Abstract
Dendritic cells (DCs) are antigen-presenting cells with the capacity to prime naive T cells for efficient cellular responses against pathogens such as HIV-1. DCs are also susceptible to HIV-1 infection, which may impair their ability to induce immunity. Here, we examined the ability of HIV-1-infected, in vitro-derived DCs to respond to CD40 ligand (CD40L) stimulation with the aim to study events during early HIV-1 infection. HIV-1BaL-infected p24+ DCs were detected after only 3 days of exposure to highly concentrated virus. We show that HIV-1-infected DCs up-regulated costimulatory molecules, but were skewed in their production of effector cytokines in response to CD40L stimulation. CD40L stimulation induced significant secretion of tumor necrosis factor α (TNFα) and interleukin 12 (IL-12) p70 from both HIV-1-exposed and unexposed DCs. Intracellular stainings of HIV-1-exposed DCs revealed that TNFα could be detected in both the p24- and p24+ DCs, but IL-12 p70 could be found only in the p24- DCs. Thus, although p24+ DCs showed a mature phenotype similar to p24- DCs after CD40L stimulation, they appeared to have an impaired cytokine profile. These observations suggest that HIV-1 infection disables DC function, a phenomenon that may be relevant for optimal induction of HIV-1-specific immune responses. (Blood. 2004;104:2810-2817)
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells with the unique capacity to stimulate naive T cells and initiate primary T-cell responses.1 Maturation of DCs is essential for efficient T-cell stimulation and involves functional and phenotypic changes of DCs.2 Mature DCs lose their capacity to capture antigens and instead up-regulate high levels of major histocompatibility complex (MHC) class I and II molecules in order to efficiently present antigens to T cells. During the DC-T-cell interaction, CD40 ligand (CD40L) expressed on T cells binds to CD40 on the surface of DCs. This may induce further maturation of the DCs, characterized by high up-regulation of costimulatory molecules such as CD80 and CD86 and induction of cytokine production. The cytokines produced by DCs dictate the subsequent T-cell response. The interaction between CD40L and CD40 may therefore be crucial for bringing DCs to a state of maturation where they can efficiently trigger immune cascades.3,4
During sexual transmission of HIV-1, the virus crosses the mucosal epithelium and finally reaches the lymphoid tissue where it can establish permanent infection.5 DCs are proposed to play a crucial role in the early events of HIV-1 transmission by transporting the virus from the peripheral site to the lymphoid compartment. The DC-T-cell interaction in the lymphoid tissue is critical for the generation of immune responses, but also creates a perfect microenvironment for HIV-1 replication and transmission between cells.6 Although several studies have reported no or small changes in DCs after HIV-1 exposure, no obvious defects are defined.7,8
In the present study, we have developed an in vitro system designed to reflect the sequence of events that occur in vivo early after HIV-1 infection. Immature in vitro-derived DCs were exposed to HIV-1BaL and then stimulated with CD40L transfected cells to mimic the DC-T-cell interaction. DCs were subsequently examined for cell surface expression of costimulatory molecules and cytokine production. Here, we report that although high numbers of HIV-1-infected p24+ DCs were found after 72 hours of exposure to highly concentrated HIV-1, infection alone failed to induce differentiation of DCs to a mature phenotype. HIV-1-exposed DCs were susceptible to CD40L stimulation and significant induction of both tumor necrosis factor α (TNFα) and interleukin 12 (IL-12) p70 was obtained after 24 hours of CD40L stimulation. However, intracellular cytokine staining at the single cell level revealed that although the HIV-1-infected p24+ DCs expressed TNFα, they failed to express IL-12 p70.
Materials and methods
In vitro differentiation and culture of dendritic cells
DCs were differentiated from peripheral blood mononuclear cells (PBMCs) as described earlier.9,10 PBMCs were separated from healthy blood donors using Lymphoprep (Nycomed, Oslo, Norway) density gradient and monocytes were allowed to adhere for 1.5 hours at 37°C. Cells were washed 3 times with phosphate-buffered saline (PBS) and adherent monocytes were cultured for 6 days in medium (RPMI 1640 supplemented with 1% HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 2 mM l-glutamine, 1% streptomycin and penicillin, 10% endotoxin-free fetal bovine serum (FBS); GIBCO Life Technologies, Paisley, United Kingdom), recombinant human cytokines IL-4 (6.5 ng/mL; R&D Systems, Minneapolis, MN), and granulocyte macrophage-colony-stimulating factor (GM-CSF; 250 ng/mL; Leucomax, Schering-Plough, Brinny, Ireland), to obtain immature DCs. The FBS was chosen based on the lack of interferon γ (IFNγ) induction in freshly isolated PBMCs.
To obtain control DCs free of contaminating cells, CD14+ monocytes were enriched from PBMCs by negative selection using RosetteSep Human Monocyte Enrichment (StemCell Technologies, Vancouver, BC, Canada). Monocytes were cultured in IL-4 and GM-CSF as described in the preceding paragraph. After 6 days, 95% CD1a+ immature DCs were obtained with low to undetectable levels (< 0.6%) of CD3+ T cells and CD14+ monocytes.
HIV-1 virus growth and preparation
The CCR5-using HIV-1BaL isolate (National Institutes of Health [NIH] AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases [NIAID], NIH) was grown on PBMC cultures stimulated with phytohemagglutinin (PHA; Sigma, St Louis, MO) and IL-2 (Chiron, Emeryville, CA). To concentrate the virus and to minimize the presence of bystander activation factors in the supernatant that could induce DC maturation, the virus was ultracentrifuged (138 000g [45 000 rpm], 30 minutes, 4°C, Beckman L-80 Ultracentrifuge, rotor 70.1; Beckman Coulter, Fullerton, CA) and the virus pellet was resuspended in RPMI 10% FBS to obtain a 10 × virus concentrate.
Characterization of HIV-1BaL stock
The viral titer of the HIV-1BaL stock was determined by p24 enzyme-linked immunosorbent assay (ELISA; Murex HIV Antigen Mab; Abbott, Abbott Park, IL) according to manufacturer's protocol. Samples were analyzed in serial dilutions in duplicate. The 10 × HIV-1BaL stock had an HIV-1 p24Gag content of 11.7 μg/mL. The HIV-1BaL was also characterized by determining the level of active reverse transcriptase (RT; Lenti RT; Cavidi Tech, Uppsala, Sweden). The 10 × HIV-1BaL stock contained 15 000 pg active RT/mL. A correlation between the concentration of active RT and the number of HIV-1 RNA copies concludes that 1 fg RT equals 167 RNA copies.11 Since one HIV-1 virion contains 2 RNA copies, 1 fg RT theoretically equals 83.5 virions. The 10 × HIV-1BaL stock contained approximately 1.3 × 109 virions/mL.
HIV-1 infection and stimulation of dendritic cells
A quantity of 300 μL of 1 × or 10 × HIV-1BaL (3.8 × 107 and 3.8 × 108 virions, respectively) or mock was added to 5 × 105 immature DCs/mL (RPMI supplemented with 10% FBS, recombinant human IL-4, and GM-CSF) in a 24-well plate (Costar Corning, Corning, NY) to a final volume of 1.3 mL per well. In control experiments the virus was removed after overnight incubation by washing the DCs 3 times in RPMI to remove all cell-free virus. After 4 to 72 hours of culture, DCs were transferred to a 24-well plate coated with 5 × 104 CD40L-transfected murine L cells12 and cultured for an additional 6 to 24 hours. These cells express CD40L in its accurate membrane-bound trimeric form and are potent inducers of DC maturation.13,14 Background stimulation was measured using nontransfected cells in comparable experiments. CD40L expression on the cells was confirmed with a monoclonal antihuman CD40L antibody (clone TRAP1; Pharmingen, San Diego, CA) and flow cytometric analysis.
Quantification of HIV-1 protein in dendritic cells
The frequency of HIV-1BaL infection in DCs was determined by intracellular staining for the HIV-1 Gag protein p24. Cells were first stained for cell surface markers, then washed in PBS and fixed in 2% formaldehyde (Sigma) for 10 minutes at room temperature. Cells were washed in PBS with 2% FBS followed by a wash in PBS with 2% FBS, 2% HEPES, and 0.1% saponin (Sigma) to allow permeabilization of the cell surface membrane. Cells were incubated for 2 hours at 4°C with the anti-p24 antibody (clone KC57; Coulter, Hialeah, FL) or the corresponding isotype control. Cells were washed in saponin solution to remove excessive antibody and resuspended in PBS. Expression was assessed by a FACScalibur flow cytometer (Becton Dickinson). Cells (1-2 × 105 per sample) were collected and results were presented as percent p24+ DCs.
Phenotypic characterization of dendritic cells
DCs were collected and the cell culture wells were thereafter visually inspected to confirm that all cells were recovered. Cell surface characterization was performed by flow cytometric analysis as previously described.9 Cells were resuspended in PBS with 2% FBS and incubated for 30 minutes at 4°C with the following cell marker-specific antihuman monoclonal antibodies: CD1a (clone NA1/34; DAKO, Glostrup, Denmark), CD14 (clone TÜK4; DAKO), CD3 (clone SK7), CD80 (clone B7.5), CD86 (clone 2331/FUN-1), CD83 (clone HB15e), HLA-DR (clone L234; all from BD Biosciences, San Diego, CA), or with the corresponding isotype controls. Cell surface expression was assessed by a FACScalibur flow cytometer (Becton Dickinson). Cells (1-2 × 105 per sample) were collected and results were presented as mean fluorescent intensity.
Intracellular cytokine stainings in dendritic cells
The frequency of cytokine-producing DCs was determined by intracellular stainings for IL-12 p70 or TNFα, as previously described.15,16 Brefeldin A (10 μg/mL; Sigma) was added to the cell cultures 6 hours prior to intracellular staining. Cells were first stained for cell surface markers and then for intracellular cytokines, as described in the preceding paragraph. Cells were stained with the FITC-labeled anti-p24 antibody to distinguish HIV-1-infected and noninfected DCs and phycoerythrin (PE)-labeled antibodies (anti-IL-12 p70 [clone 20C2] or anti-TNFα [clone mAb11]; BD Biosciences) or the corresponding isotype controls. Expression was assessed by a FACScalibur (Becton Dickinson). Cells (1-2 × 105 per sample) were collected and results were presented as percent cytokine-producing DCs.
Simultaneous quantification of cytokines secreted by dendritic cells
To quantify the levels of TNFα and IL-12 p70 secreted from DCs, a Bio-Plex assay (Bio-Rad Laboratories, Hercules, CA) was performed and the results were analyzed using a Luminex reader (Luminex, Austin, TX), as described previously.17 Samples were analyzed in serial dilutions in duplicate and presented as cytokine concentration in pg/mL.
Statistical analysis
Statistical significance was assessed by unpaired t test (parametric) or Mann-Whitney test (nonparametric) and was considered significant at a P value less than .05. Correlations were analyzed with the Spearman rank test and were considered significant at a P value less than .05.
Ethics committee approval
This study was performed on samples from healthy blood donors and was approved by the local ethics committee at Karolinska Institutet. Informed consent was provided according to the Declaration of Helsinki.
Results
HIV-1 infection of in vitro-derived dendritic cells
Initially, adherent CD14+ monocytes cultured for 6 days in the presence of IL-4 and GM-CSF differentiated into immature DCs as defined by their expression of CD1a, lack of CD14, and low expression of CD40, CD80, CD86, and CD83.9,10 The frequency of immature CD1a+ DCs ranged from 75% to 90% in the cell cultures on day 6. The frequency of CD14+ cells was 5% to 10%, whereas the frequency of contaminating CD3+ cells ranged from 5% to 15% in the cultures (Figure 1A). Control DCs generated from negatively isolated CD14+ monocytes devoid of CD3+ T cells resulted in 95% immature CD1a+ DCs with less than 0.6% CD3+ T cells and less than 0.2% CD14+ cells in the cultures on day 6 (Figure 1B). Dose-response experiments were performed over time by exposing immature DCs to 2 different doses of the CCR5-using HIV-1BaL isolate for 4 to 72 hours. Intracellular staining for HIV-1 p24 combined with surface staining against CD1a was used to investigate the frequency of HIV-1-infected DCs. In order to include only DCs in the analysis, gates were set based on cell size, CD1a expression, and lack of CD3 expression. HIV-1 p24+ DCs appeared after 48 hours of exposure with the higher dose of HIV-1BaL (defined as 10 × virus concentrate and equaled 11.7 μg p24/mL or 1.3 × 109 virions/mL) and after 72 hours following exposure to the lower dose (1 × virus, equaled 1.17 μg p24/mL or 1.3 × 108 virions/mL) (Figure 1C). The frequency of p24+ DCs increased over time in a dose-dependent manner. Using the higher dose, 10 × HIV-1BaL, the frequency of p24-expressing DCs in 5 donors ranged from 0.1% to 1.3% (4 hours), 0.1% to 1.1% (24 hours), 0.7% to 3.0% (48 hours), 1.2% to 3.9% (52 hours), and 5.6% to 36.1% (72 hours). No decreased cell viability was found after 72 hours of HIV-1BaL exposure as assessed by annexin V (data not shown). HIV-1BaL exposure for 72 hours of DCs and control DCs resulted in similar frequencies of p24+ DCs (Figure 1D). Removing the virus by extensive washing after overnight incubation and then culturing DCs for a total of 72 hours resulted in lower but significant frequencies of p24+ DCs as compared with the cultures in which the virus was left for 72 hours (Figure 1D). No p24+ DCs could be detected when 10 μM 3′-azido-3′-deoxythymidine (AZT) was added together with HIV-1BaL to the cultures (Figure 1D). Supernatants were collected and the levels of HIV-1 p24 were measured by ELISA to validate the intracellular p24 stainings by detection of increasing levels of p24 in the cell culture supernatant over time (data not shown). These data confirm previous reports on low but productive HIV-1 replication in DCs.18
HIV-1BaL infection of in vitro-differentiated, monocyte-derived, human DCs. Flow cytometry was used to phenotypically characterize immature DCs generated from plastic adherent monocytes (A) or control DCs generated from monocytes isolated from PBMCs by negative selection (B) obtained after 6 days of culture in the presence of IL-4 and GM-CSF by determining the frequencies of CD1a+, CD3+, and CD14+ cells. Immature DCs were exposed to 2 different doses (1 × or 10 ×) of the CCR5-using isolate HIV-1BaL. The frequency of HIV-1BaL-infected DCs was determined by intracellular p24 staining after 4, 24, 48, 52, and 72 hours of virus exposure (C). The figure shows median values of 5 individual donors. Immature DCs (D, top row) or control DCs (D, bottom row) were exposed to 10 × HIV-1BaL for 72 hours or the virus was washed off after overnight incubation and cultured for 72 hours in the absence or presence of 10 μM AZT. Gates were set on large CD1a+ CD3- cells. The frequency of HIV-1BaL-infected DCs was determined by intracellular p24 staining after 72 hours.
HIV-1BaL infection of in vitro-differentiated, monocyte-derived, human DCs. Flow cytometry was used to phenotypically characterize immature DCs generated from plastic adherent monocytes (A) or control DCs generated from monocytes isolated from PBMCs by negative selection (B) obtained after 6 days of culture in the presence of IL-4 and GM-CSF by determining the frequencies of CD1a+, CD3+, and CD14+ cells. Immature DCs were exposed to 2 different doses (1 × or 10 ×) of the CCR5-using isolate HIV-1BaL. The frequency of HIV-1BaL-infected DCs was determined by intracellular p24 staining after 4, 24, 48, 52, and 72 hours of virus exposure (C). The figure shows median values of 5 individual donors. Immature DCs (D, top row) or control DCs (D, bottom row) were exposed to 10 × HIV-1BaL for 72 hours or the virus was washed off after overnight incubation and cultured for 72 hours in the absence or presence of 10 μM AZT. Gates were set on large CD1a+ CD3- cells. The frequency of HIV-1BaL-infected DCs was determined by intracellular p24 staining after 72 hours.
HIV-1-exposed dendritic cells exhibit a normal phenotype and respond to CD40L stimulation
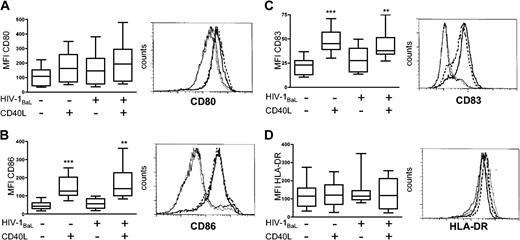
With appropriate stimulation, like CD40 ligation, DCs undergo rapid phenotypic changes such as up-regulation of MHC class II, costimulatory molecules CD80 and CD86, and CD83 expression, resulting in a mature DC phenotype.2 To investigate whether exposure to HIV-1BaL alone induced a mature DC phenotype, immature DCs were exposed to HIV-1BaL for 72 hours with or without CD40L stimulation for the final 24 hours. The cell surface expression of CD80, CD86, CD83, and HLA-DR on DCs was thereafter determined by flow cytometry and the mean fluorescent intensity (MFI) for each marker was calculated (Figure 2). Any CD3+ T cells were excluded by gating on CD1a+CD3- DCs. HIV-1BaL exposure alone did not have any significant effects on the phenotypic differentiation of the DCs compared with the unexposed DCs, confirming previous reports.19-21 However, CD40L stimulation of both HIV-1-exposed and unexposed DCs induced significant expression of CD86 (P < .01) and CD83 (P < .01), but did not significantly up-regulate the already high expression of HLA-DR. Although no significant differences could be assessed for CD80, the responsiveness to CD40L stimulation for each individual donor followed the same pattern as seen in CD86 and CD83 expression. Stimulation of DCs using the nontransfected cell line did not induce any significant phenotypic maturation of the DCs, demonstrating that the CD40-CD40L interaction induced maturation of the DCs. HIV-1BaL exposure and/or CD40L stimulation did not affect the viability of the DCs compared with unstimulated DCs as assessed by annexin V staining (data not shown). The susceptibility of HIV-1BaL-exposed DCs to CD40L stimulation was similar to that observed in the donor matched HIV-1-unexposed, unstimulated DCs. Conclusively, HIV-1BaL exposure alone did not lead to maturation of DCs and HIV-1BaL-exposed DCs retained their capability to fully mature after CD40L stimulation.
Phenotypic characterization of HIV-1BaL-exposed and CD40L-stimulated DCs. Immature DCs were exposed to 1 × HIV-1BaL for 72 hours with or without CD40L stimulation during the final 24 hours. The expression of CD80 (A), CD86 (B), CD83 (C), and HLA-DR (D) was measured by flow cytometry. Gates were set on large CD1a+ CD3- cells. The box plots (range and median) represent mean fluorescent intensity (MFI) obtained from 8 individual donors. Significant differences compared with the HIV-1BaL-unexposed, unstimulated DCs were assessed by an unpaired t test and are indicated by ** (P < .01) and *** (P < .001), respectively. The histograms show the cell surface expression of the maturation markers on DCs from one representative donor; medium (solid line), HIV-1BaL (thin dotted line), CD40L stimulation (solid bold line), and HIV-1BaL with CD40L stimulation (dashed bold line).
Phenotypic characterization of HIV-1BaL-exposed and CD40L-stimulated DCs. Immature DCs were exposed to 1 × HIV-1BaL for 72 hours with or without CD40L stimulation during the final 24 hours. The expression of CD80 (A), CD86 (B), CD83 (C), and HLA-DR (D) was measured by flow cytometry. Gates were set on large CD1a+ CD3- cells. The box plots (range and median) represent mean fluorescent intensity (MFI) obtained from 8 individual donors. Significant differences compared with the HIV-1BaL-unexposed, unstimulated DCs were assessed by an unpaired t test and are indicated by ** (P < .01) and *** (P < .001), respectively. The histograms show the cell surface expression of the maturation markers on DCs from one representative donor; medium (solid line), HIV-1BaL (thin dotted line), CD40L stimulation (solid bold line), and HIV-1BaL with CD40L stimulation (dashed bold line).
HIV-1 p24-expressing dendritic cells up-regulate CD86 after CD40L stimulation
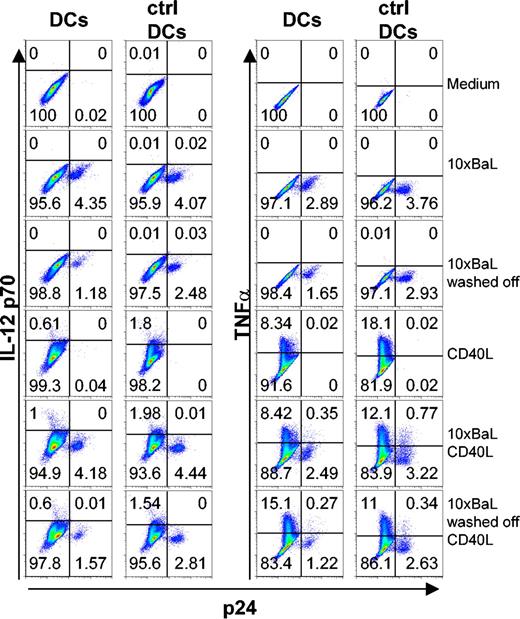
We next examined whether HIV-1BaL-infected DCs had a different phenotype compared with the p24 DCs in the same culture and if they could further mature after CD40L stimulation. Immature DCs were exposed to either 1 × or 10 × HIV-1BaL for 72 hours with or without CD40L stimulation during the final 24 hours. The DCs were thereafter examined for expression of p24, CD1a and the co-stimulatory molecule CD86 by flow cytometry. Gates were set to distinguish p24+ DCs from p24- DCs and to exclude CD3+ T cells from the analyses. The average expression of CD86 was found to be higher on the p24+ DCs than the p24- DCs. However, this difference was not statistically significant. After CD40L stimulation the CD86 expression significantly increased on the surface of both uninfected p24- (P < .01) and infected p24+ (P < .05) DCs, but was consistently higher on the p24+ DCs (Figure 3A). In addition, experiments were performed using control DCs devoid of T cells to confirm that the presence of contaminating cells did not affect the susceptibility to CD40L stimulation and subsequent up-regulation of CD86 expression on DCs (Figure 3B). We also found that DCs did not up-regulate CD86 when the virus was kept in the culture for 72 hours as compared with removing the virus after overnight incubation (10 × BaL washed off) and the medium control (Figure 3B). These data indicate that HIV-1BaL infection per se results in a certain degree of maturation of DCs and that the infected DCs remain susceptible to further maturation by CD40L stimulation.
HIV-1BaL p24+ DCs mature and up-regulate CD86 after CD40L stimulation. Immature DCs were exposed to 1 × or 10 × HIV-1BaL for 72 hours with or without CD40L stimulation during the final 24 hours. The expression of CD86 and intracellular p24 on CD1a+ DCs was determined by flow cytometry. Gates were set on large CD1a+ CD3- cells. (A) Average CD86 expression ± SEM of 5 donors in p24- DCs (▪) and p24+ DCs (□). Significant differences were assessed by an unpaired t test and are indicated by * (P < .05) and ** (P < 0.01), respectively. (B) The CD86 versus p24 expression on unstimulated (top row) and CD40L stimulated (bottom row) control DCs from 1 representative donor of 3 after 72 hours of 10 × HIV-1BaL exposure with or without CD40L stimulation during the final 24 hours.
HIV-1BaL p24+ DCs mature and up-regulate CD86 after CD40L stimulation. Immature DCs were exposed to 1 × or 10 × HIV-1BaL for 72 hours with or without CD40L stimulation during the final 24 hours. The expression of CD86 and intracellular p24 on CD1a+ DCs was determined by flow cytometry. Gates were set on large CD1a+ CD3- cells. (A) Average CD86 expression ± SEM of 5 donors in p24- DCs (▪) and p24+ DCs (□). Significant differences were assessed by an unpaired t test and are indicated by * (P < .05) and ** (P < 0.01), respectively. (B) The CD86 versus p24 expression on unstimulated (top row) and CD40L stimulated (bottom row) control DCs from 1 representative donor of 3 after 72 hours of 10 × HIV-1BaL exposure with or without CD40L stimulation during the final 24 hours.
HIV-1 exposure of dendritic cells does not alter cytokine secretion by CD40L stimulation
A controversy in the HIV-1 field has been whether DCs from HIV-1-infected individuals are defective in their ability to produce IL-12 and thereby fail to provide the help required for cytotoxic T-cell responses. To address this question we studied the cytokine profile of HIV-1BaL-exposed DCs in response to CD40L stimulation using Luminex. Again, immature DCs or control DCs were exposed to 10 × HIV-1BaL for 72 hours in the presence or absence of CD40L stimulation during the final 24 hours and the supernatants from DCs were analyzed for their content of TNFα and IL-12 p70 (Table 1). The spontaneous release of the cytokines studied from the unstimulated DCs was undetectable or low. HIV-1BaL exposure alone resulted in secretion of small amounts of TNFα that were found to be statistically significant compared with unexposed DCs (P < .01). CD40L stimulation resulted in high secretion of both cytokines measured, regardless of prior HIV-1 exposure. All groups of DCs showed a significant increase in secretion of TNFα and IL-12 p70 after 24 hours of CD40L stimulation as compared with the matched, unstimulated cultures (P < .01). TNFα increased 100- to 10 000-fold and IL-12 p70 increased 10 000-fold or more after CD40L stimulation. The small augmentation of HIV-1BaL exposure alone on TNFα secretion noted before CD40L stimulation was no longer detectable after stimulation. No significant differences could therefore be found between the CD40L-stimulated DCs and the DCs that received both HIV-1 exposure and CD40L stimulation. Analysis of supernatants from control DCs completely devoid of T cells confirmed that the presence of contaminating cells did not affect the levels of TNFα and IL-12 p70 secreted after HIV-1BaL exposure with or without CD40L stimulation (Table 1). Stimulation of DCs using the nontransfected cell line showed no induction of cytokines above spontaneous levels (data not shown). These data indicate that HIV-1BaL exposure alone resulted in induction of TNFα, but did not significantly affect IL-12 p70 secretion, as previously shown by others.20-22 In addition, virus exposure did not inhibit CD40L-induced cytokine secretion, when measured globally by detecting proteins secreted in the cell culture supernatant.
HIV-1BaL exposure does not inhibit cytokine secretion by CD40L stimulation
. | Median production, pg/mL (range) . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | IL-12 p70 . | . | TNFα . | . | |||
. | DCs . | Control DCs* . | DCs . | Control DCs* . | |||
| Medium | 0.5 (0-1.7) | 0 | 0.5 (0-1.0) | 0.3 (0-0.6) | |||
| 10 × BaL | 0.3 (0-1.0) | 0 | 12.3† (2.2-527) | 20.2 (5.6-34.7) | |||
| 10 × BaL washed off | 0.2* (0-0.4) | 0.9 (0-1.8) | 8.7* (0-17.4) | 7.6 (4.9-10.3) | |||
| CD40L | 1336‡ (658-28 258) | 1076 (1021-1160) | 7441‡ (3313-36 329) | 5576 (3967-7186) | |||
| 10 × BaL CD40L | 2061‡ (458-17 139) | 2103 (2019-2187) | 10 387‡ (6386-107 607) | 11 313 (6376-16 250) | |||
| 10 × BaL washed off CD40L | 3699* (3612-3786) | 3821 (2431-5210) | 12 852* (9454-16 250) | 15 135 (6160-24 110) | |||
. | Median production, pg/mL (range) . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | IL-12 p70 . | . | TNFα . | . | |||
. | DCs . | Control DCs* . | DCs . | Control DCs* . | |||
| Medium | 0.5 (0-1.7) | 0 | 0.5 (0-1.0) | 0.3 (0-0.6) | |||
| 10 × BaL | 0.3 (0-1.0) | 0 | 12.3† (2.2-527) | 20.2 (5.6-34.7) | |||
| 10 × BaL washed off | 0.2* (0-0.4) | 0.9 (0-1.8) | 8.7* (0-17.4) | 7.6 (4.9-10.3) | |||
| CD40L | 1336‡ (658-28 258) | 1076 (1021-1160) | 7441‡ (3313-36 329) | 5576 (3967-7186) | |||
| 10 × BaL CD40L | 2061‡ (458-17 139) | 2103 (2019-2187) | 10 387‡ (6386-107 607) | 11 313 (6376-16 250) | |||
| 10 × BaL washed off CD40L | 3699* (3612-3786) | 3821 (2431-5210) | 12 852* (9454-16 250) | 15 135 (6160-24 110) | |||
Immature DCs or control DCs devoid of T cells were exposed to 10× HIV-1BaL for 72 hours or the virus was washed off after overnight incubation and then cultured for a total of 72 hours, with or without CD40L stimulation during the final 24 hours. Cell culture supernatants were collected and analysed simultaneously by Luminex for the presence of TNFα and IL-12 p70. n = 6, if not otherwise indicated.
Data obtained from 2 individual donors (n = 2). Statistical analysis was not performed due to limited number of samples.
A significant difference between medium and HIV-1BaL -exposed DCs (P < .01, Mann-Whitney).
A significant difference between CD40L-stimulated DCs and the matched unstimulated DCs (P < .01, Mann-Whitney).
HIV-1 p24+ dendritic cells produce TNFα but not IL-12 p70 after CD40L stimulation
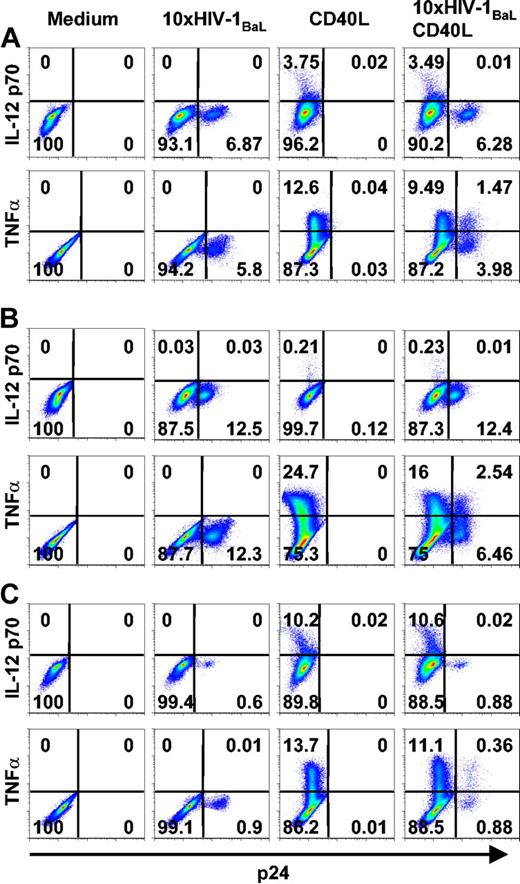
To study cytokine production in further detail, we used the intracellular p24 staining to distinguish between HIV-1-exposed but p24- DCs and HIV-1BaL-infected p24+ DCs, combined with intracellular cytokine staining. Immature DCs were exposed to 10 × HIV-1BaL for 72 hours with or without CD40L stimulation during the final 6 to 24 hours. It has been shown that stimulation of HIV-1-exposed DCs can result in a stronger induction of proinflammatory cytokines.7 To exclude the possibility that the HIV-1-infected DCs produced and secreted cytokines more rapidly than the uninfected DCs (and thereby escaped detection by intracellular staining), kinetics experiments were performed for both TNFα and IL-12 p70. DCs were stained for p24 and IL-12 p70 or TNFα after 72 hours of HIV-1BaL exposure with CD40L stimulation during the final 6 hours, 16 hours, or 24 hours. CD3+ cells were excluded from the analysis. After 6 hours of CD40L stimulation, the frequency of IL-12 p70 was similar to background levels (0.1%-0.15%). IL-12 p70 peaked after 16 hours of CD40L stimulation with frequencies of positive DCs ranging from 0.2% to 10% and thereafter plateaued, showing similar levels of IL-12 p70 after 24 hours of CD40L stimulation. TNFα production peaked already after 6 hours of CD40L stimulation showing 12% to 69% TNFα+ DCs after stimulation. TNFα could be detected also at later time points, although at lower frequencies (5%-40% after 16 hours and 3%-10% after 24 hours of stimulation; data not shown). To study the cytokine profile of the HIV-1-infected DCs in the relevant time period, we exposed DCs to 10 × HIV-1BaL for 72 hours with CD40L stimulation during the final 6 hours to measure TNFα and 24 hours to detect IL-12 p70. We found that while both the p24- and p24+ DC populations produced TNFα, only the p24- DCs colocalized with IL-12 p70-expressing cells (Figure 4). In addition, experiments were performed using control DCs devoid of T cells to confirm that the presence of contaminating cells did not affect the susceptibility to CD40L stimulation and subsequent induction of or lack of induction of cytokine production in DCs (Figure 4). Conclusively, the p24+ DCs failed to express IL-12 p70.
HIV-1BaL p24+ DCs produce TNFα but fail to produce IL-12 p70 in response to CD40L stimulation. Immature DCs and control DCs were exposed to 10 × HIV-1BaL for 72 hours with or without CD40L stimulation for the final 6 hours (TNFα) or 24 hours (IL-12 p70). As an additional control, the HIV-1BaL was washed off after overnight incubation and the DCs were cultured for 72 hours with or without CD40L stimulation for the final 6 hours (TNFα) or 24 hours (IL-12 p70). The figure shows intracellular cytokine production in p24- and p24+ DCs and control DCs after no stimulation (medium), HIV-1BaL exposure 72 hours (10× BaL), HIV-1BaL exposure overnight and cultured for 72 hours (10 × BaL washed off), CD40L stimulation alone, or HIV-1BaL exposure followed by CD40L stimulation. Gates were set on large CD1a+ CD3- cells. Numbers indicate frequency of stained DCs.
HIV-1BaL p24+ DCs produce TNFα but fail to produce IL-12 p70 in response to CD40L stimulation. Immature DCs and control DCs were exposed to 10 × HIV-1BaL for 72 hours with or without CD40L stimulation for the final 6 hours (TNFα) or 24 hours (IL-12 p70). As an additional control, the HIV-1BaL was washed off after overnight incubation and the DCs were cultured for 72 hours with or without CD40L stimulation for the final 6 hours (TNFα) or 24 hours (IL-12 p70). The figure shows intracellular cytokine production in p24- and p24+ DCs and control DCs after no stimulation (medium), HIV-1BaL exposure 72 hours (10× BaL), HIV-1BaL exposure overnight and cultured for 72 hours (10 × BaL washed off), CD40L stimulation alone, or HIV-1BaL exposure followed by CD40L stimulation. Gates were set on large CD1a+ CD3- cells. Numbers indicate frequency of stained DCs.
Dendritic cells that are highly susceptible to HIV-1BaL infection produce less IL-12 p70 than dendritic cells more resistant to infection
There was a tendency that DCs from donors more susceptible to in vitro HIV-1BaL infection produced less IL-12 p70 in response to CD40L stimulation than DCs from donors with a lower frequency of p24+ DCs. This phenomenon is illustrated in Figure 5; Donor A had an intermediate frequency of both p24+ DCs and IL-12-producing DCs and was representative of a majority of the 17 experiments performed. Donor B had a high frequency of HIV-1-infected p24+ DCs and very low numbers of IL-12-producing DCs. Donor C showed a high frequency of IL-12-producing DCs after CD40L stimulation, but had an apparent low susceptibility to HIV-1BaL infection. The frequency of TNFα-producing DCs was more consistent between different donors but the number of TNFα+ DCs tended to increase with the frequency of p24+ DCs. In summary, these data indicated that early during HIV-1BaL infection DCs produce TNFα but fail to produce IL-12 p70 after CD40L stimulation.
DCs highly susceptible to HIV-1BaL infection produce less IL-12 p70 than DCs more resistant to infection. Immature DCs were exposed to 10 × HIV-1BaL for 72 hours with CD40L stimulation during the final 6 hours (TNFα) or 24 hours (IL-12 p70). The figure shows intracellular cytokine production in p24- and p24+ DCs after no stimulation (medium), HIV-1BaL exposure alone, CD40L stimulation alone, or HIV-1BaL exposure followed by CD40L stimulation in 3 different donors (A, B, and C, respectively). The figure shows data representing donors with an intermediate susceptibility to HIV-1BaL infection (A), a high susceptibility to HIV-1BaL infection (B), and a low susceptibility to HIV-1BaL infection (C), and their IL-12 p70 and TNFα production after CD40L stimulation. Gates were set on large CD1a+ CD3- cells. Numbers indicate frequency of stained DCs.
DCs highly susceptible to HIV-1BaL infection produce less IL-12 p70 than DCs more resistant to infection. Immature DCs were exposed to 10 × HIV-1BaL for 72 hours with CD40L stimulation during the final 6 hours (TNFα) or 24 hours (IL-12 p70). The figure shows intracellular cytokine production in p24- and p24+ DCs after no stimulation (medium), HIV-1BaL exposure alone, CD40L stimulation alone, or HIV-1BaL exposure followed by CD40L stimulation in 3 different donors (A, B, and C, respectively). The figure shows data representing donors with an intermediate susceptibility to HIV-1BaL infection (A), a high susceptibility to HIV-1BaL infection (B), and a low susceptibility to HIV-1BaL infection (C), and their IL-12 p70 and TNFα production after CD40L stimulation. Gates were set on large CD1a+ CD3- cells. Numbers indicate frequency of stained DCs.
Dendritic cells that are highly susceptible to HIV-1BaL infection produce high levels of TNFα but low levels of IL-12 p70
To further study any correlation between the susceptibility to HIV-1BaL infection and the ability to produce TNFα and IL-12 p70 in response to CD40L stimulation, we compared the frequency of p24+ DCs with the amount of secreted cytokines or the frequency of intracellular cytokines. The frequency of p24+ DCs and the amounts of secreted TNFα (Figure 6A) and IL-12 p70 (Figure 6B) from DCs were compared, but no statistically significant correlations were found. Further, the frequency of p24+ DCs was compared with the frequency of DCs positive for intracellular staining against TNFα or IL-12 p70 (Figure 6C-D). While the frequency of TNFα+ DCs in response to CD40L stimulation appeared to increase with the frequency of p24+ DCs, there appeared to be a tendency to negative correlation between the number of IL-12 p70+ DCs and p24+ DCs. The potential correlations were analyzed using the Spearman rank test. The correlation between p24+ and TNFα+ DCs was statistically significant (n = 8, R = 0.738, P = .046). The correlation between p24+ and IL-12 p70+ DCs was also statistically significant (n = 17, R = -0.492, P = .045). These data indicate that although HIV-1BaL infection does not alter TNFα production in response to CD40L stimulation it does interfere with IL-12 p70 production.
Positive correlation between the frequency of HIV-1BaL p24+ DCs and TNFα-producing DCs. Immature DCs were exposed to 10 × HIV-1BaL for 72 hours with CD40L stimulation for the final 6 hours (TNFα) or 24 hours (IL-12 p70). The amount of secreted cytokines was measured in DCs from 6 donors and plotted against the frequency of p24+ DCs from each individual donor (A-B). The frequencies of intracellular cytokine (IC)-positive and p24+ DCs were determined in 8 donors for TNFα (C) and 17 donors for IL-12 p70 (D). To show any correlation between these parameters, the frequency of p24+ DCs was plotted against the frequency of TNFα+ DCs (C) and IL-12 p70+ DCs (D). Correlations were assessed using the Spearman rank test and considered statistically significant at P < .05. The correlation between p24+ and TNFα+ DCs (C) was statistically significant (n = 8, R = 0.738, P = .046). The correlation between p24+ and IL-12 p70+ DCs (D) was also statistically significant (n = 17, R = -0.492, P = .045).
Positive correlation between the frequency of HIV-1BaL p24+ DCs and TNFα-producing DCs. Immature DCs were exposed to 10 × HIV-1BaL for 72 hours with CD40L stimulation for the final 6 hours (TNFα) or 24 hours (IL-12 p70). The amount of secreted cytokines was measured in DCs from 6 donors and plotted against the frequency of p24+ DCs from each individual donor (A-B). The frequencies of intracellular cytokine (IC)-positive and p24+ DCs were determined in 8 donors for TNFα (C) and 17 donors for IL-12 p70 (D). To show any correlation between these parameters, the frequency of p24+ DCs was plotted against the frequency of TNFα+ DCs (C) and IL-12 p70+ DCs (D). Correlations were assessed using the Spearman rank test and considered statistically significant at P < .05. The correlation between p24+ and TNFα+ DCs (C) was statistically significant (n = 8, R = 0.738, P = .046). The correlation between p24+ and IL-12 p70+ DCs (D) was also statistically significant (n = 17, R = -0.492, P = .045).
Discussion
The effects of HIV-1 on DC function are poorly understood yet crucial to our knowledge of the pathogenesis of disease. The key finding in this study is that HIV-1-infected DCs matured, but failed to produce IL-12 p70 after CD40L stimulation.
Here, we show that HIV-1 exposure of in vitro-derived DCs did not lead to significant phenotypic differentiation and induced only small amounts of TNFα. Both the p24+ and p24- DCs were responsive to CD40L stimulation and showed full phenotypic maturation. CD40L stimulation also induced significant secretion of TNFα and IL-12 p70. However, intracellular cytokine stainings revealed that while TNFα was produced by both p24- and p24+ DCs, IL-12 p70 was exclusively produced by p24- DCs.
DCs may be one of the initial target cells for HIV-1 infection or virion capture at mucosal transmission sites and can therefore contribute to the dissemination of the virus to adjacent CD4+ T cells. In addition, if HIV-1-exposed DCs have impaired antigen-presenting capacity, the essential expansion of HIV-1-specific T-cell responses can be affected. Increasing evidence suggests that HIV-1 has evolved mechanisms to suppress CD40L expression,23,24 which could in part explain the lack of efficient immune response. CD40L is expressed mainly on the surface of a subset of CD4+ T cells upon cellular activation.25 The expression of CD40L declines on CD4+ T cells in patients with AIDS.26 Here, we designed an in vitro system with the aim to reflect the sequence of events that takes place in vivo early after HIV-1 exposure of DCs and the subsequent activation steps. Immature DCs were therefore first cultured with HIV-1BaL and then stimulated with CD40L to mimic the DC-T-cell interaction that occurs preferentially in the lymphoid compartments. Control experiments showed that the early kinetics of HIV-1 infection in DCs and the maturation and cytokine production of DCs were not influenced by the presence of a low frequency of contaminating cells in the DC cultures or by leaving the virus in the cultures throughout the culture. We found that CD40L further matured both HIV-1-exposed and HIV-1-infected DCs, with regard to cell surface phenotype and that the p24+ DCs had a slightly higher expression of CD86 compared with the p24- DCs in the same HIV-1BaL-exposed culture. However, whereas CD40L stimulation induced TNFα production in both p24- and p24+ DCs, IL-12 production was detected exclusively in the uninfected p24- DCs. This suggests that reconstitution of CD40L in HIV-1 infection may not fully restore the function of HIV-1-infected DCs.
As shown here, a dramatic consequence of CD40L-CD40 binding is induction of IL-12 secretion, which is important for induction of cellular responses against infections. This implies that induction of IL-12 production could constitute a functional marker of maturation in DCs in addition to up-regulation of cell surface markers.27 We show that while cytokine-producing DCs were always phenotypically mature, mature DCs did not always produce cytokines, which would point to the importance of looking at functional properties of DCs at the single cell level. Studies have revealed a severe defect in IL-12 production in PBMCs isolated from patients with AIDS.28,29 This impairment could be overcome by addition of soluble CD40L to monocyte cultures from HIV-infected patients.30 PBMCs from HIV-1 patients in different stages of the disease have shown that both the nature of stimulus and the stage of disease determine the amount of IL-12 secreted in culture.31 DCs isolated from HIV+ individuals showed similar amounts of secreted IL-12 following stimulation with CD40L, as did DCs from HIV- individuals.21 A recent report from Kawamura and co-authors shows that in vitro HIV-1 infection impairs DCs' capacity to stimulate CD4+ T cells, but the HIV-1-exposed DCs secrete increased amounts of IL-12 p70 after stimulation with soluble CD40L.32 This discrepancy between our findings and theirs may be explained by a difference in the time frame studied. While Kawamura et al extended the period of HIV-1 infection to 10 to 12 days, enriched for p24+ DCs and stimulated with CD40L for 48 hours, we aimed to study the very early onset of HIV-1 infection (72 hours) in DCs and CD40L stimulation as a model for DC-T-cell interaction. It cannot be excluded that we have examined a transient state of HIV-1-infected DCs early in HIV-1 infection where DCs are prevented from producing IL-12 p70. However, as the source of the secreted IL-12 p70 was not addressed by Kawamura et al, it may at least in part originate from the fraction of DCs that were p24-.
We found that CD40L stimulation of HIV-1-infected p24+ DCs resulted in phenotypically mature CD86+ DCs that produce TNFα but fail to produce IL-12 p70. Studies on the intracellular mechanisms of DC function have described a similar DC phenotype. Many inducers of DC maturation are also strong inducers of nuclear factor (NF)-κB transcription factors.33,34 The NF-κB family of transcription factors function as dimers of 5 proteins: p50, p52, RelA, RelB, and cRel. DCs derived from p50-/- cRel-/- mice respond to CD40L stimulation by up-regulation of MHC and costimulatory molecules, but unlike in wild-type-derived DCs, IL-12 production is abolished. These data demonstrate an essential role for p50 and cRel in CD40L-induced expression of IL-12.35 TNF receptor-associated factor 6 (TRAF6) binds to the CD40 cytoplasmic domain. In response to CD40L-CD40 binding, TRAF6 mediates the activation of NF-κB and JNK/stress activated protein kinase.36 DCs derived from mice with a mutation in the TRAF6 binding site in the cytoplasmic tail of CD40 show a normal phenotypic maturation after CD40L stimulation compared with wild-type-derived DCs, by up-regulation of MHCII, CD80, and CD86. However, disruption of the TRAF6 binding site diminishes IL-12 p70 production to background levels.37 HIV-1 infection elicits a broad range of cellular responses, many of which interfere with the regulatory pathways of IL-12 gene expression.38 One possible explanation for the DC phenotype in vitro and the IL-12 impairment revealed in patients may be that HIV-1 interferes with TRAF binding sites or other parts of the NF-κB signaling pathway.
Downmodulation of IL-12 may be a common strategy of evading the host immune system by viruses. Suppression of IL-12 has been documented in other viral infections. A recent study reported on the selective suppression of IL-12 production by another virus, human herpes virus 6.39 Human cytomegalovirus has been shown to inhibit maturation and cytokine production in DCs.40 In addition, IL-12 production is impaired both in macrophages after in vitro infection and in patients with naturally acquired measles infection.41-43 An understanding at the molecular level of the impaired IL-12 expression in HIV-1 infection may reveal novel therapeutic targets for reversing immune suppression in patients with AIDS.
Prepublished online as Blood First Edition Paper, July 1, 2004; DOI 10.1182/blood-2003-07-2314.
Supported by the Swedish Research Council; the Swedish Society of Medicine; the Swedish Foundation for Strategic Research; the Tore Nilsson, Magnus Bergvall, and Swedish Physicians Against AIDS Research Foundations; the Swedish Cancer Society; and the Swedish International Development Cooperation Agency/Department for Research Cooperation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal