Abstract
Cyclin D1 expression is deregulated by chromosome translocation in mantle cell lymphoma and a subset of multiple myeloma. The molecular mechanisms involved in long-distance gene deregulation remain obscure, although changes in acetylated histones and methylated CpG dinucleotides may be important. The patterns of DNA methylation and histone acetylation were determined at the cyclin D1 locus on chromosome 11q13 in B-cell malignancies. The cyclin D1 promoter was hypomethylated and hyperacetylated in expressing cell lines and patient samples, and methylated and hypoacetylated in nonexpressing cell lines. Domains of hyperacetylated histones and hypomethylated DNA extended over 120 kb upstream of the cyclin D1 gene. Interestingly, hypomethylated DNA and hyperacetylated histones were also located at the cyclin D1 promoter but not the upstream major translocation cluster region in cyclin D1-nonexpressing, nontumorigenic B and T cells. RNA polymerase II binding was demonstrated both at the cyclin D1 promoter and 3′ immunoglobulin heavy-chain regulatory regions only in malignant B-cell lines with deregulated cyclin D1 expression. Our results suggest a model where RNA polymerase II bound at IgH regulatory sequences can activate the cyclin D1 promoter by either long-range polymerase transfer or tracking.
Introduction
B-cell malignancies such as non-Hodgkin lymphoma and multiple myeloma (MM) are characterized by 14q32 translocations involving the immunoglobulin heavy-chain (IgH) locus.1,2 These translocations serve to juxtapose regulatory elements, such as the intronic enhancer (Eμ)2 or 3′ Cα locus control region (LCR)3 that deregulate transcription of target genes over several hundred kilobases of linear DNA. The mechanisms involved in long-distance deregulation of target genes by IgH regulatory elements are not well understood; however, regulatory elements in the IgH locus are thought to activate the transcription of target genes such as c-myc,4 bcl-2,5 and cyclin D16 that are involved in B-cell malignancies.
In some diseases such as Burkitt lymphoma (BL, c-myc),7 MM,8 and mantle cell lymphoma9 (MCL, cyclin D1), the target gene can be separated by large linear distances (up to several hundred kilobases) from the translocated immunoglobulin regulatory sequences. The long-distance deregulation observed in B-cell malignancies is analogous to long-distance regulatory control observed in other multigene domains such as human growth hormone10 and the avian and mammalian β-globin loci.11,12 Potential mechanisms involved in long-distance gene regulation and deregulation include looping, tracking, or linking.13,14 Recent evidence for looping has accumulated with the demonstration of interactions between the β-globin LCR and promoters by 2 independent techniques.15,16
Epigenetic modifications of eukaryotic gene promoters such as DNA hypomethylation and histone acetylation correlate with activation of transcription. Activation of genes may occur by local promoter DNA hypomethylation and histone hyperacetylation. In addition, treatment of silenced genes with DNA methyltransferase or histone deacetylase inhibitors transcriptionally activates silent genes.17 Alternatively, chromatin domains of multigene families have been described where patterns of hyperacetylation extend over 50 to 100 kb.18
DNA methylation plays an important role in the control of eukaryotic gene expression.19 This modification occurs largely in the CpG dinucleotides (known as CpG islands when found in high concentrations upstream of a gene) of various palindromic sequences. CpG islands are often located at the promoters of housekeeping and other tissue-specific genes. At these locations, the CG sequence is not methylated. By contrast, the CG sequences in inactive gene promoters are usually methylated to suppress their expression. DNA methylation of the CpG islands in the promoter region of a gene can interfere with the binding of transcription factors, thus suppressing gene expression.20 Methylated DNA segments can also repress the transcription of genes distant from the methylation site (located on the same chromosome) by altering the structure of chromatin domains.21
Acetylation of the lysine residues at the N terminus of histone proteins removes positive charges, thereby reducing the affinity between histones and DNA. RNA polymerase and transcription factors are then able to access the promoter region of active genes. Acetylation of histone tails correlates with transcriptional activity in many genes. The importance of histone acetylation in the regulation of transcription is reinforced by the observations that histone deacetylation causes repression of gene expression. Histone deacetylases often form complexes that bind transcriptional repressors.17,21
The activation of the cyclin D1 gene that occurs in MCL and a subset of MM was used as a model system to investigate the mechanisms responsible for long-distance gene deregulation in B-cell malignancies. Cyclin D1 is not expressed in normal lymphocytes, where unlinked family members cyclin D2 and D3 are active.22,23 The cyclin D1 promoter is composed of a CpG island that can be potentially regulated by DNA methylation.24 In B-cell malignancies, cyclin D1 gene expression is activated by the insertion or translocation of IgH regulatory elements such as the Eμ intronic or 3′ Cα enhancer/LCRs that can be as far as 100 to 300 kb away from the cyclin D1 gene.8,9 The majority of breakpoints in MCL map to the major translocation cluster (MTC) region located about 120 kb upstream (centromeric) of the cyclin D1 gene.25 The nearest gene to cyclin D1, MYEOV, is located 360 kb centromeric to cyclin D1 and is expressed in a subset of t(11;14) MM but not MCL.26
Histone hyperacetylation and DNA hypomethylation may participate in the long-distance deregulation of the cyclin D1 gene observed in these malignancies. In multigene domains such as the β-globin18 and growth hormone loci,10 domains of hypomethylated DNA and hyperacetylated histones are known to exist. To investigate whether such a domain is present in the 11q13 region in cyclin D1–expressing B-cell malignancies, a detailed analysis of the patterns of histone H3 and H4 acetylation and DNA methylation was performed using chromatin immunoprecipitation (ChIP), Southern blot, and bisulfite sequencing assays.
ChIP assays were also performed to determine whether RNA polymerase II (Pol II) was bound in vivo to the cyclin D1 promoter and IgH regulatory elements (Eμ enhancer, 3′ IgH Cα HS3 and HS4). Pol II is bound to regulatory elements in vivo in the β-globin LCR,27 major histocompatibility complex (MHC) upstream regulatory element,28 and prostate-specific antigen enhancer.29 A long-range Pol II transfer (LPT) mechanism has been proposed in the mouse β-globin locus, where Pol II is first recruited to the LCR and then is relocalized to the promoters by accessory factors.30 In cyclin D1–expressing B-cell malignancies, Pol II was present at the cyclin D1 promoter and IgH regulatory regions.
Materials and methods
Cell culture
Human MCL cell lines Granta and NCEB-1, human MM cell line U266, human myelocytic cell line HL60, human erythroleukemia cell line K562, human small cell lung cancer (SCLC) cell line H82, human breast cancer cell lines MCF7, MDA-MB-231, and Epstein-Barr virus (EBV)–immortalized human B-lymphocyte cell lines LCL1 and LCL2 were maintained in RPMI 1640 supplemented with 10% calf serum. Human Burkitt lymphoma (BL) cell line Manca31 was maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% calf serum. Cell lines HL60, K562, U266, MCF7, and MDA-MB-231 were purchased from American Type Culture Collection (ATCC; Manassas, VA). Cell lines Granta519 (Granta) and NCEB-132 were obtained from Dr Martin Dyer (Department of Hematology, University of Leicester, United Kingdom). Manca cells were obtained from Dr Mark Groudine (Fred Hutchinson Cancer Research Center, Seattle, WA). EBV-immortalized B-cell lines LCL1 and LCL2 and interleukin 2 (IL-2)–activated T-cell clone D160 cells were obtained from Dr David Lewinsohn (Oregon Health & Science University, Portland). Samples of peripheral blood and bone marrow of patients with MCL/t(11;14) B-cell malignancy were obtained through a protocol approved by the Institutional Review Board of the University of Arizona. Informed consent statement was provided according to the Declaration of Helsinki. Human normal CD19+ B cells and human normal CD4+ T cells were purchased from Cambrex Bioscience (Walkersville, MD).
B-cell separation
The RosetteSep human B-cell enrichment cocktail (50 μL; catalog no. 15024, StemCell Technologies, Vancouver, BC, Canada) was added for 1 mL peripheral blood or bone marrow. Cells and antibody were incubated at room temperature for 20 minutes with gentle swirling every 5 minutes. The cell suspension was then diluted with phosphate-buffered saline (PBS) containing 2% heat-inactivated calf serum and underlain with 1 mL Ficoll-Paque Plus (Amersham Biosciences, Piscataway, NJ) and centrifuged at 650g for 20 minutes without braking. The cells at the interface were collected with a Pasteur pipette and washed twice prior to RNA/DNA extraction.
RT-PCR
Total RNA was isolated from various cell lines by using the RNA Trizol reagents (Invitrogen, Carlsbad, CA). Then, 3 to 5 μg total RNA was reverse transcribed into cDNA by using dNTP (1 mM), 5 × reverse transcription, buffer (250 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 8.5, 150 mM KCl, 40 mM MgCl2, and 50 mM dithiothreitol [DTT]), 16 U RNasin (Promega, Madison, WI), 10 mM DTT (Promega), and 2.5 units of avian myeloblastosis virus reverse transcriptase (Roche Biotech, Basel, Switzerland). Expression of cyclin D1 and actin control were determined by semiquantitative reverse transcription–polymerase chain reaction (RT-PCR). Human β-actin and cyclin D1 genes were coamplified in both the RT reaction and the following PCR. The cyclin D1 RT-PCR has been previously described33 and β-actin was coamplified as a control for RNA concentration and integrity. We used this semiquantitative RT-PCR assay to compare the ratio of cyclin D1 gene transcripts to the ubiquitously expressed constitutive gene β-actin. Linearity of the PCR under the conditions used was demonstrated by using dilutional analysis and varying PCR cycle number followed by densitometry. The primer sequences for cyclin D1 were 5′-CTGGCCATGAACTACCTGGA-3′ (forward) and 5′-TCACACTTGATCACTCTCG-3′ (reverse). The primer sequences for actin were 5′-GTTGCTATCCAGGCTGTGC-3′ (forward) and 5′-GCATCCTGTCGGCAATGC-3′ (reverse). The PCR products were separated on a 1.6% agarose gel, stained with ethidium bromide, and examined under UV transillumination. Each experiment was performed at least 3 times and representative data shown.
Southern blotting
Genomic DNA (10 μg) was digested with HindIII plus one of the paired restriction enzymes Msp, which is methylation insensitive, and HpaII, an isoschizomer that is inhibited by CpG methylation, overnight at 37° C. DNA fragments were separated by gel electrophoresis and transferred to a nylon membrane. Prehybridization and hybridization with [γ-32P]dCTP-labeled probes were performed at 42° C in a hybridization solution containing 50% formamide. Autoradiography was then performed with intensifying screens at –80° C for several days and the blot exposed to x-ray film. Each experiment was performed 2 to 3 times and representative data are shown.
Bisulfite sequencing
To elucidate the detailed methylation status of the promoter regions of cyclin D1, bisulfite DNA sequencing was performed. PCR primers were designed to amplify the CpG-rich region of the cyclin D1 promoter (Table 1; GenBank accession no. Z29078) or other regions after sodium bisulfite (Sigma, St Louis, MO) treatment. PCRs were conducted in 2 rounds using puRe Taq Ready-To-Go PCR Beads (Amersham Biosciences). The first round used primers US1/DS1 and bisulfite-treated DNA as template. The second round used primers US2/DS2 and PCR product from the first round as template. The PCR products were cloned into the pGEM-T easy vector system (Promega). Multiple clones (approximately 10 from each PCR product) were sequenced with a DNA-sequencing module developed by Biotechnology Computing Facility of Arizona Research Labs in the University of Arizona and subsequently at the Microbiology and Molecular Immunology Research Core Facility of the Oregon Health & Science University.
PCR primers for bisulfite sequencing (5′ to 3′), from the cyclin D1 promoter region, 1 kb upstream, and 3 kb upstream
Location . | Primer . | Temperature, °C . |
|---|---|---|
| D1 promoter | ||
| US1 | TTTTTTTAGTTGTTTTTTATTGTAGA | 52 |
| DS1 | CACAAAAACTAATATTCCATAACT | 52 |
| US2 | GGGTTTTTTGTATTTTTTTTTTTTGG | 54 |
| DS2 | AAACAACTAAAAAAAACTATAAATCCT | 54 |
| 1 kb upstream of D1 promoter | ||
| US1 | TAGGGTAAATTTTAAAGGTGAA | 54 |
| DS1 | AAAAAAAAAAAATACAAAAAACCC | 54 |
| US2 | TGTTTTAGGTAGAGGGGATTAA | 58 |
| DS2 | AAAAAATAAAATAAAAATAACTCTACA | 58 |
| 3 kb upstream of D1 promoter | ||
| US1 | TTAAGGGTTTAATAATGGAAAA | 53 |
| DS1 | ACAAAAAACAAATAACCCAATC | 53 |
| US2 | GTTGTAGGTGATTTTATTTGGG | 57 |
| DS2 | CCAAAATTAACTAAAATCACCTTC | 57 |
Location . | Primer . | Temperature, °C . |
|---|---|---|
| D1 promoter | ||
| US1 | TTTTTTTAGTTGTTTTTTATTGTAGA | 52 |
| DS1 | CACAAAAACTAATATTCCATAACT | 52 |
| US2 | GGGTTTTTTGTATTTTTTTTTTTTGG | 54 |
| DS2 | AAACAACTAAAAAAAACTATAAATCCT | 54 |
| 1 kb upstream of D1 promoter | ||
| US1 | TAGGGTAAATTTTAAAGGTGAA | 54 |
| DS1 | AAAAAAAAAAAATACAAAAAACCC | 54 |
| US2 | TGTTTTAGGTAGAGGGGATTAA | 58 |
| DS2 | AAAAAATAAAATAAAAATAACTCTACA | 58 |
| 3 kb upstream of D1 promoter | ||
| US1 | TTAAGGGTTTAATAATGGAAAA | 53 |
| DS1 | ACAAAAAACAAATAACCCAATC | 53 |
| US2 | GTTGTAGGTGATTTTATTTGGG | 57 |
| DS2 | CCAAAATTAACTAAAATCACCTTC | 57 |
ChIP
ChIP was performed according to the protocol included with antidiacetylated H3 (catalog no. 17-245), and antitetracetylated H4 antibodies (catalog no. 17-229; Upstate Biotechnology, Lake Placid, NY). Antibody to RNA Pol II was obtained from Santa Cruz Biotechnology (Santa Cruz, CA; catalog no. sc-899). A cell suspension (5 × 106 cells/ChIP assay) was fixed with medium containing 1% formaldehyde for 10 minutes at 37° C. The cell suspension was washed with PBS and treated with sodium dodecyl sulfate (SDS) lysis buffer (1% SDS, 10 mM EDTA [ethylenediaminetetraacetic acid], 50 mM Tris-HCl, pH 8.1) containing protease inhibitors of 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μg/mL aprotinin, and 1 μg/mL pepstatin A (all from Sigma) for 10 minutes on ice. The suspension was then sonicated (Microson Ultrasonic Cell Disruptor; Misonix, Farmingdale, NY) for five 15-second treatments, and the supernatant was recovered by centrifugation at 15 500g for 10 minutes at 4° C. The supernatant was diluted with 9 × volume ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 150 mM NaCl) and pretreated with 30 μL salmon sperm DNA/protein A agarose/50% slurry for 30 minutes at 4° C. In addition, for the Pol II ChIP reaction, the supernatant was preabsorbed with rabbit preimmune serum overnight at 4° C before adding the salmon sperm DNA/protein A agarose/50% slurry. The pretreated supernatant (1 mL/IP condition) was incubated with antibody with rotation overnight at 4° C. To collect the antibody-histone complex, 40 μL salmon sperm DNA/protein A agarose was added, and the mixture was incubated for 2 hours at 4° C with rotation. According to the protocol, the pelleted protein A agarose-antibody-histone complex was washed. The histone complex was eluted from the antibody with 0.5 mL elution buffer (1% SDS, 0.1 M NaHCO3); 20 μL 5 M NaCl was added to the elution, and the histone-DNA complex was reverse cross-linked by heating at 65° C for 4 hours. To the reaction mixture was added 10 μL 0.5 M EDTA, 20 μL1 M Tris-HCl, pH 6.5, and 2 μL 15 mg/mL proteinase K, and this mixture was incubated for 1 hour at 45° C. The DNA was recovered by phenol/chloroform extraction and ethanol precipitation, and then dissolved with 10 mM Tris-HCl (pH 7.5)/1 mM EDTA. Control DNA was recovered from the sonication supernatant by the same procedure as for the input DNA. The average size of the control DNA recovered was 600 to 1000 bp. A total of 2.5 μL of 50 μL antibody-bound DNA or 0.5 μL of 100 μL control DNA was used as template for PCR. PCR products were resolved by agarose gel electrophoresis with ethidium bromide staining. Each experiment was performed 2 to 3 times and representative data shown. Table 2 lists all the PCR primers used for analysis. No antibody and mouse IgG (Sigma) were used as controls with similar results.
PCR primers for ChIP assays
Name . | Forward . | Reverse . | Temperature, °C . |
|---|---|---|---|
| GAPDH | TAGTGTCCTGCTGCCCACAGTCCAG | GGCGACGCAAAAGAAGATGC | 68 |
| β-globin 5′ HS2 | TGAGTGGCAGTGTTTAAAGGG | CCATCCCTGATGAGTTTTTCCTC | 63 |
| MYEOV | CTACCCTCTCCTCTTCCTTCTC | TACGCATGGCAGACATCAC | 60 |
| MTC | AGATTAAACTGCGTCTTGTTCG | TAGAGGCTTTGTCCTACCATCC | 62 |
| P519 | ACATGAGCGATCTGGTAAGGACTC | CCACATGAGAGCTCACTCTGCAA | 60 |
| P519_bkp | GAGGGAGACCTATAACATGAGC | TACTGTAAGGGAGATGTGTTGG | 62 |
| UP10K | TACAGACCATCACCGTTGCC | TTCACTCACCACCCATCCTC | 65 |
| UP3K | TTTCTCCTGACCGACCATCC | CACTGTCTTCTTAAAACCCACC | 63 |
| D1 promoter | CCTTGGGCATTTGCAACGAC | CGCATTTCCAAGAACGCCAC | 64 |
| D1 intron 1 | TTCTTTGCTACTCACCCCC | CCCTTCCTCCTTCAGAAAATAC | 61 |
| D1 intron 4 | TGGCTCAGAAACACCATCG | GGACATTCCACCCACCAAAG | 65 |
| Down 60 K | ACAATATCCCACCATCTCCTC | AACCCTAACACTCCCCATC | 61 |
| Eμ | CAGCCCTTGTTAATGGACTT | GGAAAGTTAAATGGGAGTGACC | 65 |
| 3′ Cα HS4 | TCCAGTCTGAAAAACAAGACC | ACCTCCCCCAATGCAAATC | 63 |
| 3′ Cα HS3 | AGGTCTCGACTTAGCACTG | GGCATGTTTCTCAGAACAGC | 66 |
Name . | Forward . | Reverse . | Temperature, °C . |
|---|---|---|---|
| GAPDH | TAGTGTCCTGCTGCCCACAGTCCAG | GGCGACGCAAAAGAAGATGC | 68 |
| β-globin 5′ HS2 | TGAGTGGCAGTGTTTAAAGGG | CCATCCCTGATGAGTTTTTCCTC | 63 |
| MYEOV | CTACCCTCTCCTCTTCCTTCTC | TACGCATGGCAGACATCAC | 60 |
| MTC | AGATTAAACTGCGTCTTGTTCG | TAGAGGCTTTGTCCTACCATCC | 62 |
| P519 | ACATGAGCGATCTGGTAAGGACTC | CCACATGAGAGCTCACTCTGCAA | 60 |
| P519_bkp | GAGGGAGACCTATAACATGAGC | TACTGTAAGGGAGATGTGTTGG | 62 |
| UP10K | TACAGACCATCACCGTTGCC | TTCACTCACCACCCATCCTC | 65 |
| UP3K | TTTCTCCTGACCGACCATCC | CACTGTCTTCTTAAAACCCACC | 63 |
| D1 promoter | CCTTGGGCATTTGCAACGAC | CGCATTTCCAAGAACGCCAC | 64 |
| D1 intron 1 | TTCTTTGCTACTCACCCCC | CCCTTCCTCCTTCAGAAAATAC | 61 |
| D1 intron 4 | TGGCTCAGAAACACCATCG | GGACATTCCACCCACCAAAG | 65 |
| Down 60 K | ACAATATCCCACCATCTCCTC | AACCCTAACACTCCCCATC | 61 |
| Eμ | CAGCCCTTGTTAATGGACTT | GGAAAGTTAAATGGGAGTGACC | 65 |
| 3′ Cα HS4 | TCCAGTCTGAAAAACAAGACC | ACCTCCCCCAATGCAAATC | 63 |
| 3′ Cα HS3 | AGGTCTCGACTTAGCACTG | GGCATGTTTCTCAGAACAGC | 66 |
Results
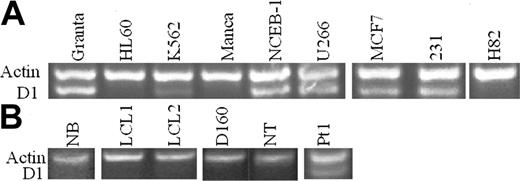
The t(11;14)(q13;q32) observed in B-cell malignancies results in the overexpression of cyclin D1 (also known as bcl-1, PRAD1, CCND1). Initially, cyclin D1 expression was analyzed by semiquantitative RT-PCR33 in human hematopoietic cell lines. Human β-actin and cyclin D1 genes were coamplified in both the RT reaction and the following PCR. Human β-actin, a housekeeping gene, was used as positive control for RNA concentration and integrity. We have confirmed that this assay is linear under the conditions used and can be used to distinguish high levels of cyclin D1 mRNA in overexpressing cell lines from nonexpressing cell lines or the low levels found in K562 erythroleukemia cells. Cyclin D1 expression was evident in 2 MCL cell lines (Granta, NCEB-1), which contain a t(11;14) translocation and one MM cell line (U266), which contains an insertion of IgH regulatory sequences near the cyclin D1 gene.34 A promyelocytic leukemia cell line (HL60) and a BL cell line (Manca) did not express cyclin D1. In contrast, a stem cell/erythroleukemia cell line (K562) with no described translocation or insertion of IgH regulatory sequences showed low-level cyclin D1 expression. Immunohistochemical (IHC) studies with anti–cyclin D1 antibody showed no evidence of heterogeneity in the K562 or Granta cell populations and no detectable expression by IHC in K562 cells (data not shown). Two human breast cancer cell lines (MCF7, MDA-MB-231) both expressed cyclin D1 mRNA, whereas a SCLC cell line H82 did not (Figure 1A). Cyclin D1 expression status was also examined in nonmalignant human B and T cells and peripheral blood B cells of a patient with lymphocytosis and t(11;14) B-cell malignancy.35 There was no cyclin D1 expression in normal B cells (NB), 2 EBV-immortalized B-cell lines (LCL1, LCL2), T-cell clone (D160), or resting T cells (NT) (Figure 1B), whereas abundant cyclin D1 mRNA expression was found in purified B cells from peripheral blood of patient 1.
Cyclin D1 RT-PCR in cell lines and cells. (A) MCL cell lines NCEB-1 and Granta and MM cell line U266 expressed cyclin D1 mRNA. Breast cancer cell lines MCF7 and MDA-MB-231 (231) were also cyclin D1+. K562 stem cell/erythroleukemia cells expressed low levels of cyclin D1. HL60 and Manca cells did not express cyclin D1 mRNA. SCLC cell line H82 was also cyclin D1–. (B) Normal human B cells CD19+ (NB), EBV-immortalized human B cells (LCL1, LCL2), normal human T-cell clone (D160), and normal human T cells CD4+ (NT) were all cyclin D1–. Pt1 indicates a sample of purified B cells from peripheral blood from a patient with t(11;14) B-cell malignancy and expressed cyclin D1 mRNA. Actin was used as control.
Cyclin D1 RT-PCR in cell lines and cells. (A) MCL cell lines NCEB-1 and Granta and MM cell line U266 expressed cyclin D1 mRNA. Breast cancer cell lines MCF7 and MDA-MB-231 (231) were also cyclin D1+. K562 stem cell/erythroleukemia cells expressed low levels of cyclin D1. HL60 and Manca cells did not express cyclin D1 mRNA. SCLC cell line H82 was also cyclin D1–. (B) Normal human B cells CD19+ (NB), EBV-immortalized human B cells (LCL1, LCL2), normal human T-cell clone (D160), and normal human T cells CD4+ (NT) were all cyclin D1–. Pt1 indicates a sample of purified B cells from peripheral blood from a patient with t(11;14) B-cell malignancy and expressed cyclin D1 mRNA. Actin was used as control.
DNA methylation
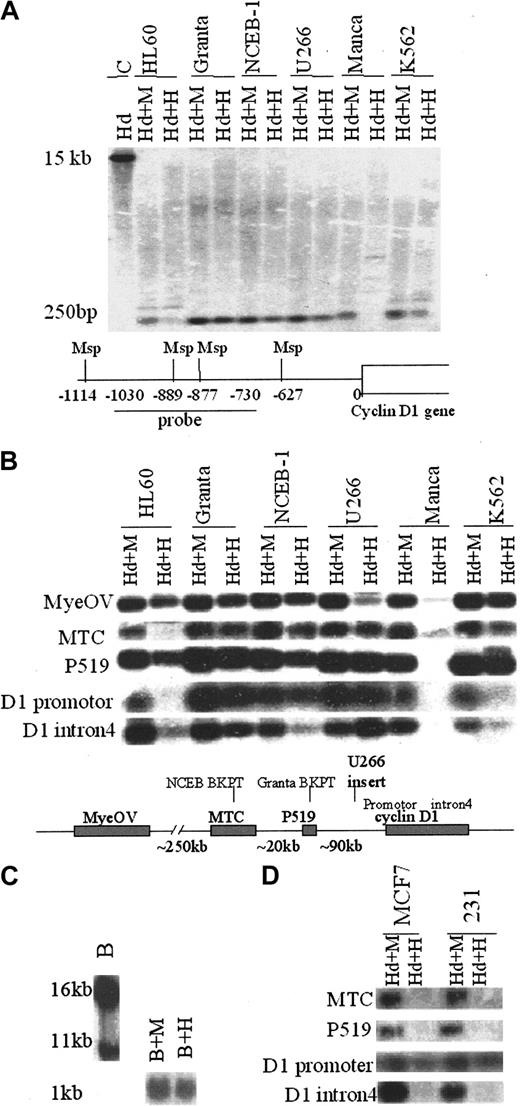
The pattern of DNA methylation in the 11q13 region near the cyclin D1 gene was first studied by a Southern blot assay using the paired restriction enzymes Msp, which is methylation insensitive, and HpaII, an isoschizomer that is inhibited by CpG methylation. Complete cleavage by Msp will produce a small–molecular weight band whose size depends on the probe used. If CpG methylation is present at the HpaII recognition site, the low–molecular weight band will not be observed and the higher–molecular weight band will be produced. Figure 2A illustrates an example using analysis of the cyclin D1 promoter region in cyclin D1-expressing and -nonexpressing cells. The lower–molecular weight band observed represents a doublet of completely cleaved fragments. When one or both of the HpaII recognition sites is methylated, the low–molecular weight 250-bp bands will disappear in the HpaII plus HindIII lane and will be replaced by larger–molecular weight bands. Figure 2A shows that hypomethylation of the cyclin D1 promoter region correlated with expression of cyclin D1 in hematopoietic cell lines. Multiple DNA probes were then used to determine the methylation structure of a large domain around the cyclin D1 gene. By comparing the smallest DNA fragment, a composite figure (Figure 2B) is shown of the hybridization patterns of 11q13 probes in cyclin D1-overexpressing MCL (NCEB-1, Granta) and MM (U266) as well as control cell lines. Most genomic regions were unmethylated and showed no correlation between expression of the cyclin D1 gene and methylation status. Some of the probes used (MYEOV, P519, and intron 4 of the cyclin D1 gene) showed variable patterns of DNA methylation in the cell lines examined. However, cyclin D1-expressing cells also exhibited complete DNA hypomethylation at the P519 and MTC regions located about 90 and 120 kb upstream of the cyclin D1 gene, respectively. The cyclin D1 promoter region and upstream P519 and MTC regions were unmethylated in overexpressing cell lines (Granta, NCEB1, U266; see map at the bottom of Figure 2B for probes used). The majority of breakpoints in MCL map to these regions. There were no high–molecular weight bands detectable in the cyclin D1–expressing cell lines (Figure 2A and data not shown). K562 cells, which express low amounts of cyclin D1 mRNA without a t(11;14) translocation, exhibited partial methylation of the cyclin D1 promoter and MTC regions. Using a probe in the P519 region that spans the translocation breakpoint in Granta cells, Southern blot analysis showed complete hypomethylation at both translocated and normal chromosomes in Granta cells (Figure 2C).
DNA methylation status in the cyclin D1 promoter and upstream regions in the 11q13 locus correlates with the cyclin D1 gene expression. (A) DNA from cyclin D1–expressing B-cell lines (Granta, NCEB-1, U266), cyclin D1–nonexpressing cell lines (HL60, Manca), and a low-expressing cell line (K562) was cleaved with HindIII (Hd) plus Msp (M) or HpaII (H), Southern blotted, and probed with a fragment of the cyclin D1 promoter. The map below the blots shows Msp sites and position of the probe relative to the cyclin D1 gene transcription start, which is included in a 15-kb HindIII fragment as shown in the first lane. (B) Southern analysis of DNA methylation in cyclin D1–expressing and –nonexpressing hematopoietic cell lines with probes in the 11q13 region. The map below the blots shows the probes used and the sites of translocation breakpoints relative to the cyclin D1 gene locus. The translocation breakpoints for Granta cells and NCEB-1 cells are indicated as well as the site of insertion of IgH regulatory sequences in U266 cells. The small DNA fragments produced by complete cleavage (plus HindIII, Hd) are shown. If there is a band in the Hd+H(HindIII + HpaII) lane, that sequence is unmethylated. In contrast, if there is no band observed, then that sequence is methylated and a higher–molecular weight band was seen (not shown). (C) Southern analysis of DNA methylation in Granta cell line using a t(11;14) translocation breakpoint probe at the P519 region. When restriction enzyme BamHI (B) was used alone for the digestion and probed with the P519 probe, two bands were produced, representing the translocated (16-kb) and normal (11-kb) chromosomes, respectively. When gDNAwas cleaved with BamHI plus Msp (B+M) or BamHI plus HpaII (B+H), there was only one small band (1 kb) observed, consistent with hypomethylation of both the translocated and untranslocated chromosomes. (D) Southern blot analysis of DNA methylation in cyclin D1+ breast cancer cell lines MCF7 and MDA-MB-231(231). The cyclin D1 promoter was unmethylated in both cell lines, but the upstream MTC and P519 regions were methylated.
DNA methylation status in the cyclin D1 promoter and upstream regions in the 11q13 locus correlates with the cyclin D1 gene expression. (A) DNA from cyclin D1–expressing B-cell lines (Granta, NCEB-1, U266), cyclin D1–nonexpressing cell lines (HL60, Manca), and a low-expressing cell line (K562) was cleaved with HindIII (Hd) plus Msp (M) or HpaII (H), Southern blotted, and probed with a fragment of the cyclin D1 promoter. The map below the blots shows Msp sites and position of the probe relative to the cyclin D1 gene transcription start, which is included in a 15-kb HindIII fragment as shown in the first lane. (B) Southern analysis of DNA methylation in cyclin D1–expressing and –nonexpressing hematopoietic cell lines with probes in the 11q13 region. The map below the blots shows the probes used and the sites of translocation breakpoints relative to the cyclin D1 gene locus. The translocation breakpoints for Granta cells and NCEB-1 cells are indicated as well as the site of insertion of IgH regulatory sequences in U266 cells. The small DNA fragments produced by complete cleavage (plus HindIII, Hd) are shown. If there is a band in the Hd+H(HindIII + HpaII) lane, that sequence is unmethylated. In contrast, if there is no band observed, then that sequence is methylated and a higher–molecular weight band was seen (not shown). (C) Southern analysis of DNA methylation in Granta cell line using a t(11;14) translocation breakpoint probe at the P519 region. When restriction enzyme BamHI (B) was used alone for the digestion and probed with the P519 probe, two bands were produced, representing the translocated (16-kb) and normal (11-kb) chromosomes, respectively. When gDNAwas cleaved with BamHI plus Msp (B+M) or BamHI plus HpaII (B+H), there was only one small band (1 kb) observed, consistent with hypomethylation of both the translocated and untranslocated chromosomes. (D) Southern blot analysis of DNA methylation in cyclin D1+ breast cancer cell lines MCF7 and MDA-MB-231(231). The cyclin D1 promoter was unmethylated in both cell lines, but the upstream MTC and P519 regions were methylated.
DNA methylation patterns were also examined in 2 cyclin D1–overexpressing breast cell lines that do not deregulate cyclin D1 expression by IgH translocation. Although the promoter was unmethylated, consistent with the expression of cyclin D1, the P519 and MTC upstream regions in both breast cancer lines were CpG methylated (Figure 2D), suggesting the hypomethylation seen at the upstream regions may be associated with B-cell malignancies that deregulate cyclin D1.
Bisulfite sequencing experiments were then conducted to confirm and extend these results by assaying the methylation status of all CpG dinucleotides biochemically. The bisulfite sequencing data corroborated the difference in methylation at the cyclin D1 promoter region in expressing and nonexpressing cells (Figure 3B). These differences in DNA methylation status were shown to extend as far as 3 kb upstream of the cyclin D1 gene transcription initiation site (Figure 3C).
The cyclin D1 promoter region showed differential cytosine methylation in cyclin D1+ and cyclin D1– cell lines. (A) Diagram of the cyclin D1 promoter region analyzed. The bent arrow represents the transcription start site, the vertical lines mark the locations of CpG dinucleotides, and filled rectangles indicate the positions of PCR primers used for sodium bisulfite genomic sequence analysis. Msp indicates the positions of restriction sites. (B). Bisulfite analysis of the cyclin D1 promoter region methylation in cyclin D1-overexpressing cell lines (Granta, NCEB1, and U266), a cyclin D1 low-expressing cell line (K562), and cyclin D1–nonexpressing cell lines (HL60 and Manca). (C) Bisulfite analysis of the upstream region of the cyclin D1 promoter in Granta and HL60 cell lines. CpG sites in the region (–2385 to –845) were not analyzed. Diagrams in A and C (upper panels) show locations of CpG dinucleotides relative to the cyclin D1 gene transcription start.
The cyclin D1 promoter region showed differential cytosine methylation in cyclin D1+ and cyclin D1– cell lines. (A) Diagram of the cyclin D1 promoter region analyzed. The bent arrow represents the transcription start site, the vertical lines mark the locations of CpG dinucleotides, and filled rectangles indicate the positions of PCR primers used for sodium bisulfite genomic sequence analysis. Msp indicates the positions of restriction sites. (B). Bisulfite analysis of the cyclin D1 promoter region methylation in cyclin D1-overexpressing cell lines (Granta, NCEB1, and U266), a cyclin D1 low-expressing cell line (K562), and cyclin D1–nonexpressing cell lines (HL60 and Manca). (C) Bisulfite analysis of the upstream region of the cyclin D1 promoter in Granta and HL60 cell lines. CpG sites in the region (–2385 to –845) were not analyzed. Diagrams in A and C (upper panels) show locations of CpG dinucleotides relative to the cyclin D1 gene transcription start.
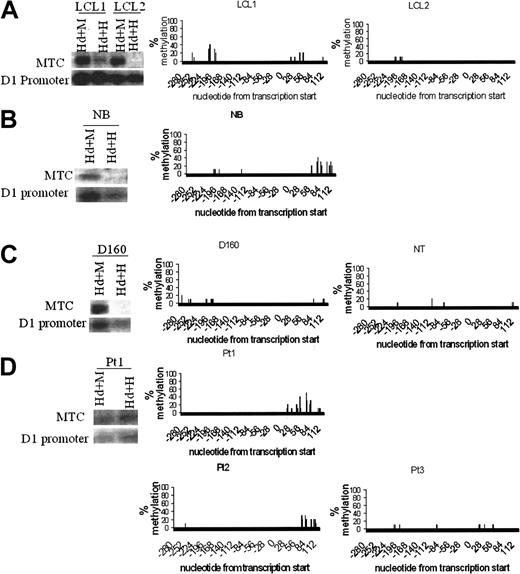
The pattern of complete hypomethylation of the cyclin D1 promoter and upstream regions in MCL and MM cell lines was surprising given the presence of both a translocated and normal chromosome 11 in these cell lines (Figure 2C and data not shown). DNA methylation patterns were then examined in nontumorigenic B and T cells, including normal CD19+ B cells, 2 EBV-transformed B-lymphocyte cell lines, normal CD4+ T cells, and an activated T-cell clone. These B and T cells did not express cyclin D1 mRNA (Figure 1B). Surprisingly, Southern blot analysis and bisulfite sequencing assays showed that the cyclin D1 promoter was unmethylated in all the nonmalignant sources of B and T cells (Figure 4A-C). However, in B and T cells, the upstream MTC region contained methylated CpG dinucleotides, similar to the results with cyclin D1-nonexpressing hematopoietic cell lines. The methylation pattern at the MTC in B and T cells differed from MCL cell lines and B cells from a t(11;14) patient sample, where the MTC was unmethylated (Figures 2B and 4D).
Cyclin D1 promoter DNA was unmethylated in normal human B and T cells. Southern blot analysis with the cyclin D1 promoter and the MTC probes and bisulfite sequencing analysis in the cyclin D1 promoter region. (A) EBV-transformed human B cells. (B) Normal human CD19+ B cells. (C) Normal human CD4+ T cells. (D) Patient samples with cyclin D1+ B-cell malignancies. For normal B and T cells, the cyclin D1 promoter was demethylated even though the gene was not expressed (Figure 1B). However, the MTC region was methylated. In 2 MCL bone marrow samples from patients (Pt 2 and Pt 3) and in a peripheral blood sample from a patient with lymphocytosis and t(11;14) B-cell malignancy (Pt 1), both the cyclin D1 promoter region (Pts 1-3) and the MTC region (Pt 1) were unmethylated in purified B cells. The methylation differences in the MTC region were confirmed by bisulfite sequencing (not shown), although the number of CpG dinucleotides in the MTC is small.
Cyclin D1 promoter DNA was unmethylated in normal human B and T cells. Southern blot analysis with the cyclin D1 promoter and the MTC probes and bisulfite sequencing analysis in the cyclin D1 promoter region. (A) EBV-transformed human B cells. (B) Normal human CD19+ B cells. (C) Normal human CD4+ T cells. (D) Patient samples with cyclin D1+ B-cell malignancies. For normal B and T cells, the cyclin D1 promoter was demethylated even though the gene was not expressed (Figure 1B). However, the MTC region was methylated. In 2 MCL bone marrow samples from patients (Pt 2 and Pt 3) and in a peripheral blood sample from a patient with lymphocytosis and t(11;14) B-cell malignancy (Pt 1), both the cyclin D1 promoter region (Pts 1-3) and the MTC region (Pt 1) were unmethylated in purified B cells. The methylation differences in the MTC region were confirmed by bisulfite sequencing (not shown), although the number of CpG dinucleotides in the MTC is small.
Histone acetylation
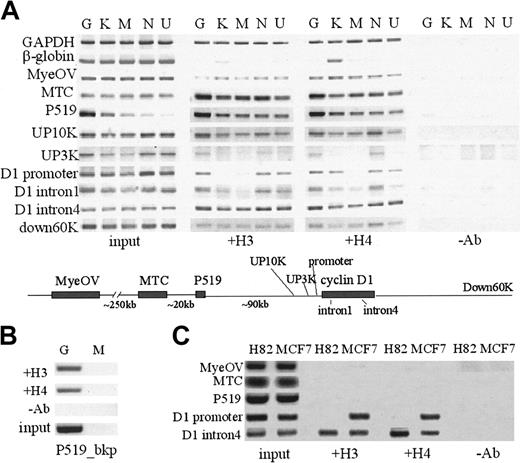
ChIP assays were then used to determine the patterns of histone H3 and H4 acetylation in the 11q13 region in cyclin D1-expressing versus -nonexpressing cell lines. The cyclin D1 promoter region was histone H3 hyperacetylated in MCL and MM cells (Granta, NCEB-1, U266) and K562, and hypoacetylated in Manca and HL60 cells. Histone H3 acetylation correlated with cyclin D1 expression status 3 kb upstream of the promoter. However, at 10 kb upstream of the cyclin D1 promoter, and continuing further upstream until the MTC, the sequences identified were hyperacetylated in all hematopoietic cell lines examined. The pattern of histone H4 acetylation was very similar in all cell lines except K562 cells, where the cyclin D1 promoter region and β-globin LCR sequences were H4 but not H3 hyperacetylated (Figure 5A). Differing patterns of H3 and H4 acetylation in the mouse β-globin locus have also been reported.27 Using a primer set specific for the translocated allele in Granta cells, the P519 breakpoint region was histone H3 and H4 hyperacetylated on the translocated chromosome (Figure 5B).
Analysis of histone H3, H4 acetylation using ChIP assays. (A) Hematopoietic cell lines: MCL Granta (G) and NCEB-1 (N), MM U266 (U), all cyclin D1+, and control K562 (K), Manca (M) cell lines. β-Globin 5′ HS2 and glyceraldehyde phosphate dehydrogenase (GAPDH) served as controls. (B) MCL cell line Granta (G) with t(11;14) translocation breakpoint at the P519 region was studied. P519_bkp included a 5′ primer on the translocation breakpoint and a 3′ primer on chromosome 11 so that the PCR detects H3 and H4 acetylation at the P519 region in a translocated chromosome-specific manner. P519_bkp region showed histone H3 and H4 acetylation in Granta cells. Manca (M) was used as negative control. (C) Nonhematopoietic cell lines: H82 (SCLC cell line, cyclin D1 nonexpressing) and MCF7 (breast cancer cell line, cyclin D1 overexpressing). Differential H3 and H4 acetylation was found in the cyclin D1 promoter region, which extended 3 kb upstream and correlated with cyclin D1 gene expression status. All the other regions were acetylated in all hematopoietic cell lines, whereas upstream regions in nonhematopoietic cell lines were hypoacetylated.
Analysis of histone H3, H4 acetylation using ChIP assays. (A) Hematopoietic cell lines: MCL Granta (G) and NCEB-1 (N), MM U266 (U), all cyclin D1+, and control K562 (K), Manca (M) cell lines. β-Globin 5′ HS2 and glyceraldehyde phosphate dehydrogenase (GAPDH) served as controls. (B) MCL cell line Granta (G) with t(11;14) translocation breakpoint at the P519 region was studied. P519_bkp included a 5′ primer on the translocation breakpoint and a 3′ primer on chromosome 11 so that the PCR detects H3 and H4 acetylation at the P519 region in a translocated chromosome-specific manner. P519_bkp region showed histone H3 and H4 acetylation in Granta cells. Manca (M) was used as negative control. (C) Nonhematopoietic cell lines: H82 (SCLC cell line, cyclin D1 nonexpressing) and MCF7 (breast cancer cell line, cyclin D1 overexpressing). Differential H3 and H4 acetylation was found in the cyclin D1 promoter region, which extended 3 kb upstream and correlated with cyclin D1 gene expression status. All the other regions were acetylated in all hematopoietic cell lines, whereas upstream regions in nonhematopoietic cell lines were hypoacetylated.
Examination of the histone acetylation patterns in the cyclin D1-overexpressing breast cancer line MCF7 showed acetylated H3 and H4 at the promoter and intron 4 regions, consistent with cyclin D1 gene expression, whereas the cyclin D1 promoter region was hypoacetylated in the cyclin D1-nonexpressing lung cancer cell line H82 (Figure 5B). However, the cyclin D1 upstream regions, including the P519 and MTC regions, were hypoacetylated in both H82 and MCF7 cell lines, in contrast to all the hematopoietic cell lines examined, where they were hyperacetylated.
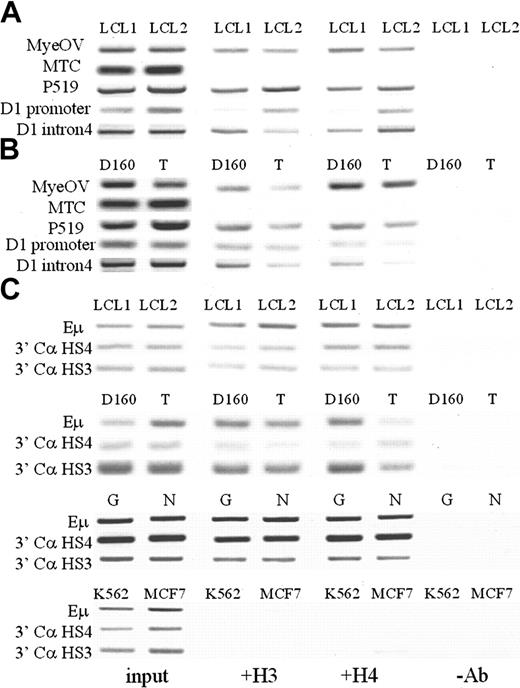
Histone acetylation patterns were then examined in nontumorigenic B and T cells, including 2 EBV-transformed B-lymphocyte cell lines (LCL1 and LCL2; Figure 6A) and normal CD4+ T cells and an activated T-cell clone (Figure 6B). These B and T cells did not express cyclin D1 mRNA (Figure 1B). ChIP assays showed that the cyclin D1 promoter and upstream regions to P519 contained acetylated histones H3 and H4. The MTC region was hypoacetylated in all the nonmalignant sources of B- and T-cell sources examined. Acetylated histones were also present at the IgH regulatory regions Eμ and 3′ Cα HS3 and HS4 in MCL cell lines and B- and T-cell sources, but not in K562 or MCF7 cells (Figure 6C).
Analysis of histone acetylation patterns at the cyclin D1 locus and IgH regulatory regions in nonmalignant human B and T cells. (A) Two EBV-immortalized human B-cell lines, LCL1 and LCL2, showed acetylation in all regions except the MTC region. (B) Activated T-cell clone (D160) and cord blood CD4+ T cells (T) showed a similar pattern. (C). IgH regulatory regions including Eμ intronic enhancer, 3′ Cα DnaseI-hypersensitive regions HS4 and HS3 from chromosome 14 were acetylated in the normal B/T cells and the MCL cell lines Granta (G) and NCEB-1 (N), but not in the stem cell/erythroleukemia cell line K562 and breast cancer cell line MCF7.
Analysis of histone acetylation patterns at the cyclin D1 locus and IgH regulatory regions in nonmalignant human B and T cells. (A) Two EBV-immortalized human B-cell lines, LCL1 and LCL2, showed acetylation in all regions except the MTC region. (B) Activated T-cell clone (D160) and cord blood CD4+ T cells (T) showed a similar pattern. (C). IgH regulatory regions including Eμ intronic enhancer, 3′ Cα DnaseI-hypersensitive regions HS4 and HS3 from chromosome 14 were acetylated in the normal B/T cells and the MCL cell lines Granta (G) and NCEB-1 (N), but not in the stem cell/erythroleukemia cell line K562 and breast cancer cell line MCF7.
RNA Pol II binding
ChIP assays were also used to study the presence of Pol II at the cyclin D1 promoter and IgH regulatory regions. Pol II was bound to the cyclin D1 promoter in MCL lines Granta and NCEB-1 and in MCF7 breast cancer cells, which expressed high levels of cyclin D1 (Figure 7A-B). Interestingly, no Pol II binding was detected at the cyclin D1 promoter in K562 erythroleukemia cells (Figure 7B), which express low levels of cyclin D1 mRNA by RT-PCR. There was no Pol II found at the cyclin D1 promoter region in nonmalignant B or T cells.
ChIP assays of RNA Pol II binding to the cyclin D1 promoter and IgH regulatory regions. (A) Pol II bound to the cyclin D1 promoter in the cyclin D1+ MCL cell lines Granta (G), NCEB-1 (N). Pol II also bound to IgH regulatory regions (Eμ, HS4, HS3) in Granta, NCEB-1, and Manca (M) cells, which contain chromosomal translocation involving IgH regulatory elements. Myeloid HL60 cells were used as a negative control. (B) MCF7, a cyclin D1+ breast cancer cell line, demonstrated Pol II binding in the cyclin D1 promoter region but not the IgH regulatory elements. EBV-immortalized human B cells, LCL1 and LCL2, showed Pol II binding in the Eμ intronic enhancer region. K562 was used as a negative control. (C) Normal human CD4+ T cells (NT) and activated T-cell clone (D160) showed no Pol II binding in either region. Granta (G) and NCEB-1 (N) were used as positive controls.
ChIP assays of RNA Pol II binding to the cyclin D1 promoter and IgH regulatory regions. (A) Pol II bound to the cyclin D1 promoter in the cyclin D1+ MCL cell lines Granta (G), NCEB-1 (N). Pol II also bound to IgH regulatory regions (Eμ, HS4, HS3) in Granta, NCEB-1, and Manca (M) cells, which contain chromosomal translocation involving IgH regulatory elements. Myeloid HL60 cells were used as a negative control. (B) MCF7, a cyclin D1+ breast cancer cell line, demonstrated Pol II binding in the cyclin D1 promoter region but not the IgH regulatory elements. EBV-immortalized human B cells, LCL1 and LCL2, showed Pol II binding in the Eμ intronic enhancer region. K562 was used as a negative control. (C) Normal human CD4+ T cells (NT) and activated T-cell clone (D160) showed no Pol II binding in either region. Granta (G) and NCEB-1 (N) were used as positive controls.
ChIP assays were then used to determine whether Pol II is bound in vivo to IgH regulatory elements. Pol II was bound in vivo to the IgH Eμ intronic enhancer, 3′ Cα LCR HS3 and HS4 in MCL cell lines Granta and NCEB-1 and the BL cell line Manca (Figure 7A). These cell lines all contain IgH translocations. Pol II was also present at Eμ in EBV-transformed B cells but not in T cells. No Pol II was bound at the 3′ LCR Cα HS3 and HS4 in nonmalignant B or T cells or in K562 and MCF7 cells (Figure 7B-C). The latter 2 cell lines express cyclin D1 but do not possess IgH translocations.
A summary of the patterns of DNA methylation, histone acetylation, and Pol II binding is shown in Table 3.
Summary of results for epigenetic changes on the 11q13 and 14q32 loci
. | G . | N . | U . | H . | M . | K562 . | MCF7 . | 231 . | H82 . | Pt . | LCL . | NB . | T . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 mRNA | + | + | + | - | - | +/- | + | + | - | + | - | - | - |
| DNA methylation | |||||||||||||
| MYEOV | - | - | +/- | - | + | - | ND | ND | ND | ND | ND | ND | ND |
| MTC | - | - | - | + | + | +/- | + | + | ND | - | + | + | + |
| P519 | - | - | - | - | + | - | + | + | ND | ND | ND | ND | ND |
| D1 promoter | - | - | - | + | + | +/- | - | - | ND | - | - | - | - |
| D1 intron 4 | - | + | - | + | + | +/- | + | + | ND | ND | ND | ND | ND |
| Histone acetylation | |||||||||||||
| MYEOV | + | + | + | ND | + | + | - | ND | - | ND | + | ND | + |
| MTC | + | + | + | ND | + | + | - | ND | - | ND | - | ND | - |
| P519 | + | + | + | ND | + | + | - | ND | - | ND | + | ND | + |
| D1 promoter | + | + | + | ND | - | + (H4) - (H3) | + | ND | - | ND | + | ND | + |
| D1 intron 4 | + | + | + | ND | + | + | + | ND | + | ND | + | ND | + |
| Eμ | + | + | ND | ND | ND | - | - | ND | ND | ND | + | ND | + |
| 3′ Cα HS4 | + | + | ND | ND | ND | - | - | ND | ND | ND | + | ND | + |
| 3′ Cα HS3 | + | + | ND | ND | ND | - | - | ND | ND | ND | + | ND | + |
| Pol II binding | |||||||||||||
| D1 promoter | + | + | ND | - | - | - | + | ND | ND | ND | - | ND | - |
| Eμ | + | + | ND | - | + | - | - | ND | ND | ND | + | ND | - |
| 3′ Cα HS4 | + | + | ND | - | + | - | - | ND | ND | ND | - | ND | - |
| 3′ Cα HS3 | + | + | ND | - | + | - | - | ND | ND | ND | - | ND | - |
. | G . | N . | U . | H . | M . | K562 . | MCF7 . | 231 . | H82 . | Pt . | LCL . | NB . | T . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 mRNA | + | + | + | - | - | +/- | + | + | - | + | - | - | - |
| DNA methylation | |||||||||||||
| MYEOV | - | - | +/- | - | + | - | ND | ND | ND | ND | ND | ND | ND |
| MTC | - | - | - | + | + | +/- | + | + | ND | - | + | + | + |
| P519 | - | - | - | - | + | - | + | + | ND | ND | ND | ND | ND |
| D1 promoter | - | - | - | + | + | +/- | - | - | ND | - | - | - | - |
| D1 intron 4 | - | + | - | + | + | +/- | + | + | ND | ND | ND | ND | ND |
| Histone acetylation | |||||||||||||
| MYEOV | + | + | + | ND | + | + | - | ND | - | ND | + | ND | + |
| MTC | + | + | + | ND | + | + | - | ND | - | ND | - | ND | - |
| P519 | + | + | + | ND | + | + | - | ND | - | ND | + | ND | + |
| D1 promoter | + | + | + | ND | - | + (H4) - (H3) | + | ND | - | ND | + | ND | + |
| D1 intron 4 | + | + | + | ND | + | + | + | ND | + | ND | + | ND | + |
| Eμ | + | + | ND | ND | ND | - | - | ND | ND | ND | + | ND | + |
| 3′ Cα HS4 | + | + | ND | ND | ND | - | - | ND | ND | ND | + | ND | + |
| 3′ Cα HS3 | + | + | ND | ND | ND | - | - | ND | ND | ND | + | ND | + |
| Pol II binding | |||||||||||||
| D1 promoter | + | + | ND | - | - | - | + | ND | ND | ND | - | ND | - |
| Eμ | + | + | ND | - | + | - | - | ND | ND | ND | + | ND | - |
| 3′ Cα HS4 | + | + | ND | - | + | - | - | ND | ND | ND | - | ND | - |
| 3′ Cα HS3 | + | + | ND | - | + | - | - | ND | ND | ND | - | ND | - |
Expression by RT-PCR, DNA methylation detectable by Southern blot or bisulfite sequencing, or in vivo binding detectable by ChIP is denoted by + or -. DNA partial methylation or trace expression is denoted by +/-.
G indicates Granta; N, NCEB1; U, U266; H, HL60; and M, Manca.
Discussion
The pattern of DNA methylation and histone acetylation was examined in the 11q13 region surrounding the cyclin D1 gene in B-cell malignancies. Long-range activation of cyclin D1 expression by IgH regulatory elements was associated with hyperacetylated histones H3 and H4 and hypomethylated CpGs at the cyclin D1 promoter, whereas the promoter region of nonexpressing cell lines was CpG methylated and histone H3 and H4 hypoacetylated. The MTC region was CpG hypomethylated and histone H3 and H4 hyperacetylated in cyclin D1–expressing cell lines. In several nonmalignant B- and T-cell sources that did not express cyclin D1, the cyclin D1 promoter was surprisingly hypomethylated and hyperacetylated, similar to cell lines and patient samples that expressed cyclin D1.
Complete hypomethylation at the cyclin D1 promoter and upstream regions in malignant B cells was surprising. However, the finding of complete hypomethylation at the cyclin D1 promoter in normal B cells and EBV-transformed B lymphocytes suggests that although the cyclin D1 gene is inactive transcriptionally, the cyclin D1 promoter is still hypomethylated in lymphoid cells that do not contain a translocation. Upstream regions such as the MTC, although unmethylated in established hematopoietic cell lines, are methylated in lymphocytes.
The cyclin D1 promoter was devoid of acetylated histones H3 and H4 in cyclin D1–nonexpressing established cell lines. In contrast and surprisingly, in B and T lymphocytes, the cyclin D1 promoter region contained acetylated histones H3 and H4. Sequences enriched in acetylated histones extended from the cyclin D1 promoter to the P519 but not the MTC region approximately 100 kb upstream. The ChIP assays used to assay histone acetylation are “positive assays,” which cannot distinguish between binding at only the translocated versus both the translocated and normal chromosomes. Specific primers at the P519 translocation breakpoint region in Granta cells showed histone hyperacetylation on the translocated chromosome. The paucity of polymorphic differences between the translocated chromosome and normal chromosome precluded further allele-specific analyses at this time.
Acetylated histones are usually associated with active genes or may be found in domains of several multigene loci such as the chicken, mouse, and human β-globin, and the growth hormone locus.18 These studies have defined 2 general patterns of histone acetylation, a broad domain-specific acetylation pattern and a local promoter-dependent pattern that was correlated with gene expression. Our results in the cyclin D1/11q13 region in B-cell malignancies suggest a similar organization with a broad acetylation pattern that included the cyclin D1 gene locus and sequences over 90 kb upstream (P519) in both normal and malignant B cells. A local pattern of histone hyperacetylation at the cyclin D1 promoter correlated with cyclin D1 expression status in cell lines but was also found in nonmalignant sources of B and T cells that did not express cyclin D1. The histone acetylation patterns defined in the genomic regions upstream of the cyclin D1 gene extending to the P519 but not the 5′ MTC regions in nonmalignant B/T cells suggests that these regions adopt different chromatin structures and could be related to the location of translocations involving the 11q13 region. Perhaps the MTC region defines a boundary of hyperacetylated sequences that is permissive for translocations involving IgH regulatory regions. The differences observed in patterns of DNA methylation and histone acetylation between hematopoietic cell lines and nonmalignant B and T cells also emphasize the limitations of using cell line analyses without normal, nonmalignant cell sources as controls.
ChIP assays were also used to study Pol II binding to the cyclin D1 promoter and IgH regulatory regions. Several recent publications have reported binding of RNA polymerase to upstream regulatory regions in addition to promoters in the mouse β-globin and human class II MHC loci.27,28 Our results indicate that Pol II was bound to the cyclin D1 promoter only in cell lines in which the gene was actively transcribed, in contrast to promoter histone acetylation and DNA hypomethylation, which could be observed in nonexpressing B and T cells. Thus, DNA hypomethylation, histone hyperacetylation, and RNA Pol II binding at the cyclin D1 promoter together were required for deregulated expression of cyclin D1 in B cells. These data are consistent with a transcriptional mechanism of cyclin D1 gene activation in B-cell malignancies, in contrast to the recently reported LCR-mediated regulation of mouse β-globin gene expression by transcriptional elongation.36
Pol II was also found in vivo at the IgH regulatory regions, including the Eμ intronic enhancer, 3′ Cα LCR HS3 and HS4. Sterile germline transcripts originating from the Eμ enhancer have been described,37 so the presence of Pol II at the Eμ enhancer in B cells was not surprising. However, in the other IgH regulatory sequences (3′ Cα LCR), no RNA transcripts or Pol II binding has been reported. Recent targeted deletion experiments in the mouse 3′ Cα LCR have identified 3′ HS3 and HS4 as important elements for germline transcription and class switch recombination,38 but their role in deregulation of genes such as cyclin D1 in B-cell malignancies remains obscure. In addition to DNA hypomethylation and histone hyperacetylation, Pol II binding at the cyclin D1 promoter and IgH regulatory sequences was associated with cyclin D1 expression in B-cell malignancies.
Although the mechanism of long-range gene deregulation in B-cell malignancies is unclear, models including tracking, looping, and linking have been proposed in the β-globin loci for long-range gene regulation by the β-globin LCR.13,14 The presence of Pol II at the IgH regulatory regions in MCL cell lines is consistent with an LPT mechanism that has been proposed in the mouse β-globin locus.31 In this locus, looping has been implicated in the juxtaposition of the LCR and β-globin promoters, allowing access of LCR-bound factors to the β-globin promoters.15,16 Pol II binds the mouse β-globin LCR independent of its recruitment to the promoter. The LPT model proposes that Pol II is first recruited to the β-globin LCR with the aid of the transcription factor GATA-1. Another erythroid factor, NF-E2, provides a signal for Pol II relocalization to the promoter, initiating transcription.18,40
Histone acetyltransferases have been shown to interact directly with elongating RNA Pol II,39 so, in the tracking model, Pol II and associated proteins could track from translocated IgH sequences to the cyclin D1 promoter. Which of these 2 models, Pol II transfer or tracking, is applicable to the long-distance deregulation of genes such as cyclin D1 in B-cell malignancies is under investigation.
Prepublished online as Blood First Edition Paper, June 29, 2004; DOI 10.1182/blood-2004-02-0483.
Supported by National Institutes of Health grant DK56798, grants from the International Myeloma Foundation, and the University of Arizona Dean's Research Fund (E.M.E).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Martin Dyer provided the Granta and NCEB-1 cells and Mark Groudine the Manca cells. David Lewinsohn provided the LCL1, LCL2, and D160 cells. We thank Mark Groudine, Matt Lorincz, Mirit Aladjem, William Forrester, and Matt Thayer for discussions and comments on the manuscript. We are also grateful to Bernard Futscher for expert assistance with bisulfite sequencing and ChIP experiments.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal