR]} ×100. X indicates telomerase amplification signal of sample; X0, background; C, internal control signal of sample; R, telomerase amplification signal of TSR8 positive control (1 μL = 0.1 amole); and CR, internal control signal of TSR8. It is normal that the intensity of the internal control (IC) is very low in the TSR8 reaction, however, this was previously demonstrated not to affect quantification as linear range of this assay is 0.001 amole to 10 amole.32 In our assay, 0.1 amole TSR8 was used and corresponds to 100% of TGP.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/8/10.1182_blood-2003-12-4251/6/m_zh80200468170001.jpeg?Expires=1769188539&Signature=Oa1JVHt4HsBn0ifk-ftpfaoYf3tjDhOk-3u2Y8xvLMisK6jxaGr0iKgdfWpzGLVKuD1NVxWWB2sYbYHrJWVpzrKPF6QFr-ydbyLlHKYhaaiuNaeFhoPqtW31OtWaHSX44UiLy2RRyS~KdJQnVEm1GCEepWOhbTmASZwjkhOExGZ4yEWQ9qw-SCTXz-yo0T7FSEcrrws3ZP17gvxl52APjTorRm1yvjJWR6qH9FsHPXcEfMBrOoFXtsJQprx6LcmIpwLiH8rB8LhAjKSOvN2X2IDtXj4sp0fozo3WUSiejBbwt6bH1hynZxKehyZr8pR1eOIVPngTMLtHC8MpP1gNZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. HTLV-I–associated up-regulation of hTERT mRNA expression. (A) Expression of hTERT mRNA was investigated by real-time RT-PCR at time 0 and at 3, 5, 7, and 12 weeks after coculture of PHA-stimulated PBMCs with HTLV-I–transformed lethally irradiated MT-2 cells (100 Gy). Human β-2-microglobulin was used as endogenous control. Reactions were in triplicate for each sample. Experimental variations are indicated by error bars. The expression level in nonactivated PBMCs was defined as 1.0 and the expression levels were expressed as fold induction relative to the PBMCs. Expression of hTERT mRNA in MT-2 is shown for comparison. (B) Western blot analysis of viral protein expression in MT-2–derived cocultured MU04 cell line and MT-2 control. (C) Telomeric amplification protocol (TRAP) assay was performed as described in “Materials and methods” using 125 ng protein lysate in CHAPS buffer. IC indicates internal 36-bp control. Quantification of telomerase activity, telomerase generated product (TGP) was calculated according to the standard formula32: TGP (units) = {[(X – X0)/C]/[R – X0/CR]} ×100. X indicates telomerase amplification signal of sample; X0, background; C, internal control signal of sample; R, telomerase amplification signal of TSR8 positive control (1 μL = 0.1 amole); and CR, internal control signal of TSR8. It is normal that the intensity of the internal control (IC) is very low in the TSR8 reaction, however, this was previously demonstrated not to affect quantification as linear range of this assay is 0.001 amole to 10 amole.32 In our assay, 0.1 amole TSR8 was used and corresponds to 100% of TGP.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/8/10.1182_blood-2003-12-4251/6/m_zh80200468170001.jpeg?Expires=1769785008&Signature=ZRIJJgm4fOgoof91jiivUbFnHlu7V7fEO2kZHUt6WEDUURw9C1FEc3Im4n5sfrJbaxgSazyay3U~Q0Cl2EnqnxKni0zCI3v5PNmabLcIMvpsZFPM9bHowu2vYGaDIwbWeaJXnfrx5FgEhguv80B0enPbYjemArpPESDpJZ0Hcfib~sbJ~Q7faOYTCI0eEmhvamj4FCkx-K55XoXgLhGglefEV8Roj30iaKoQmhbKIiIHPXP5JbzYLaVAOr72V15bjssW9bYaSmRI4~Alx7Xl5wyT7-ErSZ1RNWAUDLyuFUMSBFmnlF7ZS0ZRy93j9~St4k7hH8cSnl2iMrCYLDU0oA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)