Abstract
For activation T cells engage antigen-presenting cells (APCs) in lymphatic tissues. The contact duration and kinetics (static versus dynamic) vary considerably in different model systems; however, it is unclear whether T cells, APCs, or the environment are responsible for the observed discrepancies. Using 3-D collagen matrices as structural scaffold, we directly compared the kinetics of T-cell engagement and activation by functionally major APC types, ie, dendritic cells (DCs) and resting or activated B cells. Resting B cells engaged T cells in long-lived (several hours), adhesive, and leukocyte function-associated antigen-1 (LFA-1)-dependent conjugates in 3-D collagen as well as in intact lymph nodes in vivo. DCs and preactivated B cells, however, supported predominantly dynamic, short-lived (minutes), and sequential contacts to T cells that were dependent on high cytoskeletal activity of the APCs but could not be inhibited by anti-LFA-1 treatment. Naive T cells were most strongly activated by DCs and activated B cells, whereas resting B cells were 100-fold less efficient to induce T-cell proliferation. Thus, in the same 3-D environment, naive T cells respond with a spectrum of different interaction modes dependent on the type and activation state of the APCs. Thereby, more dynamic interaction kinetics is positively correlated with higher T-cell priming efficiency. (Blood. 2004;104: 2801-2809)
Introduction
For activation, T cells must physically engage antigen-presenting cells (APCs).1 With B cells as APCs, the interaction plane, termed “immunologic synapse,”2 is a highly organized structure of signaling and adhesion molecules.3 A mature synapse requires at least 30 minutes of stable cell-cell interaction to form2 and can last for several hours in vitro4 and in explanted lymph nodes.5 Although investigated mostly with B cells or surrogate APCs in liquid cultures, the concept of long-term contacts has previously been considered a universal feature of all cognate T cell-APC (T-APC) interactions.6 However, in promigratory collagen matrices as environment for T-APC interaction also dynamic and short-lived encounters to dendritic cells (DCs) are able to induce the full spectrum of T-cell signaling and activation.7 Here, T cells engage DCs only for a few minutes, detach, and establish new contacts to neighboring DCs until activation is achieved.8 Migrating T cells can drag a mature synapse over the surface of an APC, showing the principal compatibility of T-cell migration and synapse maintenance.9 Furthermore, studies on APC-T interactions in explanted lymph nodes10 or thymic organ cultures11 have observed both static and dynamic interactions between T cells or thymocytes and local APCs. Intravital imaging revealed a 3-phase model of T-APC interaction in a lymph node, in which CD8 T cells displayed a period of 8 hours, characterized by short-lived and dynamic DC scanning, followed by 12 hours with stable interactions to single DCs and a return to high motility afterward.12 During adhesive contacts, T cells maintained considerable cytoskeletal activity and shape change.12 Weaver's group (Hurez et al13 and Saparov et al14 ) described a heterogeneity in naive T cells, leading to a majority of short-interacting cells, which could proliferate but did not produce interleukin 2 (IL-2), the latter only being produced by T cells making long contacts to APCs. T-cell receptor (TCR)-proximal signals such as phosphorylation of zeta-associated protein 70 (ZAP-70) or Lck15 are only detectable for the first 15 minutes after the onset of a contact and are already absent before a synapse has fully matured.16 In contrast, active phosphatidyl inositol 3 (PI3)-kinase is located in an initial segment (IS) over the entire period of T-APC interaction and interference with this process inhibits T-cell activation.17,18 However, besides a continuous TCR signal, also discontinuous signaling can lead to T-cell activation and effector function.19
Together, these observations suggest that a spectrum of interaction modes, reaching from static adhesion and clustering to dynamic migratory encounters with APCs, can lead to the activation of T cells.8,20 So far no systematic analysis on the biophysical and molecular principles underlying static versus dynamic interactions has been performed. According to one hypothesis, the nature of the APC, which is encountered by the T cell, is decisive for the mode of interaction,8 which is supported by studies comparing different types of DCs.21 Another theory suggests an instructive role of the tissue environment, especially collagenous scaffolds,22 and the latest concept, based on imaging experiments in lymph nodes, suggests that the T cells themselves control the duration of contacts by way of the phosphorylation status of their TCR signaling machinery.12
To better understand the influence of the APC type on the duration of T-cell contacts, we tested in an in vitro model and in vivo how the same CD4 T cells engaged with strong and weak APCs in the same environment. Therefore, the biophysical mode of interaction with T cells was analyzed in relation to its potency for T-cell activation. We describe here that T-cell activation can be achieved by a spectrum of biophysical T-APC interaction modes. Thereby, the duration of the contact seems not to be correlated with its efficiency for T-cell activation. In contrast, the most efficient APCs were those with prominent cytoskeletal activity engaging T cells in dynamic, multifocal contacts. Thus, in a cognate pair with T cells the APCs can be the partner controlling interaction kinetics and efficiency, irrespective of the surrounding environment.
Materials and methods
Mice
DO11.10 mice, which carry a transgenic TCR recognizing a peptide of chicken ovalbumin (AA 323-33923 ) in context with I-Ad, have been described24 and were used as the source for DO11.10 T cells. DO11.10 mice bred on a RAG2-deficient background25 were used for highly pure DO11.10 T cells. Balb/c mice were from Bomholtgard (Ry, Denmark). Animal care was in accordance with institutional guidelines.
Cell preparation
Naive CD4+ T cells from spleens of DO11.10 mice were enriched to a purity of 96% or more (75%-85% TCR transgenic) by negative isolation as described.26 These cells were used for quantitative assays (fluorescence-activated cell scanning [FACS], [3H]-thymidine incorporation). For direct observation of T cell-APC interactions, spleens of RAG2-deficient DO11.10 mice were used, yielding TCR transgenic T cells of up to 95% purity by the same isolation technique.26 Both cell types yielded identical responses to the same stimuli as tested in control experiments. Naive splenic B cells from Balb/c mice were isolated by negative isolation by using antibodies against CD43, CD11b, CD4, CD8, GR-1 (all from Pharmingen, San Diego, CA) and BM8 and ER-BMDM1 (BMA, Basel, Switzerland) followed by immunomagnetic depletion (Miltenyi, Bergisch Gladbach, Germany). The purity was 90% to 95% B220/MHCII+/+. Bone marrow DCs were generated in 8-day cultures as described27 and activated with CD40-L (a kind gift of Immunex, Seattle, WA) during the final 2 days. Cell lines secreting murine granulocyte-macrophage colony-stimulating factor (GM-CSF) or IL-4 were kindly provided by Thomas Blankenstein, MDC Berlin.
OVA323-339 peptide (100 μg/mL; Applied Biosystems, Weiterstadt, Germany) was added to the cultures of APCs overnight before use. In pilot studies it was found that at 100 μg/mL OVA peptide B cells induced a significant response in DO11.10 T cells. In contrast, DCs required 0.1 to 1 μg/mL OVA to induce maximal T-cell responses.7 At this dose of antigen, resting B cells were almost unable to induce measurable T-cell proliferation. Therefore, an antigen dose of 100 μg/mL was used for all experiments shown.
Activated B cells were generated by a 72-hour coculture of B cells and DO11.10 T cells in the presence of OVA peptide. Then, CD4 T cells were removed immunomagnetically, and the remaining activated B cells (∼98% B220/MHCII++, CD69/80/86high, data not shown) were cultured overnight in the presence or absence of OVA peptide before they were used for activation of freshly prepared DO11.10 T cells.
Analysis of cell-cell interactions within 3-D collagen gels
DC-T interactions within 3-D collagen gels were analyzed as described.7 B-T interactions were analyzed by labeling B cells with CFSE (5,6-carboxyfluorescein diacetate, succinimidyl ester; 0.5 μM, 4 minutes; Molecular Probes, Leiden, The Netherlands) before coculture with CD4+ T cells in 3-D collagen gels.7 Cell migration and fluorescence were monitored simultaneously by time-lapse confocal or fluorescence microscopy28 using an HC Plan Apo lens (20 ×, NA 0.7) with Leica TCS software and scanhead or an Olympus BX61 microscope with a UAPO lens (20 ×/340, NA 0.75) and an FView camera with AnalySIS software from SIS (Muenster, Germany). For analysis of Ca2+ signaling in T cells, B cells were labeled with SNARF (seminaphthorhodafluor; 5 μM, 4 minutes; Molecular Probes), and T cells were loaded with Fluo-4.7 Fluorescence and transmission images were recorded simultaneously by time-lapse confocal microscopy7 using a Plan Apo lens (63 ×, NA 1.4, oil immersion) with Zeiss Meta software (Carl Zeiss, Jena, Germany) and scanhead. The imaging medium was RPMI + 10% FCS and additives as described.7
Intravital imaging
B cells were labeled with 5 μM CFSE. B cells (1 × 107) were injected intravenously in the tail veins of Balb/c mice 16 hours before imaging. DO11.10 cells were stained with cell tracker orange (5 μM; Molecular Probes), and 1 × 107 of the cells were injected in the tail vein of the same mice 2 hours before imaging. Mice were anesthetized with intubation narcosis receiving a mixture of oxygen and isoflurane. Inguinal lymph nodes were surgically exposed, taking extreme care not to disrupt the blood vessels feeding the node. The successful procedure was checked by light microscopy, visualizing a functional blood flow after surgery. The animal was put into a bath containing warmed PBS, and imaging was performed by using a confocal laser microscope (Zeiss Meta with Zeiss software) and an Achroplan water dipping lens (20 ×, NA 0.5). Images were recorded every 45 to 60 seconds, reading the red and green channel simultaneously. Imaging was performed up to 3 hours permanently at the same spot without obvious bleaching or inhibition of cell motility.
APC-T contact interruption
T cells were mixed with APCs (2 × 105 T cells + 2 × 105 B cells or 2 × 104 DCs) in round-bottom 96-well microtiter plates and centrifuged at 250g for 60 seconds. APCs were inactivated by Mitomycin C before use (100 μg/mL, 90 minutes, 37°C). APC-T contacts were broken by resuspending the wells with an antibody cocktail, consisting of saturating amounts of anti-MHC II (major histocompatibility complex class II; M5/114) and 10 μg/mL each of azide free anti-CD18 (GAME-46) and anti-CD54 (3E2, both from Pharmingen). After 48 hours of coincubation, cultures were pulsed with 1 μCi (0.037 MBq) [3H]-thymidine for the final 12 hours, before cell-bound radioactivity was assessed by liquid scintillation counting. A similar assay was performed, and T-cell proliferation was measured with CFSE as described in “CFSE-based T-cell proliferation.”
CFSE-based T-cell proliferation
DO11.10 T cells were labeled with CFSE and embedded with peptide-loaded or unloaded APCs within 3-D collagen matrices (4 × 105 DO11.10 T cells + 4 × 104 DCs or 1.2 × 105 B cells or activated B cells in 100 μL collagen). After different time points, gels were digested by collagenase (30 U/100 mL gel for 30 minutes at 37°C, type VII collagenase; Sigma, Deisenhofen, Germany), and the cells were stained with allo-phycocyanine-labeled CD4 antibody before analysis on a fluorescence-activated cell sorting (FACS)-Calibur. CFSE staining of CD4+ cells was analyzed. For the comparison of T-cell proliferation in the presence of certain inhibitors, CFSE-labeled DO11.10 T cells were embedded with peptide-loaded or unloaded APCs (2 × 105 T cells + 2 × 104 APC/100 μL gel). After polymerization of the gels, inhibitors were added to the supernatant (all antibodies at 10 μg/mL, BIRT377,29 a gift of Boehringer-Ingelheim, [Ridgefield, CT] at the indicated concentrations), and the proliferation of T cells was measured after 72 hours of coincubation. To correct for the much higher degree of proliferation in the presence of DCs, all events were normalized to their controls as 100%.
APC-T contact assay
DO11.10 T cells were labeled with CFSE. DC or B cells were labeled with SNARF. A mixture of 2.5 × 105 APCs with 2.5 × 105 T cells/200 μL medium was incubated in 96-well flat-bottom plates in liquid medium in the presence of inhibitors for 1 hour or 8 hours at 37°C. Then cells were fixed for 20 minutes with warm 2% paraformaldehyde (PFA) before FACS analysis. SNARF was detected on FL3. Individual cells were identified by their single fluorescence. Cell-cell pairs were identified both by red-green double fluorescence as well as an increase in forward light scatter (FSC). Data were normalized to their controls as 100%.
Scanning electron microscopy
T cells and APCs were embedded within collagen gels on coverslips. After polymerization, gels were covered with medium. After 6 hours of incubation at 37°C the gels were fixed with warm 2.5% glutardialdehyde for 15 minutes at 37°C. After overnight incubation at 4°C, the glutardialdehyde was replaced by phosphate-buffered saline (PBS). For analysis, gels were washed in 50 mM cacodylate-buffer at 4°C, then dehydrated in a series of ethanol-water washes (from 30% to 100% and finally in a 50/50 mixture of 100% ethanol and hexamethydisilazane). After air drying, gels were platin-sputtered and analyzed on a Zeiss EM-10 microscope.
Statistical analysis
Student t test was applied to assess significant differences.
Results
B cells form long-lasting, unipolar contacts to T cells
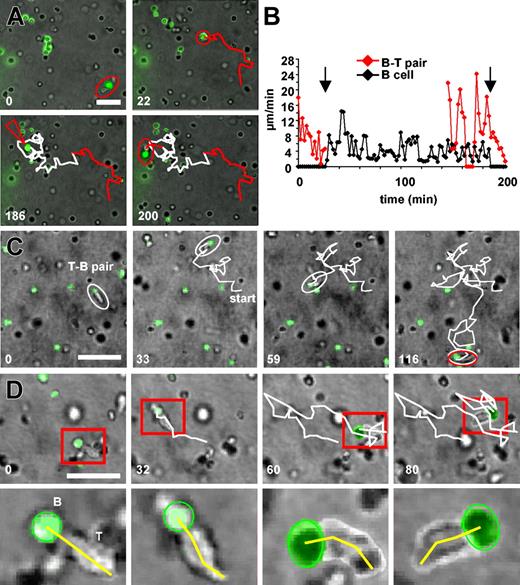
To observe the physical interactions of B cells and T cells on antigen presentation, we embedded freshly prepared splenic B cells or bone marrow-derived DCs, each loaded with cognate antigen together with naive DO11.10 T cells within a 3-D collagen matrix. The analysis of cellular movements revealed that T cells contacting B cells were engaged within stable, adhesive, and long-lived contacts (Figure 1A). B-T pairs typically consisted of a resting B cell placed at the leading edge of an elongated T cell (Figure 1A). During B-T interaction B cells remained spherical in shape, suggesting a lack of prominent cytoskeletal activity (Figure 1A,C). Because there was no obvious stop in the migratory activity of T cells on contact formation with B cells, B-T pairs were often motile, with the B cell being pushed through the collagen matrix by the T cell (Figure 1A, and Supplemental Movie 1, available on the Blood website; see the Supplemental Movies link at the top of the online article). These results show that the binding force between T cells and B cells is high enough to withstand considerable shear forces imposed by the tight collagen network on migration (Figure 1D). Like in T-DC pairs,7 the contact to B cells was able to induce calcium transients in T cells despite the constant motility of the T cell around the region of contact (Figure 1C; Supplemental Movie 2). As described earlier,7 DCs engaged T cells only for short periods. Two modes of contact formation to DCs were observed. T cells were either migrating toward the DC, engaging and leaving it without stopping (Figure 1B; Supplemental Movie 3), or DCs used their active cytoskeleton to catch nearby T cells with the tips of fine dendrites and then drag them along their path (Supplemental Movie 4). In both cases DCs constantly maintained an active cytoskeletal motion. A close inspection of B-T or DC-T pairs by scanning electron microscopy (SEM) showed that T cells made an intimate unipolar contact with their leading edge, forming a stable interaction plane with the B-cell body without the participation of the hind part of the T cell (Figure 1D). T cells in DC-T pairs engaged the surface of the DCs with the entire DC-oriented part of their body and also got additional contacts from the sides and above by individual dendrites of the DCs, thus forming a tight, multifocal interaction (Figure 1E). Recent studies in explanted,10,30 as well as in situ lymph nodes,12 have shown the ability of DCs to engage T cells in short-lived, dynamic, and sequential fashion also in their natural environment. However, thus far no studies on antigen presentation by B cells have been performed in vivo. To this end we established intravital imaging in the inguinal lymph node (LN) to observe the interaction of naive DO11.10 T cells with peptide-loaded naive B cells around the time point, whereby transient interactions with DCs had been observed in vivo by others.12 We were able to monitor a similar organization of established T-B pairs in vivo as we had previously done in 3-D collagen gels (Figure 1A,C-D). T-B pairs were observed to migrate through the LN parenchyma with the T cell following a B cell, which, however, displayed more vigorous cytoskeletal activity, compared with collagen lattices (Figure 1E; Supplemental Movie 11). Low resolution imaging of entire B-cell follicles revealed that such T-B pairs were rather frequent (Figure 1G) and maintained over periods of more than 3 hours (Supplemental Movie 12; data not shown).
Cognate interactions of DO11.10 T cells with B cells within a 3-D collagen gel and in a lymph node in situ are long-lasting. Naive B cells or mature DCs were loaded with 100 μg/mL OVA peptide and embedded within a 3-D collagen matrix together with freshly prepared DO11.10 T cells. B cells had been labeled with CFSE or SNARF before embedding. Cell movements were recorded by time-lapse video-microscopy. (A) Formation and migration of a T cell-B cell pair (Supplemental Movie 1). The white line indicates the migration path taken over time. The B-T pair is stable over the entire observation period. For better visibility the relevant B-T pair has been enlarged by 50%. Five hours after culture set-up. (B) Interaction of a T cell with a DC (Supplemental Movies 3 and 4). Note that the interaction lasts only for 13 minutes. Twenty-five hours after culture set-up. (C) T cells receive a calcium signal on cognate interaction with naive B cells. A T cell is shown, which fluxes intracellular calcium while moving around a spot of contact with a naive B cell (Supplemental Movie 2). Thirty minutes after culture set-up. (D) SEM analysis of a cognate B-T pair fixed in the process of migration within 3-D collagen. (E) As in panel D, for a cluster of 3 T cells and 1 DC. B cells were stained with CFSE and injected into a Balb/c mouse together with cell tracker orange-stained DO11.10 T cells. Intravital imaging was performed in the inguinal lymph node. (F) A T-B pair migrating within the B cell follicle. Note the similarity of morphology as compared with the appearance in 3-D collagen. The path of the cell pair during migration is indicated (Supplemental Movie 11). Four hours after injection of T cells. (G) Multiple T-B pairs form at the T zone border and within a B-cell follicle (arrowheads, Supplemental Movie 12). Seven hours after injection of T cells. Scalebar: 100 μm (G), 50 μm (A,B,F), 10 μm (C-E). Numbers in panels A-C and F indicate minutes of real time.
Cognate interactions of DO11.10 T cells with B cells within a 3-D collagen gel and in a lymph node in situ are long-lasting. Naive B cells or mature DCs were loaded with 100 μg/mL OVA peptide and embedded within a 3-D collagen matrix together with freshly prepared DO11.10 T cells. B cells had been labeled with CFSE or SNARF before embedding. Cell movements were recorded by time-lapse video-microscopy. (A) Formation and migration of a T cell-B cell pair (Supplemental Movie 1). The white line indicates the migration path taken over time. The B-T pair is stable over the entire observation period. For better visibility the relevant B-T pair has been enlarged by 50%. Five hours after culture set-up. (B) Interaction of a T cell with a DC (Supplemental Movies 3 and 4). Note that the interaction lasts only for 13 minutes. Twenty-five hours after culture set-up. (C) T cells receive a calcium signal on cognate interaction with naive B cells. A T cell is shown, which fluxes intracellular calcium while moving around a spot of contact with a naive B cell (Supplemental Movie 2). Thirty minutes after culture set-up. (D) SEM analysis of a cognate B-T pair fixed in the process of migration within 3-D collagen. (E) As in panel D, for a cluster of 3 T cells and 1 DC. B cells were stained with CFSE and injected into a Balb/c mouse together with cell tracker orange-stained DO11.10 T cells. Intravital imaging was performed in the inguinal lymph node. (F) A T-B pair migrating within the B cell follicle. Note the similarity of morphology as compared with the appearance in 3-D collagen. The path of the cell pair during migration is indicated (Supplemental Movie 11). Four hours after injection of T cells. (G) Multiple T-B pairs form at the T zone border and within a B-cell follicle (arrowheads, Supplemental Movie 12). Seven hours after injection of T cells. Scalebar: 100 μm (G), 50 μm (A,B,F), 10 μm (C-E). Numbers in panels A-C and F indicate minutes of real time.
No correlation of APC-T contact time and T-cell priming efficiency
Quantification of a large number of individual contacts of T cells to different APCs made clear that most T cells engaged DCs in a short-term manner in this experimental setting (Figure 2A; Supplemental Movie 3). There was no large effect of cognate antigen for the duration of contacts to DCs (Figure 2A), and only a minor increase in contact duration was seen from 19 to 23 hours of coincubation, which was less pronounced than during phase 2 of T-APC interactions in vivo.12 In contrast, in the absence of antigen on B cells, most detectable contacts were in the range of a few minutes, but in the presence of high loads of antigen, we exclusively observed long interactions (individual contacts lasting up to > 17 hours; Figure 2A; Supplemental Movie 5), irrespective of the time of observation. Strikingly, when we preactivated the B cells and then measured the duration of interaction with fresh DO11.10 T cells, we found a predominance of short and dynamic interactions, similar to DCs (Figure 2A).
The duration of APC-T contact does not correlate with APC effectiveness. Naive DO11.10 T cells were embedded in 3-D collagen matrices together with different APCs, labeled or not with OVA peptide. (A) Cell movements were recorded by time-lapse video microscopy, and the duration of individual T cell-APC contacts was analyzed. Each dot represents one observed contact. Bars represent the median. Dots at more than 60 minutes are cell pairs that have been observed for more than 60 minutes without releasing the contact. Data are pooled from 4 independent experiments (or 2 experiments, for activated B cells). (B) T-cell proliferation within such gels was analyzed by CFSE down-regulation after 72 hours of coincubation in the presence of antigen followed by collagenase digestion of the gels. The numbers indicate the percentage of cells that have undergone one or more divisions. (C) The kinetics of T-cell proliferation was studied by CFSE-dilution analysis. To this end, CFSE-labeled DO11.10 T cells were embedded together with APCs in 3 individual gels at time point 0. After 24, 48, and 72 hours gels were digested and analyzed for dilution of CFSE. All data were gated on CD4+ cells, which were identified by simultaneous staining with an anti-CD4 antibody. Open symbols indicate APCs without OVA; closed symbols, APCs with OVA. Data are representative of 5 experiments performed (activated B cells 2).
The duration of APC-T contact does not correlate with APC effectiveness. Naive DO11.10 T cells were embedded in 3-D collagen matrices together with different APCs, labeled or not with OVA peptide. (A) Cell movements were recorded by time-lapse video microscopy, and the duration of individual T cell-APC contacts was analyzed. Each dot represents one observed contact. Bars represent the median. Dots at more than 60 minutes are cell pairs that have been observed for more than 60 minutes without releasing the contact. Data are pooled from 4 independent experiments (or 2 experiments, for activated B cells). (B) T-cell proliferation within such gels was analyzed by CFSE down-regulation after 72 hours of coincubation in the presence of antigen followed by collagenase digestion of the gels. The numbers indicate the percentage of cells that have undergone one or more divisions. (C) The kinetics of T-cell proliferation was studied by CFSE-dilution analysis. To this end, CFSE-labeled DO11.10 T cells were embedded together with APCs in 3 individual gels at time point 0. After 24, 48, and 72 hours gels were digested and analyzed for dilution of CFSE. All data were gated on CD4+ cells, which were identified by simultaneous staining with an anti-CD4 antibody. Open symbols indicate APCs without OVA; closed symbols, APCs with OVA. Data are representative of 5 experiments performed (activated B cells 2).
Because B cells were able to induce a Ca2+ signal in attached T cells (Figure 1C; Supplemental Movie 2), we investigated the proliferative response of DO11.10 T cells in the presence of different APCs. Despite their short-lived nature in 3-D collagen, contacts to both DC and activated B cells induced a higher rate (Figure 2B) as well as faster induction of T-cell proliferation (Figure 2C). In both parameters resting B cells were inferior to DCs and activated B cells (Figure 2B-C). These results showed that, despite a predominance of short and dynamic encounters, DCs and activated B cells were much more efficient inducers of T-cell activation than resting B cells, which relied on long-lived T-cell encounters, only.
T cells reach an activation threshold faster after contact with DCs than with resting B cells
The long duration of T-B contacts prompted us to investigate whether such long contact times were really necessary to fully activate T cells by resting B cells or whether T cells might already be activated earlier but still remained trapped on the B cell, eg, for the delivery of B-cell help. By adding activation-blocking antibodies at various time points after initiation of an APC-T cell coculture, we found that B cells required contact to T cells for up to 60 hours to fully activate naive T cells (Figure 3A). In contrast, T cells became independent of further antigen presentation after 2 to 6 hours of contact to DCs (Figure 3A). The early onset of T-cell proliferation was not due to abortive activation, because after 4 to 6 hours of signaling by DCs most of the T cells were driven into repetitive cycling (Figure 3B), which is in accordance with published findings.16 B cells needed at least 12 to 24 hours of signaling to induce significant cycling in T cells, and even after 48 hours a significant proportion of the T cells had not been activated (Figure 3B). On reduction of the peptide concentration on APCs to 1 μg/mL, naive T cells still required only 4 to 6 hours of contact to DCs for repetitive cycling (Figure 3B), whereas B cells were largely incapable of stimulating T cells at these peptide concentrations (not shown). Thus, resting B cells had to maintain long-lived interactions with T cells to induce a threshold signaling level in attached T cells.
DC need less signaling time than B cells to fully activate T cells. DO11.10 T cells (with or without CFSE) were mixed with OVA-loaded B cells or DCs within liquid cultures in round-bottom multiwell plates. At different time points a cocktail of antibodies that inhibited further APC signaling was added to individual wells. (A) After 48 hours, cultures were pulsed with 1 μCi (0.037 MBq) [3H]-thymidine for 12 hours. Subsequently, cell-bound radioactivity was assessed by liquid scintillation counting. Data are means of duplicate measurements for each time point. (B) T-cell proliferation after 48 hours was assessed by FACS, measuring down-regulation of the CFSE label.
DC need less signaling time than B cells to fully activate T cells. DO11.10 T cells (with or without CFSE) were mixed with OVA-loaded B cells or DCs within liquid cultures in round-bottom multiwell plates. At different time points a cocktail of antibodies that inhibited further APC signaling was added to individual wells. (A) After 48 hours, cultures were pulsed with 1 μCi (0.037 MBq) [3H]-thymidine for 12 hours. Subsequently, cell-bound radioactivity was assessed by liquid scintillation counting. Data are means of duplicate measurements for each time point. (B) T-cell proliferation after 48 hours was assessed by FACS, measuring down-regulation of the CFSE label.
Motile B cells stop migration at the time of T-cell contact formation
Besides a fraction of nonmotile cells, many B cells showed a considerable degree of autonomous motility within collagen matrices with velocities ranging between 4 and 8 μm/minute (Figure 4B), which is in accordance with migration speeds of B cells in lymph nodes (Miller et al10 and data no shown). Nevertheless, all observed T cell-B cell pairs were characterized by a round and immobile B cell on top of a motile T cell. Because most B cells engaged in long-lived clusters with T cells were viable (not shown), we wanted to clarify whether T-B contact formation could only occur between motile T cells and a subpopulation of previously immobile B cells or whether 2 independently motile cells could form functional conjugates. Therefore, we obtained frame-to-frame analyses of video sequences showing the process of conjugate formation. Previously motile B cells immediately stopped autonomous migration and rounded, when they contacted a T-cell surface and were passively transported through the collagen matrix or lymph node parenchyma as long as they stayed in contact with the T cell (Figure 4A; Supplemental Movies 6 and 12). Before and after contacting a T cell, B cells could exhibit autonomous motility and movement (Figure 4A). Velocity plots of B cells and B-T pairs showed (Figure 4B) that during T-cell contact B cells did not migrate, whereas cell-cell pairs were moving at speeds of up to 20 μm/minute, which is similar to the velocities measured for free T cells within lymph nodes.10 Thus, the same antigen-specific signal induced motility in T cells, but a cessation of motility in resting B cells, ultimately leading to a stable cell-cell pair.
T-cell contact induces a stop signal in B cells. B-T pairs are pushed by T-cell movements and maintain a fixed contact plane. Naive OVA-loaded B cells were stained with CFSE and embedded within 3-D collagen together with fresh DO11.10 T cells. (A) A B cell is observed establishing 2 successive contacts to 2 distinct T cells and is only motile in the intervening time, whereas no T-cell contact is maintained (Supplemental Movie 6). An established B-T pair moves through the collagen pushed forward by T-cell movements (red path). Twenty-two minutes later, the B-T pair breaks up, and the B cell now moves by itself for the next 160 minutes (white path). Then the B cell is caught by a moving B-T pair (red arrowhead), whereby the T cell exchanges the previously contacted B cell for the new one. Again, the previously motile B cell stops all autonomous migration and is transported passively by the motile T cell (red path) 30 minutes after culture set-up. (B) Cell tracking analysis of the velocities of the cell-cell pairs and the free B cell during the events described in panel A. The B cell develops autonomous migration immediately after breaking of the contact to a T cell (arrow) and ceases migration immediately after engagement by another T cell (arrow). B-T pairs move at velocities of free T cells.7,10 (C) An exceptionally motile B-T pair covering a large distance (white path) within 116 minutes of observation. Note that the path makes many twists and turns (Supplemental Movie 7). Day 2 after culture set-up. (D) A close inspection of a motile B-T pair shows that, despite the pair's many turns (white path), the B cell (green) remains round and forms a fixed unit with the leading edge of the T cell. The uropod of the T cell is often at an angle relative to the B-T contact plane (yellow line). These movements of the T-cell uropod appear to generate the force for the movement of the entire B-T pair (Supplemental Movie 8). The lower row of panel D is an enhanced view of the cell-cell pair in the red box in the upper row of the panel. Day 3 after culture set-up. Scalebar: 50 μm (A,D), 100 μm (B). Numbers indicate minutes of real time.
T-cell contact induces a stop signal in B cells. B-T pairs are pushed by T-cell movements and maintain a fixed contact plane. Naive OVA-loaded B cells were stained with CFSE and embedded within 3-D collagen together with fresh DO11.10 T cells. (A) A B cell is observed establishing 2 successive contacts to 2 distinct T cells and is only motile in the intervening time, whereas no T-cell contact is maintained (Supplemental Movie 6). An established B-T pair moves through the collagen pushed forward by T-cell movements (red path). Twenty-two minutes later, the B-T pair breaks up, and the B cell now moves by itself for the next 160 minutes (white path). Then the B cell is caught by a moving B-T pair (red arrowhead), whereby the T cell exchanges the previously contacted B cell for the new one. Again, the previously motile B cell stops all autonomous migration and is transported passively by the motile T cell (red path) 30 minutes after culture set-up. (B) Cell tracking analysis of the velocities of the cell-cell pairs and the free B cell during the events described in panel A. The B cell develops autonomous migration immediately after breaking of the contact to a T cell (arrow) and ceases migration immediately after engagement by another T cell (arrow). B-T pairs move at velocities of free T cells.7,10 (C) An exceptionally motile B-T pair covering a large distance (white path) within 116 minutes of observation. Note that the path makes many twists and turns (Supplemental Movie 7). Day 2 after culture set-up. (D) A close inspection of a motile B-T pair shows that, despite the pair's many turns (white path), the B cell (green) remains round and forms a fixed unit with the leading edge of the T cell. The uropod of the T cell is often at an angle relative to the B-T contact plane (yellow line). These movements of the T-cell uropod appear to generate the force for the movement of the entire B-T pair (Supplemental Movie 8). The lower row of panel D is an enhanced view of the cell-cell pair in the red box in the upper row of the panel. Day 3 after culture set-up. Scalebar: 50 μm (A,D), 100 μm (B). Numbers indicate minutes of real time.
Motile B-T pairs maintain a stable contact plane
Mobile B-T pairs covered distances of several 100 μm per hour while frequently changing the orientation of their movement (Figures 4C and 1E; Supplemental Movies 7, 11, and 12). Because we had observed functional calcium signaling in T cells despite this high motility (Figure 1C; Supplemental Movie 2), we next wondered how T cells maintained a functional contact to B cells during this extended motility. A close inspection of moving B-T pairs revealed that T cells had a dynamic uropod, which generated the protruding force, whereas the leading edge remained relatively immobile and oriented toward the B cell, thus maintaining a fixed cell-cell contact plane (Figure 4D; Supplemental Movie 8).
Activated B cells develop high cytoskeletal activity and engage T cells in a multifocal manner
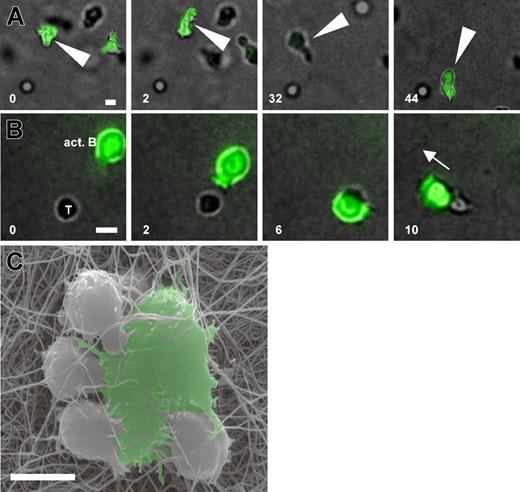
Preactivated B cells engaged T cells for shorter periods than naive B cells (Figure 2A). Because B blasts were much larger and more granulated than resting B cells by FACS (not shown), we wondered whether this altered morphology could explain the shortened contact times. In comparison to resting B cells the cytoskeletal activity of activated B cells was much more enhanced, leading to a DC-like morphology of activated B cells migrating within 3-D collagen (Figure 5A; Supplemental Movie 9). For contact formation activated B cells were able to grab nearby T cells with prominent cytoskeletal protrusions (Figure 5B; Supplemental Movie 10), finally dragging T cells away. This was reminiscent of the way DCs interacted with T cells (Figure 1B). A close inspection of the resulting cell-cell pairs by SEM showed that T cells made large contact areas to the surface of activated B cells, and the B cells themselves touched the T cells from the side with individual membrane protrusions. This formed a multifocal B-T contact, which also frequently included more than 1 T cell (Figure 5C).
Activated B cells develop high cytoskeletal activity and actively engage T cells in a multifocal manner. OVA-loaded naive B cells were activated for 72 hours in the presence of DO11.10 T cells, purified, reloaded with OVA, and embedded with fresh DO11.10 T cells in 3-D collagen. (A) Activated B cells (green) display effective migration and a prominent cytoskeletal activity (Supplemental Movie 9) 30 minutes after culture set-up. (B) The formation of a contact to a DO11.10 T cell (T) involves active grabbing by B-cell membrane protrusions (white arrowheads), the movement of the B cell around the T-cell body, and its subsequent dragging by the motile B cell in its direction of movement (white arrow, and Supplemental Movie 10) 30 minutes after culture set-up. (C) SEM of DO11.10 T cells in contact with an activated B cell. The activated B cell is able to contact 4 T cells simultaneously, thereby making intense multifocal contacts to the T cells by the generation of membrane protrusions. Scale bar: 10 μm. White numbers indicate minutes of real time.
Activated B cells develop high cytoskeletal activity and actively engage T cells in a multifocal manner. OVA-loaded naive B cells were activated for 72 hours in the presence of DO11.10 T cells, purified, reloaded with OVA, and embedded with fresh DO11.10 T cells in 3-D collagen. (A) Activated B cells (green) display effective migration and a prominent cytoskeletal activity (Supplemental Movie 9) 30 minutes after culture set-up. (B) The formation of a contact to a DO11.10 T cell (T) involves active grabbing by B-cell membrane protrusions (white arrowheads), the movement of the B cell around the T-cell body, and its subsequent dragging by the motile B cell in its direction of movement (white arrow, and Supplemental Movie 10) 30 minutes after culture set-up. (C) SEM of DO11.10 T cells in contact with an activated B cell. The activated B cell is able to contact 4 T cells simultaneously, thereby making intense multifocal contacts to the T cells by the generation of membrane protrusions. Scale bar: 10 μm. White numbers indicate minutes of real time.
Naive B cells depend on functional LFA-1 for both T-cell engagement and activation
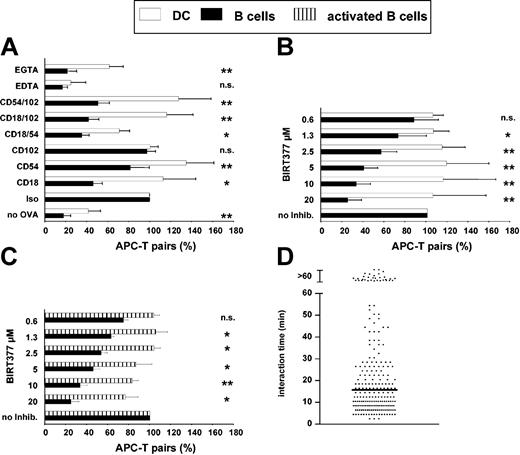
Which molecular differences might account for the strong discrepancies in the physicodynamics of DC-T and B-T interaction? In a contact-formation assay we found that any kind of interference with the intercellular adhesion molecule (ICAM)-LFA-1 pair of adhesion molecules by antibodies (Figure 6A), or by BIRT377, a small molecule inhibitor of LFA-1 activation (Figure 6B29,31 ), significantly reduced the ability of B cells to bind T cells, whereas DCs were almost functioning normally (Figure 6A-B). This was also evident shortly after onset of the cocultures, in which DCs might still be depending on LFA-1-mediated pathways (not shown). Also, activated B cells were largely resistant to BIRT377 inhibition of their T-cell binding capabilities (Figure 6C). The strongly decreased APC effectivity of B cells in the presence of BIRT377 was paralleled by the inability of B cells to maintain stable contacts to T cells in 3-D collagen matrices (Figure 6D), pointing to a strict dependence of B cells on stable T-cell interactions mediated by way of LFA-1 for the induction of T-cell activation. Analysis of T-cell proliferation in the presence of the same inhibitors showed a similar response. In the presence of antibodies against CD18 and CD54 the ability of B cells to induce T-cell proliferation was reduced to 40%, whereas DCs were still able to induce the same level of T-cell proliferation as without inhibiting antibodies (Figure 7B). Likewise, much lower concentrations of BIRT377 were necessary to inhibit T-cell activation by B cells than activation by way of DCs (Figure 7A). Thus, static and long-lived binding of T cells by way of LFA-1 is of critical importance for the function of resting B cells as APCs, whereas both DCs and activated B cells are not dependent on this adhesion pathway.
Naive B cells strongly depend on a functional LFA-1-ICAM interaction for T-cell engagement. Naive and activated B cells or DCs were labeled with OVA, stained with SNARF, and coincubated with fresh CFSE-stained DO11.10 T cells in 96-well flat-bottom plates in the presence of different antibodies or cation chelators (A) or BIRT377, an inhibitor of LFA-1 activation (B-C). After 8 hours the cultures were fixed by PFA and analyzed for the formation of cell pairs by FACS. The absolute number of cell pairs without inhibition varied between 10% and 20%. The relative number of cell pairs in relation to the control (“Iso” or “no Inhib”) is shown. Data are means from at least 3 independent experiments. *P < .025; **P < .0004; n.s. = not significant. (D) Contact duration between DO11.10 T cells and naive B cells loaded with 100 μg/mL OVA was measured as described in Figure 2, albeit in the presence of 10 μg/mL BIRT377. Data were acquired over 16 hours after setting up of the culture. The horizontal bar indicates the median.
Naive B cells strongly depend on a functional LFA-1-ICAM interaction for T-cell engagement. Naive and activated B cells or DCs were labeled with OVA, stained with SNARF, and coincubated with fresh CFSE-stained DO11.10 T cells in 96-well flat-bottom plates in the presence of different antibodies or cation chelators (A) or BIRT377, an inhibitor of LFA-1 activation (B-C). After 8 hours the cultures were fixed by PFA and analyzed for the formation of cell pairs by FACS. The absolute number of cell pairs without inhibition varied between 10% and 20%. The relative number of cell pairs in relation to the control (“Iso” or “no Inhib”) is shown. Data are means from at least 3 independent experiments. *P < .025; **P < .0004; n.s. = not significant. (D) Contact duration between DO11.10 T cells and naive B cells loaded with 100 μg/mL OVA was measured as described in Figure 2, albeit in the presence of 10 μg/mL BIRT377. Data were acquired over 16 hours after setting up of the culture. The horizontal bar indicates the median.
Naive B cells strongly depend on a functional LFA-1-ICAM interaction for T-cell activation. For analysis of T-cell proliferation in the presence of LFA-1-ICAM inhibitors, DO11.10 T cells were stained with CFSE before embedding with OVA-loaded APCs within 3-D collagen in the presence of antibodies against LFA-1-ICAM (A) or BIRT377 (B). Seventy-two hours after beginning of the coculture, gels were digested by collagenase, stained with anti-CD4, and analyzed by flow cytometry for cell division in the CD4 compartment. The rates of T-cell proliferation relative to the controls (anti-CD102 for antibodies, no inhibition for BIRT377) are shown. Data are representative of 3 independent experiments.
Naive B cells strongly depend on a functional LFA-1-ICAM interaction for T-cell activation. For analysis of T-cell proliferation in the presence of LFA-1-ICAM inhibitors, DO11.10 T cells were stained with CFSE before embedding with OVA-loaded APCs within 3-D collagen in the presence of antibodies against LFA-1-ICAM (A) or BIRT377 (B). Seventy-two hours after beginning of the coculture, gels were digested by collagenase, stained with anti-CD4, and analyzed by flow cytometry for cell division in the CD4 compartment. The rates of T-cell proliferation relative to the controls (anti-CD102 for antibodies, no inhibition for BIRT377) are shown. Data are representative of 3 independent experiments.
Discussion
Our study compares different APCs during the process of antigen presentation within a 3-D collagen environment and in vivo. In earlier studies we had observed short-lived interactions between T cells and DCs,7 whereas others, using different experimental models, have defined stable synapselike interactions as the prototype interaction of T cells and B cells.4,6,22,32,33 From the results obtained we conclude that long-lived APC-T interactions are also possible within 3-D collagen. However, they are dependent on the nature of the used APCs. Periods of dynamic T-DC interactions were recently observed in vivo12 and the static adhesion of T cells to naive B cells in lymph nodes observed in this study suggest that a similar spectrum of interaction modes is of physiologic relevance.
The absence of a T-cell stop signal after the contact initiation with naive B cells was unexpected and contrasts with previous evidence.34 Mechanistically, persistent migration was associated with the maintenance of the uropod as well as longitudinal shape change along the T-cell length axis, whereas the T-B contact plane was stable, showed little lateral shift, and was fully functional for T-cell activation. This is consistent with the finding that the leading edge is the area of highest sensitivity in T cells.35 Investigations are currently under way to elucidate whether the basis for this unusual T-cell activation system is a conventional IS or a new, previously undefined structure adding to the already described diversity of IS.36 SEM images of T-cell pairs with DCs or activated B cells showed a multifocal engagement of T cells by the APCs. Recently, it was shown that DCs can engulf more than half of the surface of a T cell on contact formation in lymph nodes.30 Because T cells are also sensitive to signaling in the middle and rear part of their body,35 such multifocal contacts might more efficiently use the potential of T cells to receive information. Thus, we propose that an effective APC requires the combination of an active cytoskeleton37 and high densities of signaling molecules, enabling the APCs to generate multifocal contact areas to T cells. It will be necessary to analyze whether such individual foci of contact contain independent minisynapses that might be the basis for effective signal generation.
Despite the ability of naive B cells to engage T cells for long periods of time, their effectivity as APCs was low as compared with DCs or activated B cells, which is in line with previous observations.1,38 DCs were both more potent APCs and required up to 10-fold less time for T-cell activation than B cells. In contrast to naive B cells complete T-cell activation by DCs and activated B cells was achieved in the absence of long-contact stabilization. This is consistent with a serial interaction model that accumulates serial yet discrete signals over a series of on-off states.19
The slight increase in contact duration around 19 to 23 hours observed here is less pronounced than previously described in vivo for a comparable time period. It is currently unclear whether this obvious difference is mainly due to the use of CD8 cells in the study by Mempel et al,12 because phase 2 was less pronounced in the CD4 compartment, or whether 3-D collagen matrices are missing important components that are necessary to completely model natural behavior. In any case, dynamic interaction of T cells in 3-D collagen always leads to full T-cell activation.7
It is open whether the life history of the minor fraction of T cells undergoing long-term interactions with DCs in 3-D collagen is different from short-term interactors. Although new imaging studies have now firmly established the existence of long-lived T-DC interactions in LN,5,12 a series of in vitro studies7,13,16 also show that long-lived interactions can be prematurely disrupted without an apparent loss in effector cytokine production13 as well as proliferation.7,16 Thus, the question no longer is whether long-lived interactions exist, but for what reason and by which mechanisms. In an attempt to account for the different stages of short-lived and long-lived contacts Mempel at al12 have suggested that those T cells that show short-lived interactions are about to resensitize their TCR, which after passage through peripheral blood is desensitized.39 Once resensitized, the cells now engage cognate APCs for hours. This would imply that exclusively the T cell decides whether or not a contact is made stable, whereas the APC plays a passive role. The interpretation of these data presented in our study also indicates a prominent role of the APCs for the duration of a T-cell contact. Thus, neither the importance of long-lived T-APC interactions for T-cell activation nor the mechanisms by which they are established are clearly defined until now.
In an attempt to define molecular events, which might explain the differences in stickiness between DCs and B cells, we found that the LFA-1-ICAM pair of adhesion molecules played an important role in this process. LFA-1 becomes activated on the T-cell surface to bind much more effectively to ICAM, when a TCR-mediated signal is induced.40,41 Signals mediated by way of MHCII molecules on B cells can also induce their LFA-1-dependent binding.42 Two mechanisms are responsible for this behavior of LFA-1, namely an affinity change of single LFA-1 molecules mediated by Mg/Mn2+40,41,43 and an avidity change induced by Ca2+-dependent polymerization of LFA-1 in the cell membrane.40 The formation of B-T pairs appeared to be dependent on both mechanisms of LFA-1 activation, given the strong inhibitory effect of cation chelators. In contrast, divalent cations were important for DC-mediated adhesion, but the lack of ethylene glycol tetraacetic acid (EGTA) inhibition suggests that Ca2+ appeared to be largely dispensable. Thus, DCs also rely on cation-dependent adhesion molecules43 for DC-T binding. However, the identity of these molecules can differ from those used by B cells. Interestingly, the preactivation of B cells induced changes that now also allowed them to bind T cells in the absence of active LFA-1. Currently, it is not possible to decide whether DCs and activated B cells make use of their LFA-1 for the binding of T cells at all, but, if so, they do it in a way distinct from naive B cells, because we did see dynamic T-APC interactions without LFA-1 inhibition where naive B cells were strictly sticking. This is corroborated by studies in CD18-deficient DCs, which have no defect in T-cell interaction and activation44 (S.G. and G.V., manuscript in preparation). In contrast to DCs, not only binding but also activation of T cells by B cells was sensitive to direct inhibition of LFA-1 or its ligands. Similar differences in LFA-1 dependency of B cell- and DC-induced mixed leukocyte reaction (MLR) have also been observed in liquid cultures.45 Interestingly, both DCs and activated, but not naive, B cells expressed Cytip (not shown), a protein that down-regulates the binding capacity of LFA-1.46 Thus, potent APCs might rely on a robust cytoskeleton, expression of molecular tools to overcome too tight binding to T cells, and high levels of costimulatory molecules and MHC.
In summary, we show that different APCs interact with T cells in entirely different physicodynamic modes. Naive B cells as APCs can only establish long synapselike interactions with T cells, which are not as efficient for T-cell activation as dynamic interactions to DCs or activated B cells, at least, if they are the only mode of T-APC interaction available. An important mechanism for the observed differences is a distinct use of LFA-1-mediated adhesion events by resting B cells and DCs or activated B cells. T cells apparently display and need a spectrum of biophysical interaction modes that culminate in the same end point, T-cell activation. Future studies need to address whether the type of T cell, which is generated by stable contacts to weak or dynamic interactions with strong APCs, is identical or whether antigen presentation by naive B cells, which would happen, eg, after peptide immunization in vivo,47 might even lead to immune deviation toward a regulation of T-cell dependent responses.
Prepublished online as Blood First Edition Paper, July 15, 2004; DOI 10.1182/blood-2004-03-1193.
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank P. Reichardt, C. Figdor, R. Germain, B. Schraven, and K. Loser for helpful discussions and critical comments on the manuscript. We also thank T. Blankenstein for providing GM-CSF- and IL-4-transfected cell lines, D. Vestweber for providing access to a confocal microscope, and M. Steinert and S. Schröter for expert technical assistance. We thank Martina Jossberger for expert technical assistance with SEM.



![Figure 3. DC need less signaling time than B cells to fully activate T cells. DO11.10 T cells (with or without CFSE) were mixed with OVA-loaded B cells or DCs within liquid cultures in round-bottom multiwell plates. At different time points a cocktail of antibodies that inhibited further APC signaling was added to individual wells. (A) After 48 hours, cultures were pulsed with 1 μCi (0.037 MBq) [3H]-thymidine for 12 hours. Subsequently, cell-bound radioactivity was assessed by liquid scintillation counting. Data are means of duplicate measurements for each time point. (B) T-cell proliferation after 48 hours was assessed by FACS, measuring down-regulation of the CFSE label.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/9/10.1182_blood-2004-03-1193/6/m_zh80210468430003.jpeg?Expires=1768271759&Signature=C4VZZ8CwNkLcUgiHsiCBthDfDrVoGLjGSu0OlKxfxYnkib-3b8RTl1fr-pm3YJCEYS-p6nre7ktdNFy3n1G0-GdB-nyuiUCmsh5yAuT4clsNI-HQpe5BZUj6InaW5Tjk2Zg~z1sq69QJILXvr1jGYa2Bmp09531InKtvRdTP~lgJzQa8y0lCMZNkhmHiJg8uWCk3lYPNEsFX~BLFac~ps6g19IqJUg38vXU7rGae3dTNss0rMhoy5yavdHvsF5C-9ztnSWocOZv3u8embY3gqCTim56vBDWEIPan3~wksjssMH1hS2QO1m9jW7OPDVEXNjbSJigvcBs45Nb74sgO-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)