Abstract
We report that chlorogenic acid (Chl) induces apoptosis of several Bcr-Abl–positive chronic myelogenous leukemia (CML) cell lines and primary cells from CML patients in vitro and destroys Bcr-Abl–positive K562 cells in vivo. In contrast, this compound has no effect on the growth and viability of Bcr-Abl–negative lymphocytic and myeloid cell lines and primary CML cells. Sodium chlorogenate (NaChl) exhibits 2-fold higher efficiency in killing K562 cells compared with Chl. NaChl also induces growth inhibition of squamous cell carcinoma (HSC-2) and salivary gland tumor cells (HSG), although at 50-fold higher concentration. NaChl inhibits autophosphorylation of p210Bcr-Abl fusion protein rapidly. We demonstrate that p38 phosphorylation is increased in Bcr-Abl–positive cells after treatment with NaChl and closely paralleled the inhibition of Bcr-Abl phosphorylation. NaChl did not increase phosphorylation of p38 in Bcr-Abl–negative cells including HSC-2 and HSG that are responsive to this compound, indicating that p38 activation by NaChl is dependent on Bcr-Abl kinase inhibition. Inhibition of p38 activity by SB203580 significantly reduced NaChl-induced apoptosis of K562 cells, whereas activation of p38 by anisomycin augmented the apoptosis. These findings indicate that inhibition of Bcr-Abl kinase leading to activation of p38 mitogen-activated protein (MAP) kinase may play an important role in the anti-CML activity of Chl.
Introduction
Chronic myelogenous leukemia (CML) is a malignant clonal disorder of hematopoietic stem cells leading to massive expansion of myeloid lineage cells.1 The natural fate of CML is to progress from a benign chronic phase into the fatal blast crisis between approximately 3 and 5 years. Development of CML is associated with a specific chromosomal translocation known as the Philadelphia (Ph) chromosome that is detectable throughout the course of the disease.2 Somatic mutation in Ph chromosome originates from reciprocal translocation between the long arms of chromosomes 9 and 22 and fuses Bcr with c-Abl genetic sequences. Both the Bcr-Abl fusion proteins p210 and p185 can cause CML or acute leukemia.3,4 The p210 form of Bcr-Abl is seen in 95% of CML and in 20% of acute lymphocytic leukemia, whereas the p185 form is identified in about 10% of acute lymphocytic leukemia patients.5,6 The Bcr-Abl fusion proteins are constitutively active non–receptor tyrosine kinases whose activity is essential for transforming abilities.7 An almost universal presence of Bcr-Abl in CML patients made this fusion protein an attractive target for drug development. Bcr-Abl inhibitors, STI571, adaphostin, and PD173955, are capable of inducing a variable degree of apoptosis in human CML cells.8-10 The signal transduction pathways involved in mediating apoptosis by Bcr-Abl inhibitors are poorly defined. In the current study, we describe a novel Bcr-Abl kinase inhibitor that triggers p38 mitogen-activated protein (MAP) kinase–dependent apoptosis of Bcr-Abl–positive CML cells.
Materials and methods
Cells and reagents
The Ph chromosome+ CML cell line K562,11 Ph chromosome–negative T-cell line Molt 4,12 myeloid cell lines U93713 and THP-1,14 and B-cell line REH15 were purchased from the American Type Culture Collection (Manassas, VA). Bcr-Abl–positive cell lines KU81216 and KCL2217 were generously provided by Dr Carlo Gambacorti-Passerini (Instituto Nazionale Tumori, Milan, Italy). Unless otherwise stated, all cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum and 100 units/mL penicillin-streptomycin (Life Technologies, Grand Island, NY). Human oral squamous cell carcinoma HSC-218 and human salivary gland tumor HSG18 were kindly provided by Prof Hiroshi Sakagami (Department of Dental Pharmacology, Meikai University School of Dentistry, Sakado, Japan). HSC-2 and HSG cell lines were maintained in Dulbecco modified Eagle medium (Life Technologies) containing 10% fetal bovine serum and antibiotics. Antibodies were purchased from the following suppliers: Specific antibodies to c-Abl, Bcr, phospho–extracellular signal-related kinase 1/2 (ERK1/2, Tyr-204), phospho-cellular protein kinase C (p-cPKCα) (Ser657), p-cPKCβII/δ (Ser660), phospho-Lyn (Tyr-508), Lyn, phospho-HCK (Tyr-411), HCK, phospho-Fyn (Thr-12), Fyn, phospho– Janus kinase 1 (JAK1, Tyr-1022/1023), JAK1, phospho-JAK2 (Tyr-1007/1008), JAK2, phospho-JAK3 (Tyr-980), and JAK3 were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–phospho-c-Abl (Tyr-245), anti-ERK, and anti–cleaved caspase-3 were from Cell Signaling Technology (Beverly, MA). Anti–caspase-3 that recognizes unprocessed pro–caspase-3 (32 kDa) and the subunit of the active caspase-3 (17 kDa), and anti–phospho p38 (Thr 180/Tyr 182) were from BD Biosciences (Mountain View, CA). Anti-p38 was purchased from Calbiochem (San Diego, CA). The p38-specific inhibitor SB203580 and ERK1/2-specific inhibitor PD98059 were from Calbiochem. Anisomycin and 12-O-tetradecanoylphorbol-13-acetate (TPA) were from Sigma Chemical (St Louis, MO). Matrigel was obtained from BD Biosciences.
Purification of Chl from Piper betel leaves
The leaves of Piper betel (Piperaceae) were collected from different areas of West Bengal, India. A voucher specimen has been deposited at the Department of Medicinal Chemistry, Indian Institute of Chemical Biology, Kolkata. Fresh leaves (5.3 kg) were extracted with distilled water in a mixture blender. The extract was lyophilized to a semisolid mass (110 g), which was tested for killing activity of K562 cells. A portion (10 g) of the extract was fractionated through Sephadex LH-20 (Sigma Chemical) column chromatography using water, water-methanol (1:1), and methanol as eluent to give 5 fractions. The active fraction (0.24 g) was then subjected to preparative high-performance liquid chromatography using μ-Bondapak column (Waters, Milford, MA) (19 × 300 mm) with solvent methanol–water–acetic acid (23:76:1) flow rate 12 mL/min and the detection at 280 nm. A purified compound (5 mg) was isolated, which showed the activity. The structure of the active compound was determined as chlorogenic acid,19 C16H18O9, melting point 205° Cto206° C, αD-33.25 (H2O). Its identity was confirmed by comparing its physical data as well as its infrared (IR), nuclear magnetic resonance (1HNMR), 13CNMR, and mass spectral data with those of an authentic sample. Sodium chlorogenate (0.112 g) was prepared by the reaction of chlorogenic acid (0.1 g) with sodium hydrogen carbonate (0.24 g) in water (5 mL) followed by lyophilization of the resulting solution.
Clinical samples
Fresh peripheral blood samples were donated by 4 CML patients with stable phase of the disease admitted to The Institute of Haematology and Transfusion Medicine, Medical College, Kolkata before receiving any treatment. Peripheral blood samples were collected with due approval from the Human Ethics Committee of the respective institutes and all experiments with human blood were conducted under an approved institutional Human Ethics Committee protocol. Of the patients, 3 were Ph chromosome positive and 1 was negative as determined by bone marrow cytogenetics analysis. Ph chromosome–negative patient diagnosis of CML was confirmed by clinical and hematologic parameters20 (ie, huge splenomegaly, sternal tenderness, anemia, leukocytosis, increased platelet count, and low leukocyte alkaline phosphatase score). Bcr-Abl fusion protein was undetectable in this patient by Western blot analysis. Peripheral blood samples were also collected from 3 healthy donors. Mononuclear cells were separated by Ficoll/Hypaque density gradient centrifugation. Informed consent was provided according to the Declaration of Helsinki.
Cell viability assays
K562, KU812, KCL22, THP-1, U937, REH, and Molt 4 cells (1 × 104) in triplicate were incubated in 0.2 mL RPMI 1640–10% fetal calf serum containing varying concentrations of Chl and NaChl. At the indicated times, cells were collected by centrifugation (at 1000g for 5 minutes). Cell viability was determined by the trypan blue exclusion assay. At least 200 cells were examined in each sample. Viability of primary CML cells was determined in the same way except that recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF, 100 ng/mL; R & D Systems, Minneapolis, MN) was included. Because of monolayer culture, treated and untreated HSC-2 and HSG cells were detached from the wells by treatment with Cell Dissociation Solution (Sigma Chemical) before counting.
Annexin V–PI binding and cell cycle assays
Cells were seeded at 1.5 × 105 cells/mL in the presence or absence of NaChl (10 μg/mL). Evaluation of apoptosis by the annexin V–propidium iodide (PI) binding assay21 was performed using the annexin V–fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (BD Biosciences) according to the supplier's instructions. Apoptotic cells stained by annexin V are in the lower-right quadrant. Late-stage apoptotic cells stained with both annexin V and PI are in the upper-right quadrant. Apoptosis of K562 cells in the presence or absence of MAP kinase inhibitors and activators was measured as follows. Cells were pretreated with PD98059 (10 μM), SB203580 (10 μM), TPA (50 nM), or anisomycin (100 ng/mL) for 30 minutes; NaChl (10 μg/mL) was added and incubated for 24 hours. Apoptosis was determined by annexin V binding. For cell cycle analysis, cells were permeabilized and stained with PI for DNA content analysis21 in a flow cytometer (BD LSR; Becton Dickinson, San Jose, CA).
Confocal microscopy
Cells (K562 and Molt 4) were incubated with NaChl (10 μg/mL) for 24 hours, stained with annexin V–Alexa568 (Boehringer Mannheim, Mannheim, Germany), seeded onto poly-L-lysine (Sigma Chemical)–coated coverslips, and analyzed with a Leica TCS SP2 confocal laser scanning microscope (DMIRB, 40× objective, Mannheim, Germany).
Immunoblotting
Cells (1.0 × 105 cells/mL) were incubated with medium alone or with NaChl (10 μg/mL) as indicated, collected by centrifugation, and boiled for 5 minutes in sodium dodecyl sulfate (SDS) buffer. Aliquots containing 80 μg total cellular protein were separated by SDS–polyacrylamide gel electrophoresis (PAGE) containing 10% polyacrylamide, transferred to nitrocellulose, and probed with primary antibodies indicated in the figure legends followed by horseradish peroxidase–coupled secondary antibodies.
In vitro immune-complex kinase assay
p210Bcr-Abl was immunoprecipitated from K562 cells using anti–c-Abl antibody followed by incubation with protein A–Sepharose. Immune complexes were washed, and autophosphorylation was measured in immune complexes by resuspension in kinase buffer as described.22 NaChl at a concentration of 50 ng/mL was preincubated with immune complexes for 30 minutes. Kinase reactions (autophosphorylation) were initiated by the addition of 10 μCi (0.37 MBq) [γ32P]–adenosine triphosphate and were incubated for 30 minutes at room temperature. Kinase reactions were quenched by the addition of SDS sample buffer, and after heating at 100° C for 5 minutes, phosphoproteins were resolved by SDS-PAGE and detected by autoradiography. For determining the kinase activity of unfused Abl, K562 cell lysates were first immunodepleted of Bcr-Abl with antibody against Bcr followed by immunoprecipitation with antibody to c-Abl.
Flow cytometry
For intracellular staining, treated and untreated cells were immediately fixed with 4% paraformaldehyde, permeabilized by fluorescence-activated cell-sorter permeabilizing solution (BD Biosciences), and stored at –20° C until all time points were collected to avoid day-to-day variation in staining. At this stage, the fixed cells can be stored for at least one week before staining. Before staining, permeabilized cells were treated with heat-inactivated 2% normal goat serum to block nonspecific staining. After blocking, cells were stained with indicated antibodies (0.2 μg/105 cells) for 30 minutes. Isotype-matched control mouse antibodies and normal rabbit sera were used as controls for specific mouse and rabbit antibodies, respectively. After washing, cells were incubated with multiple adsorbed FITC-conjugated secondary antibody (anti–mouse or anti–rabbit immunoglobulin G, depending on the primary antibody), washed, and analyzed in a flow cytometer (BD LSR, Becton Dickinson).
In vivo studies on K562 xenografts
K562 cells were suspended to 5 × 107 cells/mL in Matrigel (1 volume of cells with 1 volume of cold Matrigel). Nude female mice 6 to 7 weeks of age (National Institute of Nutrition, Hyderabad and National Institute of Immunology, New Delhi, India) were given injections of 0.2 mL of this suspension. Animals were left untreated until K562 xenografts reached 200 to 300 mm3. NaChl at varying doses (25-150 mg/kg) was administered intraperitoneally once a day for 10 days (5 mice per group). Phosphate-buffered saline (PBS, 0.2 mL/mouse) was used as control. Animal studies were conducted under an approved institutional Animal Care and Use Committee protocol.
Statistical analysis
The statistical significance of variants between groups and within each group of experiments with MAP kinase activators and inhibitors was evaluated by one-way analysis of variance.
Results
NaChl reduces viability of Bcr-Abl–positive cells in vitro and in vivo
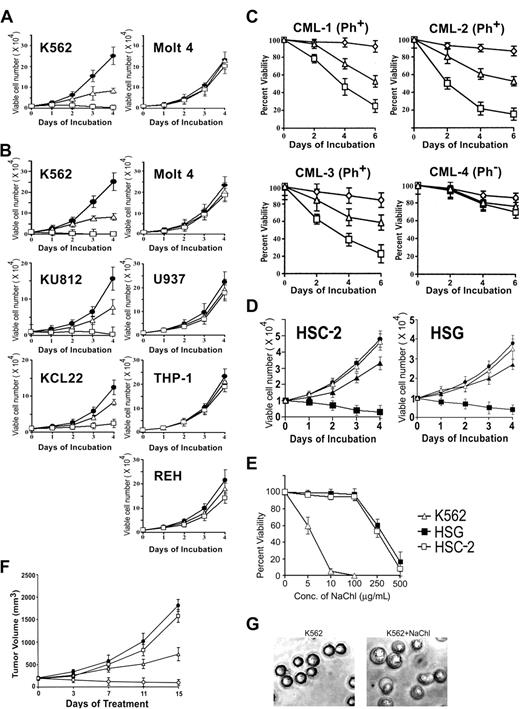
In the process of searching for antileukemic activity from medicinal plants of India, chlorogenic acid (Chl) isolated from Piper betel leaves was found to kill Bcr-Abl–positive K562 cells in vitro in a dose-dependent manner without any appreciable effects on Bcr-Abl–negative acute T-lymphoblastic leukemia cell line Molt 4 (Figure 1A). Interestingly, its sodium salt (NaChl) was twice as potent in killing K562 cells (Figure 1A-B). To evaluate the specificity of the findings, effects of NaChl on 2 additional Bcr-Abl–positive cell lines (KU812, KCL22) and 3 additional Bcr-Abl–negative cell lines (2 of myeloid origin and 1 of B-lymphocytic origin) were investigated. Like K562, Bcr-Abl–positive cells KU812 and KCL22 were destroyed in vitro by NaChl in a dose-dependent manner, although sensitivity of KCL22 to NaChl was slightly lower than K562 or KU812 (Figure 1B). In contrast, Bcr-Abl–negative myeloid (U937, THP-1) and B-lymphocytic cell line (REH) were insensitive to NaChl (Figure 1B). Specific destruction of Bcr-Abl–positive cell lines by NaChl prompted us to test this compound on leukemic cells collected from Bcr-Abl–positive and –negative CML patients. These primary CML cells were cultured in the presence of rhGM-CSF and graded concentrations of NaChl. NaChl reduced the viability of leukemic cells from all 3 Bcr-Abl–positive CML patients in a time- and dose-dependent manner, while viability of primary CML cells from a Bcr-Abl–negative patient was unaffected (Figure 1C). Chl has been shown to inhibit the growth of squamous cell carcinoma, HSC-2, and salivary gland tumor cell line, HSG.18 We therefore investigated the effects of NaChl on these oral tumor cell lines. In agreement with a previous report,23 a high concentration of NaChl (> 250 μg/mL) was required to have detectable growth inhibitory effects on HSC-2 and HSG cell lines (Figure 1D). In contrast, 50-fold less concentrations of NaChl (5-10 μg/mL) were sufficient to inhibit the growth of K562 cells (Figure 1E). Administration of NaChl in nude mice bearing K562 xenografts reduced the tumor growth in a dose-dependent manner (Figure 1F).
Effect of chlorogenic acid (Chl) and its sodium salt (NaChl) on Bcr-Abl–positive and –negative cells. (A) K562 and Molt 4 cells were cultured in medium (•) or with graded concentrations of Chl (10 μg/mL, ▵; 25 μg/mL, □) for indicated times, and viability was determined by trypan blue dye exclusion test. (B) Cells were cultured with NaChl (medium, •; 5 μg/mL NaChl, ▵; 10 μg/mL NaChl, □). (C) Mononuclear cells from 3 Bcr-Abl–positive and 1 Bcr-Abl–negative CML patient were cultured in medium containing rhGM-CSF (⋄) or with the same medium containing NaChl (5 μg/mL, ▵; 10 μg/mL, □). (D) Squamous cell carcinoma (HSC-2) and salivary gland tumor cell line (HSG) were cultured in the absence (•) or presence of varying concentrations of NaChl (100 μg/mL, ▵; 250 μg/mL, ▴; 500 μg/mL, ▪). (E) Indicated cells were cultured with varying concentrations of NaChl for 4 days and viability was determined. (F) K562 cells embedded in Matrigel were staged in nude mice until tumors reached 200 to 300 mm3. PBS (•) and NaChl (25 mg/kg, □; 100 mg/kg, ▵; 150 mg/kg, ○) were administered intraperitoneally once a day for 10 days. (G) Morphologic changes of K562 cells after 24 hours of treatment with 5.0 μg/mL NaChl (phase contrast micrographs, × 400 using Olympus CK40 microscope and Olympus SC35 Type 12 camera). Error bars denote standard deviation of 3 experiments.
Effect of chlorogenic acid (Chl) and its sodium salt (NaChl) on Bcr-Abl–positive and –negative cells. (A) K562 and Molt 4 cells were cultured in medium (•) or with graded concentrations of Chl (10 μg/mL, ▵; 25 μg/mL, □) for indicated times, and viability was determined by trypan blue dye exclusion test. (B) Cells were cultured with NaChl (medium, •; 5 μg/mL NaChl, ▵; 10 μg/mL NaChl, □). (C) Mononuclear cells from 3 Bcr-Abl–positive and 1 Bcr-Abl–negative CML patient were cultured in medium containing rhGM-CSF (⋄) or with the same medium containing NaChl (5 μg/mL, ▵; 10 μg/mL, □). (D) Squamous cell carcinoma (HSC-2) and salivary gland tumor cell line (HSG) were cultured in the absence (•) or presence of varying concentrations of NaChl (100 μg/mL, ▵; 250 μg/mL, ▴; 500 μg/mL, ▪). (E) Indicated cells were cultured with varying concentrations of NaChl for 4 days and viability was determined. (F) K562 cells embedded in Matrigel were staged in nude mice until tumors reached 200 to 300 mm3. PBS (•) and NaChl (25 mg/kg, □; 100 mg/kg, ▵; 150 mg/kg, ○) were administered intraperitoneally once a day for 10 days. (G) Morphologic changes of K562 cells after 24 hours of treatment with 5.0 μg/mL NaChl (phase contrast micrographs, × 400 using Olympus CK40 microscope and Olympus SC35 Type 12 camera). Error bars denote standard deviation of 3 experiments.
NaChl induces apoptosis of Bcr-Abl–positive cells
Nuclear condensation, a sign of apoptosis, was evident in NaChl-treated K562 cells (Figure 1G). We therefore investigated whether NaChl-induced killing of Bcr-Abl–positive cells was due to apoptosis. Apoptosis was determined by the appearance of phosphatidylserine on the external surface of the plasma membrane (binding to annexin V, Figure 2A), appearance of apoptotic nuclei (sub G0/G1 peak in DNA cell cycle analysis, Figure 2B), and activation of caspase-3 (Figure 2C). Treatment with NaChl induced apoptosis in K562 cells and leukemic cells of a Bcr-Abl–positive CML patient, while it had no effect on either Molt 4 cells or mononuclear cells of a healthy donor. Staining of K562 cell membrane with annexin V and its absence in treated Molt 4 cells support a specific apoptotic effect of NaChl (Figure 2D). In K562 cells, annexin V binding was detectable as early as 10 hours after treatment with NaChl, while caspase-3 activation and appearance of apoptotic nuclei were detectable after 48 hours (Figure 2E). This could be explained by the fact that phosphatidylserine externalization occurs very early in apoptosis, before any nuclear changes have occurred.24
NaChl induces apoptosis of Bcr-Abl–positive cells. (A) Flow cytometric analysis of annexin V binding. Cells were left untreated (NT) or were treated with NaChl (10 μg/mL) for 24 hours (T) before analysis. Values in the quadrants represent percent positive cells. (B) DNA cell cycle analysis after treatment of cells with NaChl (10 μg/mL) for 48 hours. Gates were set to assess the percentage of dead (< 2n DNA, M1), G0/G1 (2n DNA, M2), and S + G2 + M(> 2n DNA, M3). Bars denote boundaries of cell cycle phases. (C) Immunoblot analysis of caspase-3 activation. Cells were treated with NaChl (10 μg/mL) for 48 hours, harvested, and lysed by boiling in SDS sample buffer, and an equivalent amount of lysates was separated by SDS-PAGE and electrotransferred. The filters were probed with anti–caspase-3 that recognizes both procaspase-3 (32 kDa) and caspase-3 cleavage product (17 kDa). (D) Confocal fluorescence images of NaChl-treated (10 μg/mL for 24 hours) K562 and Molt 4 cells after staining with annexin V–Alexa. Molt 4 cells were used as negative control. (E) Time kinetics of apoptosis determined by different techniques. K562 cells were treated with NaChl (10 μg/mL) for indicated times and analyzed for annexin V binding, DNA cell cycle (apoptotic nuclei [Sub G0/G1 peak]), and cleaved caspase-3 by flow cytometry.
NaChl induces apoptosis of Bcr-Abl–positive cells. (A) Flow cytometric analysis of annexin V binding. Cells were left untreated (NT) or were treated with NaChl (10 μg/mL) for 24 hours (T) before analysis. Values in the quadrants represent percent positive cells. (B) DNA cell cycle analysis after treatment of cells with NaChl (10 μg/mL) for 48 hours. Gates were set to assess the percentage of dead (< 2n DNA, M1), G0/G1 (2n DNA, M2), and S + G2 + M(> 2n DNA, M3). Bars denote boundaries of cell cycle phases. (C) Immunoblot analysis of caspase-3 activation. Cells were treated with NaChl (10 μg/mL) for 48 hours, harvested, and lysed by boiling in SDS sample buffer, and an equivalent amount of lysates was separated by SDS-PAGE and electrotransferred. The filters were probed with anti–caspase-3 that recognizes both procaspase-3 (32 kDa) and caspase-3 cleavage product (17 kDa). (D) Confocal fluorescence images of NaChl-treated (10 μg/mL for 24 hours) K562 and Molt 4 cells after staining with annexin V–Alexa. Molt 4 cells were used as negative control. (E) Time kinetics of apoptosis determined by different techniques. K562 cells were treated with NaChl (10 μg/mL) for indicated times and analyzed for annexin V binding, DNA cell cycle (apoptotic nuclei [Sub G0/G1 peak]), and cleaved caspase-3 by flow cytometry.
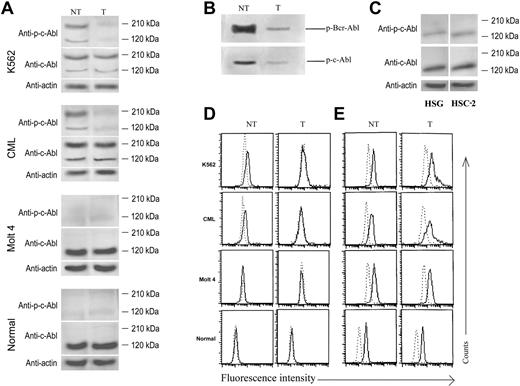
NaChl inhibits autophosphorylation of Bcr-Abl
NaChl induced apoptosis of Bcr-Abl–positive cells at much lower concentrations (5-10 μg/mL) than that observed with oral tumor cell lines (> 250 μg/mL). Blocking of Bcr-Abl tyrosine kinase activity is known to result in apoptosis of Bcr-Abl–expressing cells.8,9 We therefore evaluated the effects of NaChl on autophosphorylation of Bcr-Abl and unfused Abl (c-Abl) by Western blot. Cells were treated with 10 μg/mL NaChl for 30 minutes, harvested, and lysed, and equal amounts of total proteins were subjected to immunoblot analysis using antibodies to phospho-cAbl and cAbl. NaChl inhibited autophosphorylation of both fused (Bcr-Abl) and unfused Abl in Ph+ cells without affecting protein expression (Figure 3A). Inhibition of Bcr-Abl phosphorylation by NaChl was further confirmed in in vitro assays with immune-complexed kinases. NaChl reduced autophosphorylation of Bcr-Abl and unfused Abl (Figure 3B). An intracellular staining technique has recently been reported to detect phosphorylated MAP kinases and signal transducer and activator of transcription molecules.25,26 We applied the same technique to substantiate the results of Western blot. Intracellular phosphorylated Abl was demonstrated only in K562 cells and mononuclear cells of Ph+ CML patients, and in both cases NaChl treatment abolished the phosphorylation (Figure 3D). Abl protein was detectable in Ph+ and normal cells and remained unaffected after NaChl treatment (10 μg/mL) for 30 minutes (Figure 3E). Of note, unlike Western blot, phosphorylation and protein expression of Bcr-Abl and c-Abl could not be distinguished by flow cytometry assay. Anti–phospho-c-Abl antibody produced positive intracellular staining only in Bcr-Abl–positive cells, whereas anti–c-Abl antibody stained both Bcr-Abl–positive and –negative cells. This is in agreement with our Western blot results. Therefore, reduction in phospho-c-Abl staining detected by flow cytometry reflected reduction of Bcr-Abl phosphorylation. Moreover, Bcr-Abl protein constitutes more than 70% of total Abl protein in both K562 cells and Bcr-Abl–positive primary CML cells (Western blot); thus drastic reduction in total Abl expression detectable by flow cytometry indicates mainly the reduction in Bcr-Abl expression. Our data also indicate that a flow cytometry–based assay of phosphorylation status of proteins correlates closely and reliably with that of conventional Western blot.
NaChl inhibits Bcr-Abl and c-Abl autophosphorylation in Ph+ cells. (A) Immunoblot-based determination of Abl expression and phosphorylation status. Cells were treated with NaChl (10 μg/mL) for 30 minutes, harvested, and lysed, and equivalent amount of lysates were separated by SDS-PAGE and electrotransferred. The filters were probed with anti–phospho-c-Abl (Tyr 245) (top panels) or anti–c-Abl antibody (middle panels). β-Actin was used as loading control (bottom panels). (B) In vitro kinase assay of fused (Bcr-Abl) and unfused Abl in the presence (T) or absence (NT) of NaChl (50 ng/mL, 30 minutes). (C) HSG and HSC-2 cells do not express Bcr-Abl. Cells were harvested and lysed, and equivalent amount of lysates were separated by SDS-PAGE and electrotransferred. The filters were probed with indicated antibodies. (D) Flow cytometric determination of Abl phosphorylation status. Indicated cells were left untreated (NT) or incubated with NaChl (10 μg/mL) for 30 minutes (T), fixed, permeabilized, and stained with rabbit anti–phospho-c-Abl (Tyr 245) antibody. Dotted line indicates staining with normal rabbit sera; solid line, staining with anti–phospho-c-Abl antibody. (E) Flow cytometric determination of intracellular Abl protein expression status. Cells were fixed, permeabilized, and stained with rabbit anti–c-Abl antibody. Dotted line indicates staining with normal rabbit sera; solid line, staining with anti–c-Abl antibody.
NaChl inhibits Bcr-Abl and c-Abl autophosphorylation in Ph+ cells. (A) Immunoblot-based determination of Abl expression and phosphorylation status. Cells were treated with NaChl (10 μg/mL) for 30 minutes, harvested, and lysed, and equivalent amount of lysates were separated by SDS-PAGE and electrotransferred. The filters were probed with anti–phospho-c-Abl (Tyr 245) (top panels) or anti–c-Abl antibody (middle panels). β-Actin was used as loading control (bottom panels). (B) In vitro kinase assay of fused (Bcr-Abl) and unfused Abl in the presence (T) or absence (NT) of NaChl (50 ng/mL, 30 minutes). (C) HSG and HSC-2 cells do not express Bcr-Abl. Cells were harvested and lysed, and equivalent amount of lysates were separated by SDS-PAGE and electrotransferred. The filters were probed with indicated antibodies. (D) Flow cytometric determination of Abl phosphorylation status. Indicated cells were left untreated (NT) or incubated with NaChl (10 μg/mL) for 30 minutes (T), fixed, permeabilized, and stained with rabbit anti–phospho-c-Abl (Tyr 245) antibody. Dotted line indicates staining with normal rabbit sera; solid line, staining with anti–phospho-c-Abl antibody. (E) Flow cytometric determination of intracellular Abl protein expression status. Cells were fixed, permeabilized, and stained with rabbit anti–c-Abl antibody. Dotted line indicates staining with normal rabbit sera; solid line, staining with anti–c-Abl antibody.
A much higher (50-fold) concentration of NaChl was required to inhibit the growth of HSC-2 and HSG cells compared with that of Bcr-Abl–positive K562 cells. We therefore evaluated the status of phosphorylated and unphosphorylated Bcr-Abl (∼ 210 kDa) and wild-type Abl (∼ 140 kDa) in these cells by immunoblots with anti–phospho-c-Abl and anti–c-Abl antibodies, respectively. In both cell lines (HSC-2, HSG), wild-type Abl as well as its phosphorylated form in basal level were detectable, while Bcr-Abl (phosphorylated or unphosphorylated) was undetectable (Figure 3C).
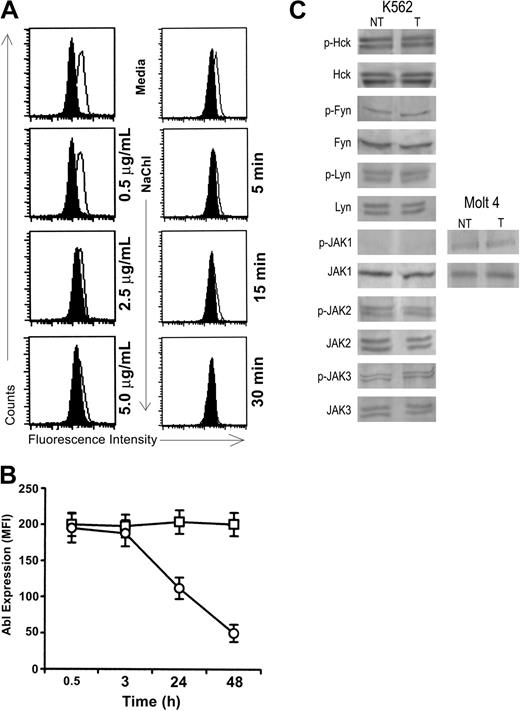
NaChl treatment reduced the expression of phosphorylated Abl in K562 cells in a dose- and time-dependent manner; maximum inhibition (almost 100%) was achieved at 30 minutes at a concentration of 10 μg/mL (Figure 4A). Treatment with NaChl (10 μg/mL) also resulted in a gradual decrease of total Abl protein after 3 hours (Figure 4B).
NaChl inhibits phosphorylation of Abl in K562 cells in a time- and dose-dependent manner without affecting Src-family kinases and JAK kinases. (A) K562 cells were treated with varying concentrations of NaChl for 30 minutes (left panel) or with 10 μg/mL NaChl for varying time periods (right panel), fixed, permeabilized, stained with anti–phospho-c-Abl (solid line) or normal rabbit sera (filled histogram), and analyzed in a flow cytometer. (B) NaChl treatment reduces Abl-protein expression in a time-dependent manner in K562 cells. Cells were treated with NaChl (10 μg/mL) for indicated times, fixed, permeabilized, and stained with anti–c-Abl antibody (□, cultured in medium; ○, cultured in the presence of NaChl). The level of expression is presented as mean fluorescence intensity (MFI). Data represent mean ± SD of 3 experiments. (C) Immunoblot-based determination of protein expression and phosphorylation status of indicated kinases in indicated cells before (NT) and after treatment for 30 minutes with NaChl (T). Experiments were performed as mentioned in the legend of Figure 3A using indicated antibodies.
NaChl inhibits phosphorylation of Abl in K562 cells in a time- and dose-dependent manner without affecting Src-family kinases and JAK kinases. (A) K562 cells were treated with varying concentrations of NaChl for 30 minutes (left panel) or with 10 μg/mL NaChl for varying time periods (right panel), fixed, permeabilized, stained with anti–phospho-c-Abl (solid line) or normal rabbit sera (filled histogram), and analyzed in a flow cytometer. (B) NaChl treatment reduces Abl-protein expression in a time-dependent manner in K562 cells. Cells were treated with NaChl (10 μg/mL) for indicated times, fixed, permeabilized, and stained with anti–c-Abl antibody (□, cultured in medium; ○, cultured in the presence of NaChl). The level of expression is presented as mean fluorescence intensity (MFI). Data represent mean ± SD of 3 experiments. (C) Immunoblot-based determination of protein expression and phosphorylation status of indicated kinases in indicated cells before (NT) and after treatment for 30 minutes with NaChl (T). Experiments were performed as mentioned in the legend of Figure 3A using indicated antibodies.
To determine whether the effects of NaChl are specific for fused and unfused Abl or whether this agent also inhibits other known kinases, several members of the Src-kinase family and JAK kinases were evaluated in K562 cells by immunoblots with phosphospecific antibodies. Neither phosphorylation nor protein expression of Lyn, HCK, Fyn, JAK2, and JAK3 was affected by NaChl (Figure 4C). The phosphorylated form of JAK1 was undetectable in K562 cells, which continued to be undetectable after treatment with NaChl. To investigate whether the anti–phospho-JAK1 antibody is not working or whether K562 cells do not express the phosphorylated form of this kinase, the effect of NaChl on this kinase was studied in Molt 4 cells known to express this kinase27 (Figure 4C). Similarly, flow cytometry–based assays indicated that phosphorylation status and protein expression of PKC α and PKC βII/δ serine kinases were unaffected by this compound (data not shown).
NaChl modulates MAP kinase pathways in K562 cells
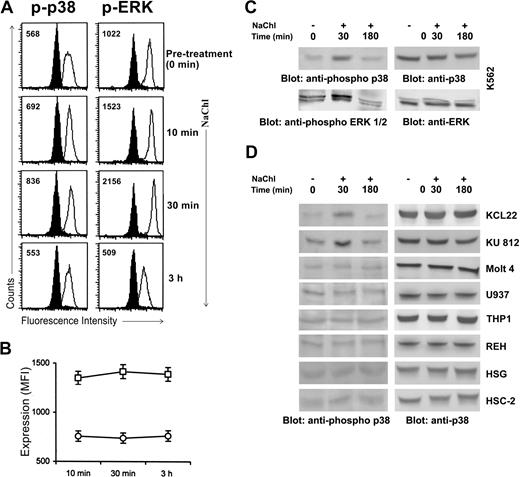
We have demonstrated that NaChl is able to induce apoptosis of Bcr-Abl–positive cells. We next examined the possible involvement of the MAP kinases. The ERK1/2 MAP kinase was analyzed because of its importance for growth and survival in many cell types.28,29 The p38 MAP kinase was examined because of its importance in the mediation of stress signals.30 Flow cytometric analysis using phospho-specific antibodies against respective MAP kinases was performed. These antibodies specifically recognize the activated phosphorylated form of ERK1/231 and p38.32 Increased phosphorylation of both p38 and ERK1/2 MAP kinases was observed in K562 cells starting at 10 minutes after NaChl addition, lasting only up to 30 minutes, and then declining to the basal level at 3 hours (Figure 5A). Expression of ERK and p38 protein levels remained unchanged throughout this incubation period (Figure 5B). NaChl induced enhanced phosphorylation of p38 and ERK1/2 MAP kinases in K562 cells by 30 minutes, followed by a decline at 3 hours that did not affect protein expression, which was confirmed by immunoblot analysis (Figure 5C). The panel of Bcr-Abl–positive and –negative cells was then evaluated for p38 activation after NaChl treatment. NaChl induced p38 phosphorylation by 30 minutes, followed by a decline at 3 hours in all Bcr-Abl–positive cells tested, while basal level of phosphorylated p38 remained unchanged by NaChl in Bcr-Abl–negative cells including HSG and HSC-2 cell lines (Figure 5D). Taken together, these data indicate that the differences in detectable phospho-activities are not due to changes in respective MAP kinase expression levels. The time kinetics of enhanced phosphorylation of p38 and ERK1/2 in K562 cells by NaChl treatment closely coincided with reduced phosphorylation of Bcr-Abl (Figures 4A and 5A,C). Thus, NaChl-induced enhanced phosphorylation of p38 in Bcr-Abl–positive cells is dependent on inhibition of Bcr-Abl phosphorylation.
NaChl treatment enhances phosphorylation of p38 and ERK1/2 MAP kinases in K562 cells. (A) Cells were treated with NaChl (10 μg/mL) for indicated time periods, fixed, permeabilized, and stained with isotype-matched control mouse monoclonal antibodies (filled histogram) or anti–phospho-p38 and anti–phospho-ERK1/2 monoclonal antibodies (solid line). Values within histograms represent the specific MFI (after subtracting the control values). (B) NaChl treatment does not alter MAP kinase protein expression in K562 cells. Expression of ERK (□) and p38 (○) is presented as mean fluorescence intensity (MFI). Data presented are mean ± SD of 3 experiments. (C) Immunoblot-based determination of p38 and ERK phosphorylation in K562 cells before and after NaChl treatment. Cells were left untreated or treated with NaChl (10 μg/mL) for 30 minutes and 3 hours before analysis. (D) NaChl treatment enhances p38 phosphorylation only in Bcr-Abl–positive cells. A panel of Bcr-Abl–positive CML cell lines (KCL22, KU812), Bcr-Abl–negative lymphoid (Molt 4, REH), myeloid (U937, THP-1), squamous cell carcinoma (HSC-2), and salivary gland tumor cell line (HSG) were left untreated or treated with NaChl (500 μg/mL for HSG and HSC-2 cells and 10 μg/mL for other cells) for 30 minutes and 3 hours before analysis by immunoblots with indicated antibodies.
NaChl treatment enhances phosphorylation of p38 and ERK1/2 MAP kinases in K562 cells. (A) Cells were treated with NaChl (10 μg/mL) for indicated time periods, fixed, permeabilized, and stained with isotype-matched control mouse monoclonal antibodies (filled histogram) or anti–phospho-p38 and anti–phospho-ERK1/2 monoclonal antibodies (solid line). Values within histograms represent the specific MFI (after subtracting the control values). (B) NaChl treatment does not alter MAP kinase protein expression in K562 cells. Expression of ERK (□) and p38 (○) is presented as mean fluorescence intensity (MFI). Data presented are mean ± SD of 3 experiments. (C) Immunoblot-based determination of p38 and ERK phosphorylation in K562 cells before and after NaChl treatment. Cells were left untreated or treated with NaChl (10 μg/mL) for 30 minutes and 3 hours before analysis. (D) NaChl treatment enhances p38 phosphorylation only in Bcr-Abl–positive cells. A panel of Bcr-Abl–positive CML cell lines (KCL22, KU812), Bcr-Abl–negative lymphoid (Molt 4, REH), myeloid (U937, THP-1), squamous cell carcinoma (HSC-2), and salivary gland tumor cell line (HSG) were left untreated or treated with NaChl (500 μg/mL for HSG and HSC-2 cells and 10 μg/mL for other cells) for 30 minutes and 3 hours before analysis by immunoblots with indicated antibodies.
The effects of specific MAP kinase inhibitors on NaChl action
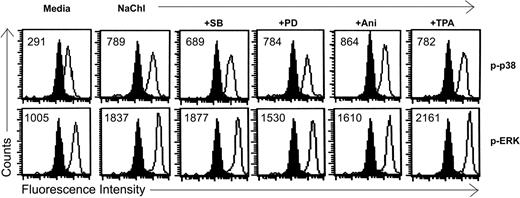
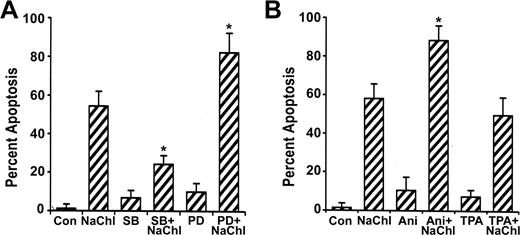
To investigate the role of ERK and p38 pathways on NaChl-induced apoptosis of K562 cells, we examined the effect of the specific ERK inhibitor PD9805933-35 and p38 inhibitor SB20358036-38 on p38 and ERK1/2 phosphorylation and apoptosis. NaChl-induced enhanced phosphorylation of p38 in K562 cells was partially reduced when cells were pretreated for 30 minutes with 10 μM SB203580 (Figure 6). ERK1/2 phosphorylation remained more-or-less unchanged under the same experimental condition (Figure 6). Similarly, NaChl-induced enhanced phosphorylation of ERK1/2 in K562 cells was partially reduced after pretreatment of cells with 10 μM PD98059 without affecting phosphorylation of p38 (Figure 6). Thus, inhibition of p38 is not linked to ERK signaling and vice versa at the dose levels of SB203580 and PD98059 tested. We next examined the effect of specific ERK inhibitor PD98059 and p38 inhibitor SB203580 on NaChl-induced apoptosis. K562 cells were incubated with 10 μg/mL NaChl in the presence or absence of SB203580 or PD98059 (10 μM each) for 24 hours. Apoptosis was measured by annexin V binding. PD98059 further increased annexin V positivity of NaChl-treated K562 cells (P < .01 vs NaChl alone), whereas SB203580 inhibited (P < .01 vs NaChl) the apoptosis (Figure 7A). These results indicate that activation of p38 but not ERK1/2 is essential for NaChl-induced apoptosis of K562 cells and that inhibition of ERK potentiates the apoptotic process. These findings were further confirmed by treating K562 cells with NaChl in the presence or absence of MAP kinase activators.
Effects of MAP kinase inhibitors and activators on NaChl-induced enhanced phosphorylation of p38 and ERK1/2 in K562 cells. Cells were pretreated with inhibitors or activators of MAP kinases for 30 minutes as described in “Materials and methods” before treatment with NaChl (10 μg/mL) for an additional 30 minutes. Filled histograms indicate staining with isotype-matched control mouse monoclonal antibodies. Solid lines indicate staining with anti–phospho p38 or anti–phospho ERK1/2 monoclonal antibody. Values within the histograms represent the specific MFI (after subtracting the respective control values).
Effects of MAP kinase inhibitors and activators on NaChl-induced enhanced phosphorylation of p38 and ERK1/2 in K562 cells. Cells were pretreated with inhibitors or activators of MAP kinases for 30 minutes as described in “Materials and methods” before treatment with NaChl (10 μg/mL) for an additional 30 minutes. Filled histograms indicate staining with isotype-matched control mouse monoclonal antibodies. Solid lines indicate staining with anti–phospho p38 or anti–phospho ERK1/2 monoclonal antibody. Values within the histograms represent the specific MFI (after subtracting the respective control values).
Effects of MAP kinase inhibitors and activators on the NaChl-induced apoptosis of K562 cells. (A) Cells were pretreated with the inhibitors for 30 minutes; then NaChl (10 μg/mL) was added and incubated for 24 hours. Apoptosis was determined by flow cytometric assay based on annexin V binding. (B) Cells were preincubated with the MAP kinase activators for 30 minutes before addition of NaChl, followed by determination of apoptosis at 24 hours by annexin V binding. Data represent mean ± SD of 3 experiments. *P < .01 versus NaChl treatment alone. SB indicates SB203580; PD, PD98059; Ani, anisomycin; and Con, control.
Effects of MAP kinase inhibitors and activators on the NaChl-induced apoptosis of K562 cells. (A) Cells were pretreated with the inhibitors for 30 minutes; then NaChl (10 μg/mL) was added and incubated for 24 hours. Apoptosis was determined by flow cytometric assay based on annexin V binding. (B) Cells were preincubated with the MAP kinase activators for 30 minutes before addition of NaChl, followed by determination of apoptosis at 24 hours by annexin V binding. Data represent mean ± SD of 3 experiments. *P < .01 versus NaChl treatment alone. SB indicates SB203580; PD, PD98059; Ani, anisomycin; and Con, control.
The effect of MAP kinase activators on NaChl action
Numerous studies have indicated that anisomycin and TPA are strong activators of p3839,40 and ERK41-43 signaling pathways, respectively. As demonstrated in previous experiments, if the activation of p38 plays an important role in mediating apoptosis of NaChl-treated K562 cells, then agents capable of stimulating p38 activity, when combined with NaChl treatment, should potentiate apoptosis. This possibility was addressed by pretreating K562 cells with anisomycin (100 ng/mL) for 30 minutes followed by treatment with NaChl (10 μg/mL) for 24 hours. Anisomycin further increased p38 phosphorylation (Figure 6) and the induction of apoptosis by NaChl (P < .01 vs NaChl) (Figure 7B). In contrast to anisomycin, pretreatment of K562 cells with TPA (50 nM) inhibited NaChl-induced apoptosis only marginally (Figure 7B). These results indicate that p38 activation potentiates NaChl-induced apoptosis of K562 cells, while ERK activation mediates the opposite effect.
Discussion
In the present study, a Bcr-Abl kinase inhibitor was identified from an herbal source. The compound was characterized to be chlorogenic acid (Chl). Chl inhibits Bcr-Abl and c-Abl kinases and induces apoptosis of Bcr-Abl–positive cells including Bcr-Abl–positive primary leukemic cells of CML patients in vitro. The compound was also effective in destroying K562 xenografts in nude mice. Sodium chlorogenate (NaChl) appears 2 times more potent than Chl, possibly because of higher solubility. NaChl has no effect on some known kinases including several members of the Src-kinase family (Lyn, HCK, Fyn), JAK-kinases (JAK2, JAK3), and serine kinases (PKCα, PKCβII/δ). Chlorogenic acid has diverse biologic activities including anti-HIV activity,44 antioxidant activity,45 anticarcinogenic activity,46 modulating activity of cytochrome P450-linked enzyme,47 antiallergic activity,48 and apoptosis-inducing activity in human oral squamous cell carcinoma and salivary gland tumor cell lines.18,23 Therefore, possible additional effects of Chl on cellular systems other than Bcr-Abl and c-Abl kinases cannot be ruled out. Since Chl is known to inhibit growth of squamous cell carcinoma and the salivary gland tumor cell line, we evaluated whether these cells express Bcr-Abl kinase and whether NaChl-induced growth inhibition of these cells is associated with p38 activation. This was addressed to clarify whether the effects of NaChl on Bcr-Abl–positive cells result from inhibition of Bcr-Abl kinase or from a Bcr-Abl–independent activation of p38 in these cells. In agreement with a previous report,23 a 50-fold higher concentration of NaChl is required to detect growth inhibition of these Bcr-Abl–negative cells compared with that of Bcr-Abl–positive cells. Importantly, basal level of phosphorylated p38 detectable in these Bcr-Abl–negative oral tumor cells was not up-regulated by NaChl. To date, a number of Bcr-Abl kinase inhibitors have been reported that are capable of inducing apoptosis of Bcr-Abl–positive cells, but there is little information on MAP kinase signaling pathways activated by these Bcr-Abl inhibitors.8-10 We explored the role of MAP kinase signal transduction system in NaChl-mediated apoptosis of Bcr-Abl–positive K562 cells. Our data show that although ERK and p38 signal transduction pathways are activated rapidly in K562 cells after NaChl treatment, activation of the p38 pathway, which is dependent on inhibition of Bcr-Abl kinase, plays a critical role in inducing apoptosis. This explanation has support from the following observations: (a) NaChl induced enhanced phosphorylation of both p38 and ERK MAP kinases in K562 cells very rapidly; (b) NaChl-induced activation of p38 and ERK is dependent on inhibition of Bcr-Abl phosphorylation. NaChl neither induces apoptosis of Bcr-Abl–negative cells nor does it activate p38 in these cells; (c) and inhibition of p38 signaling by specific inhibitor inhibits NaChl-mediated apoptosis of K562 cells. In contrast, inhibition of ERK signaling by a specific inhibitor potentiates apoptosis. (d) Further activation of p38 by p38 activator potentiates NaChl-induced apoptosis of K562 cells, while more activation of ERK mediates the opposite effect.
In K562 cells, a Bcr-Abl kinase inhibitor activated ERK, and inhibition of the ERK pathway synergistically potentiated apoptosis induced by the Bcr-Abl kinase inhibitor.49 This is consistent with our finding that ERK activation does not play a crucial role in inducing apoptosis in these Bcr-Abl–positive cells. The general consensus on ERK in regulating apoptosis is that activation of the ERK pathway delivers a survival signal that counteracts proapoptotic effects associated with JNK and p38 activation.29 Our data are in agreement with this view as NaChl-induced apoptosis of K562 cells was inhibited by ERK activation, while inhibition of ERK potentiated the apoptotic process.
p38 MAP kinase is primarily activated by various environmental stresses and has been suggested to play a critical role in apoptosis.29,30 However, the role of p38 MAP kinase in inducing apoptosis of CML cells by Bcr-Abl kinase inhibitors has not been clearly documented. A recent report demonstrated the role of p38 MAP kinase in mediating growth inhibitory effects of interferonalpha in Bcr-Abl–expressing cells.50 We report that NaChl is a new addition to the list of Bcr-Abl kinase inhibitors. We also demonstrate that activation of p38 MAP kinase is a consequence of Bcr-Abl kinase inhibition and plays a crucial role in mediating apoptosis induced by a Bcr-Abl kinase inhibitor. How Bcr-Abl kinase inhibition activates p38 MAP kinase is yet to be determined. We propose that the p38 MAP kinase pathway contributes to the apoptotic effect of other Bcr-Abl inhibitors that exhibit selective growth inhibitory effects on Bcr-Abl–positive CML cells. In agreement with our hypothesis, a recent report indicated that a well-characterized Bcr-Abl kinase inhibitor STI571 induces apoptosis of Bcr-Abl–positive cells by activating the p38 MAP kinase.51 It is possible that Bcr-Abl may exhibit constitutive negative regulatory effects on the activation of the growth inhibitory p38 MAP kinase pathway. Inhibition of Bcr-Abl kinase activity by an inhibitor, such as NaChl, may overcome the negative regulatory effects of Bcr-Abl on p38 MAP kinase that lead to apoptosis. Alternatively, inhibition of Bcr-Abl kinase may be recognized by the cell as a stress that leads to activation of p38 and results in induction of apoptosis.
Prepublished online as Blood First Edition Paper, June 29, 2004; DOI 10.1182/blood-2003-11-4065.
Supported by the Council of Scientific and Industrial Research, New Delhi.
G.B., T.B., K.C.R., S.M, C.M, B.C.P., S.B., and S.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Shelley Bhattacharya and Mr Sasi Mukherjee for critically reviewing the manuscript, and Dr Basudeb Achari for chemical identification. We deeply appreciate Dr Carlo Gambacorti-Passerini for providing KU812 and KCL22 cell lines and extend our thanks to Mr Edoardo Marchesi for their transport. We are also indebted to Prof Hiroshi Sakagami for generously providing HSC-2 and HSG cell lines. Our work has been greatly expedited with the help from Dr Partha Banerjee of Georgetown University Medical Center, Washington, DC, and Dr Sibsankar Roy of our institute; we are grateful to them. We thank Mr Anirban Manna for preparation of the manuscript.
The information provided in this report is protected by pending US & Patent Cooperation Treaty patent applications.

![Figure 2. NaChl induces apoptosis of Bcr-Abl–positive cells. (A) Flow cytometric analysis of annexin V binding. Cells were left untreated (NT) or were treated with NaChl (10 μg/mL) for 24 hours (T) before analysis. Values in the quadrants represent percent positive cells. (B) DNA cell cycle analysis after treatment of cells with NaChl (10 μg/mL) for 48 hours. Gates were set to assess the percentage of dead (< 2n DNA, M1), G0/G1 (2n DNA, M2), and S + G2 + M(> 2n DNA, M3). Bars denote boundaries of cell cycle phases. (C) Immunoblot analysis of caspase-3 activation. Cells were treated with NaChl (10 μg/mL) for 48 hours, harvested, and lysed by boiling in SDS sample buffer, and an equivalent amount of lysates was separated by SDS-PAGE and electrotransferred. The filters were probed with anti–caspase-3 that recognizes both procaspase-3 (32 kDa) and caspase-3 cleavage product (17 kDa). (D) Confocal fluorescence images of NaChl-treated (10 μg/mL for 24 hours) K562 and Molt 4 cells after staining with annexin V–Alexa. Molt 4 cells were used as negative control. (E) Time kinetics of apoptosis determined by different techniques. K562 cells were treated with NaChl (10 μg/mL) for indicated times and analyzed for annexin V binding, DNA cell cycle (apoptotic nuclei [Sub G0/G1 peak]), and cleaved caspase-3 by flow cytometry.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/8/10.1182_blood-2003-11-4065/6/m_zh80200467670002.jpeg?Expires=1769081520&Signature=3nVQCoARvdY0jzzRixZfbhx8sBE5w3buipWioitu8DjKdy1oy5oXjNHWq0Ps04vDIzP3mi5nNnLFhiPXM67I8~thxwlYmvDIpG1dR-DwvNqaESYCDmUkj4PB-Mvotvw1U1kj9SPgxvYLGw-R6HAVnnd4REqrdBiOE6Yn5fBIyqR830Xmim6NKgAExEb33ASaiw6rEwFDOT84TjVDWnH3w7YUu0919OMoAZ668tTXI4kv0k5hqV20N-iaRmsg~JqnQRTOWRH2xV4KS9Jk7T93qxpvIOqkRUZkPiQz8-gg71pOclyS191Jl0jPDN0fNGsUNPVmeQX1gbBxYsfJolOg3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal