Abstract
Leukocyte α4β1 integrins regulate hematopoietic and lymphoid development, as well as the emigration of circulating cells to sites of inflammation. Because vascular cell adhesion molecule-1 (VCAM-1) binding to high-affinity α4β1 is stable, these integrins can be detected and selectively precipitated from cell lysates using VCAM-1/Fc. With this approach, high-affinity α4β1 integrin expression was demonstrated on lymphocytes in the bone marrow, thymus, spleen, and the peritoneal cavity of normal mice, but not in peripheral lymph nodes. Immature lymphocytes preferentially expressed high-affinity α4β1 in the bone marrow and thymus. Paxillin is a cytoplasmic adaptor molecule that can bind to the α4 tail and initiate signaling. Paxillin was associated selectively with high-affinity integrins that were isolated from human Jurkat T cells or from murine tissues, and blotting with a phospho-specific antibody demonstrated that Ser988 in the α4 cytoplasmic tail was dephosphorylated in high-affinity but not low-affinity integrins. A rapid and transient α4β1 affinity up-regulation in formyl peptide receptor-transfected U937 cells stimulated with N-formyl-methyonyl-leucyl-phenylalanine (fMLP) correlated temporally with induced paxillin binding to α4 integrins. These data suggest that ligand binding to high-affinity α4β1 integrins may initiate outside-in signaling cascades through paxillin that regulate leukocyte maturation and emigration. (Blood. 2004;104:2818-2824)
Introduction
Integrins are expressed on virtually every cell in the body and mediate the attachment of cells to the extracellular matrix and to other cells. The α4β1 integrins, expressed on mononuclear leukocytes, bind primarily to vascular cell adhesion molecule-1 (VCAM-1) and the CS1 fragment of fibronectin. VCAM-1, a member of the immunoglobulin gene superfamily, is expressed by vascular endothelium in sites of inflammation, lymphoid tissues, bone marrow stroma, and cortical epithelium of the thymus.
Integrin adhesive capacity is regulated by complex intracellular signal transduction pathways, conformational changes, and clustering and lateral associations with other cell surface proteins and cytoskeletal elements.1 On most circulating leukocytes, α4 integrins have a low-binding affinity for VCAM-1.2 Inside-out signaling pathways activated by chemokines at a site of inflammation rapidly increase α4 integrin affinity and promote leukocyte emigration from blood into tissues.3,4 Alterations in integrin affinity are a consequence of conformational changes in the integrin extracellular and cytoplasmic domains and are regulated by associations with cytoplasmic proteins such as talin.5,6 The α4 integrins can also initiate outside-in signals and regulate cell spreading, migration, survival, and hematopoiesis.2,7-10 Integrin cytoplasmic domains have no enzymatic functions and activation of signaling cascades requires interactions with cytoplasmic signaling proteins and signaling adaptors. The β chain of integrins, including α4β1, associates with a variety of cytoskeletal and adaptor proteins such as talin, filamin, and α actinin.11 The cytoplasmic domain of the α4 integrin is unique because it specifically binds paxillin when Ser988 is dephosphorylated.12,13 Paxillin is a 68-kDa signaling adaptor molecule that contains LIM protein-protein interaction motifs and LD motifs that mediate protein-protein interactions.14,15 Paxillin interactions with α4β1 integrin may influence signaling through a variety of signaling pathways. Paxillin binds to tyrosine kinases including focal adhesion kinase (FAK), proline-rich tyrosine kinase (Pyk2), Src and Abl, SH2-domain containing proteins such as Crk and PTPPEST, and p95PKL, which can interact with PIX (a guanine nucleotide exchange factor) and PAK (a serine, threonine-kinase). Through these interactions and associations with actin-binding proteins talin, vinculin, and actopaxin, α4 integrin-paxillin complexes are likely to play an important role in the regulation of cytoskeletal remodeling and signaling events that are necessary for cell migration, activation, and development.
In this report, we characterize the expression of high-affinity α4 integrins in murine blood and tissues and establish that paxillin preferentially associates with high-affinity α4 integrins, which are expressed constitutively by Jurkat T cells in vitro and murine leukocytes in vivo. We also demonstrate that transient upregulation of α4 integrin affinity in U937 cells stimulated through a G protein-coupled receptor induces paxillin binding.
Materials and methods
Animals
C57BL/6 and BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME) and a colony was maintained. Mice were group-housed and fed a standard chow in a specific pathogen-free environment in accordance with institutional and governmental guidelines.
Cells and reagents
The human Jurkat T lymphoma cell line (E6-1) and U937 myelomonocytic cell line were obtained from American Type Culture Collection (ATCC; Manassas, VA). U937 cells transfected with the human formyl peptide receptor were obtained from Dr G. Downey (University of Toronto). All cell lines were routinely maintained in RPMI 1640 (Life Technologies, Burlington ON, Canada) supplemented with 10% fetal bovine serum (FBS; Life Technologies) and tested negative for mycoplasma.
Murine peritoneal exudates were obtained by flushing the peritoneal cavity with 10 mL phosphate-buffered saline (PBS) containing 10 mM EDTA (ethylenediaminetetraacetic acid). Lymphocytes were enriched by depletion of monocytes/macrophages by adherence to tissue culture plastic for 30 minutes in RPMI 5% FBS. Lymphocytes were collected from mouse spleen, thymus, and inguinal lymph nodes by passage through a 0.117-mm wire mesh. Murine bone marrow lymphocytes were obtained by flushing femurs with 3 mL PBS using a 25-gauge needle. Spleen, thymus, bone marrow, and peritoneal exudate lymphocytes were concentrated by centrifugation at 300g, 4°C for 5 minutes and resuspended in PBS containing 0.5% FBS (PBS-FBS).
Antigen-activated T cells were obtained from Dr L. Zhang (University Health Network, University of Toronto). Briefly, splenocytes from (2Cxdm2)F1 mice were isolated and incubated with irradiated (20 Gy) Ld splenocytes. The cells were cultured in α-minimum essential medium (MEM) supplemented with 10% FBS and 30 U/mL recombinant interleukin 2 (rIL-2) for 4 days. These cells express a transgenic T-cell receptor (TCR) reactive against Ld class I major histocompatibility complex (MHC).16
Chimeric VCAM-1 (VCAM-1/Fc) and intracellular adhesion molecule-1 (ICAM-1/Fc) were a gift from Dr D. Staunton (ICOS, Bothwell, WA) and have been previously characterized.17 VCAM-1/Fc consisted of the5NH2-terminal extracellular immunoglobulin-like domains of VCAM-1 linked to the Fc portion of human IgG. ICAM-1/Fc included all 5 extracellular immunoglobulin-like domains of ICAM-1. Heparin, EDTA, manganese chloride, magnesium chloride, ammonium chloride, sodium bicarbonate, rat IgG, and human IgG1 (isotype matched to the VCAM-1/Fc and ICAM-1/Fc) were purchased from Sigma (St Louis, MO).
Antibodies
Primary antibodies for flow cytometry were purchased from BD PharMingen Canada (Mississauga, ON, Canada) and included biotin-conjugated anti-mouse pan natural killer (NK) cells (DX5; rat IgM), fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD3 (17A2; rat IgG2b), biotin-conjugated anti-mouse CD3 (145-2C11, hamster IgG), biotin-conjugated anti-mouse CD19 (1D3; rat IgG2a), anti-mouse CD11b-biotin or CD11b-FITC (M1/70; rat IgG2b), FITC-conjugated anti-mouse Ly-6G (GR-1; RB6-8C5, rat IgG2b), FITC-conjugated anti-mouse IgD (11-26c.2a; rat IgG2a), and cyanine (Cy)-chrome-conjugated anti-mouse CD5 (53-7.3; rat-IgG2a). Antihuman α4 integrin (HP2/1; mouse IgG1) was purchased from Serotec (Raleigh, NC); mouse Fc block (anti-CD16/CD32; rat IgG2b) from BD PharMingen Canada; function-blocking anti-mouse α4 integrin (PS/2; rat IgG2b) from Chemicon (Temecula, CA), and function-blocking anti-mouse α4β7 integrin (DATK32; rat IgG2a) from BD PharMingen Canada.
VCAM-1/Fc and ICAM-1/Fc were detected with phycoerythrin (PE)-conjugated (Fab′)2 fragments of goat antihuman Fcγ and HP2/1 was detected with FITC-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Biotinylated primary antibodies were detected with streptavidin-FITC or streptavidin-Cy-chrome (BD PharMingen Canada).
Antibodies for Western blotting included anti-paxillin (BD Transduction Laboratories, Mississauga, ON, Canada), anti-α4 integrin (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-talin (Sigma). Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories. A monoclonal antibody raised against a peptide mimicking the α4 cytoplasmic tail phosphorylated on Ser988 was a gift from Dr M. Ginsberg (Scripps Research Institute, La Jolla, CA).18
Subtyping murine leukocytes
Leukocytes were subtyped based on the expression of cell surface markers. Neutrophils (PMNs) were identified as GR1high, CD11b+; monocytes GR1low, CD11b+; B cells CD19+; T cells CD3+; NK cells DX5+. B cells were further subtyped using antibodies against CD11b, CD5, IgD, and IgM.
Binding assay for VCAM-1/Fc
Cultured cells or lymphocytes collected from mouse lymphoid tissues were suspended at a concentration of 5 × 106/mL in Hanks balanced salt solution (HBSS; 1 mM Ca2+, 1 mM Mg2+) and supplemented with 1 mM Mn2+ where indicated. Cells were incubated with VCAM-1/Fc, ICAM-1/Fc, or human IgG (Fc containing control protein) for 30 minutes at 22°C. Cells were washed with HBSS and bound ligand was detected using fluorochrome-conjugated anti-human Fcγ (30 minutes at 4°C). After a final wash step with HBSS, cells were analyzed using a Coulter EPICS 2 flow cytometer (Hialeah, FL). Data were expressed as mean fluorescence intensity (MFI) of the entire cell population or the percentage of positive cells where the negative population was identified using IgG as a control protein.
The flow cytometry assay was modified for use with mouse peripheral blood. Peripheral blood was obtained by cardiac puncture from male and female C57BL/6 and BALB/c mice and anticoagulated using heparin. Aliquots of blood were preincubated with an equal volume of PBS containing Fc block and rat IgG for 20 minutes at 22°C prior to the addition of 20 μg/mL VCAM-1/Fc, ICAM-1/Fc, or human IgG. Mouse leukocyte subtypes were identified simultaneously by coincubation with subtype-specific antibodies conjugated to fluorochromes or biotin for 30 minutes at 22°C. Cells were washed and resuspended in PBS-FBS. Samples were incubated with the appropriate secondary antibodies plus streptavidinfluorochrome as required at 4°C for 30 minutes. Following incubation with the secondary antibody, erythrocytes were removed by hypotonic lysis and leukocytes were resuspended in PBS-FBS. VCAM-1/Fc binding was assessed by gating on individual leukocyte subtypes. For antibody inhibition experiments, blood samples were preincubated with 20 μg/mL anti-mouse α4 or α4β7 antibodies for 10 minutes prior to addition of VCAM-1/Fc or IgG.
Cell lysis and immunoprecipitation
Unstimulated Jurkat cells or leukocytes isolated from mouse tissues were washed 3 times with ice-cold PBS and resuspended in 1× lysis buffer for 15 minutes on ice. Lysis buffer consisted of 20 mM Tris (tris(hydroxymethyl)aminomethane), 150 mM NaCl, 10 mM EDTA, 1 mM sodium orthovanadate, 1 mM NaF, 1% Triton X-100, 0.05% Tween 20, and protease inhibitors (complete mini protease inhibitor cocktail and phenylmethylsulfonyl fluoride [PMSF]). Phalloidin (10 μM) was included in the lysis buffer to stabilize actin and actin-containing complexes. For lysate collection from chemokine-stimulated cells, cells were incubated with the stimulating agent for the appropriate time period followed by addition of an equal volume of ice-cold 2× lysis buffer. Lysates were centrifuged at 14 000g for 15 minutes at 4°C to remove insoluble cellular components. Protein concentration in lysate supernatants was determined using a DC-protein assay kit (Bio-Rad, Hercules, CA). For precipitations, 1 to 10 mg total lysate was precleared with mouse or human IgG and protein G-agarose beads for 30 minutes at 4°C. Anti-α4 integrin or VCAM-1/Fc was incubated with the precleared lysates for 2 hours followed by protein G-agarose overnight (4°C with rocking). Beads were washed 3 times in PBS containing protease inhibitors and boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. After precipitation, VCAM-1/Fc lysates were subjected to further immunoprecipitations after 2 successive incubations with protein G-agarose to remove any remaining VCAM-1/Fc.
SDS-PAGE and Western blotting
SDS-PAGE was carried out using 8% acrylamide gels (100 V, 22°C) and proteins were transferred to polyvinylidene difluoride (PVDF) membrane (22 V, 4°C, 18 hours). Membranes were probed for paxillin, talin, α4 integrin or phospho-α4 integrin (Ser988) and developed using HRP-conjugated secondary antibodies and the enhanced chemiluminescence (ECL) system (Amersham, Arlington Heights, IL). Blots were stripped in 20 mM glycine (pH 2.2) before reprobing.
Results
Paxillin associates with high-affinity α4 integrins
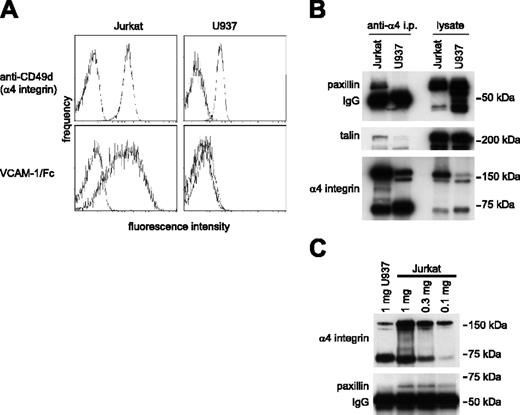
Paxillin is a cytoplasmic signaling adaptor protein that associates directly with the α4 integrin cytoplasmic tail13 and can bind only when Ser988 is dephosphorylated.12 Previous studies have not determined how paxillin binding relates to α4β1-integrin affinity. Therefore, we investigated paxillin association with α4β1 integrins in Jurkat T cells and U937 cells because both cell lines express α4β1 integrin, yet only Jurkat cells express α4β1 with constitutively high affinity for VCAM-1. The latter was demonstrated by measuring binding of soluble VCAM-1/Fc using flow cytometry (Figure 1A). Paxillin coprecipitated with α4 integrins from Jurkat cells but not from U937 cells, despite comparable or even higher paxillin expression in U937 cells (Figure 1B). In Jurkat cell lysates, paxillin association with α4 integrin was detected even when lower levels of α4 integrin were precipitated relative to a U937 cell lysate (Figure 1C). These data indicate that paxillin association with α4β1 integrins correlates directly with ligand-binding affinity. Talin also coprecipitated with α4 integrins expressed by Jurkat cells but not U937 cells, consistent with recent data that high-affinity ligand binding is induced by talin association with integrin-β cytoplasmic tails.5,6
Paxillin coprecipitation with α4 integrins correlates with the expression of high-affinity α4 integrins. (A) Flow cytometry profiles reveal slightly higher cell surface expression of α4 integrins on unstimulated Jurkat compared with U937 cells (top row), whereas VCAM-1/Fc binding to Jurkat cells is much higher (bottom row). (B) Western blotting for paxillin (top panel), talin (middle panel), and α4 integrins (bottom panel) of Jurkat and U937 cell lysates (1 mg) immunoprecipitated with HP2/1 antibody to α4 integrins (left blots) demonstrates coprecipitation of paxillin and talin with Jurkat, but not U937 α4 integrins. Western blotting of total cell lysates (20 μg/lane, right blots) illustrates abundant expression of all 3 proteins in both cell types. Representative data from 1 of 5 experiments are shown. (C) Coprecipitation of paxillin with α4 integrins was detected in 1, 0.3, and 0.1 mg Jurkat cell lysate, but not in the U937 cell lysate, even though the quantity of α4 integrins precipitated from U937 cells was comparable, or even exceeded that in the 0.3- and 0.1-mg Jurkat cell lysates. Representative data from 1 of 4 blots are shown.
Paxillin coprecipitation with α4 integrins correlates with the expression of high-affinity α4 integrins. (A) Flow cytometry profiles reveal slightly higher cell surface expression of α4 integrins on unstimulated Jurkat compared with U937 cells (top row), whereas VCAM-1/Fc binding to Jurkat cells is much higher (bottom row). (B) Western blotting for paxillin (top panel), talin (middle panel), and α4 integrins (bottom panel) of Jurkat and U937 cell lysates (1 mg) immunoprecipitated with HP2/1 antibody to α4 integrins (left blots) demonstrates coprecipitation of paxillin and talin with Jurkat, but not U937 α4 integrins. Western blotting of total cell lysates (20 μg/lane, right blots) illustrates abundant expression of all 3 proteins in both cell types. Representative data from 1 of 5 experiments are shown. (C) Coprecipitation of paxillin with α4 integrins was detected in 1, 0.3, and 0.1 mg Jurkat cell lysate, but not in the U937 cell lysate, even though the quantity of α4 integrins precipitated from U937 cells was comparable, or even exceeded that in the 0.3- and 0.1-mg Jurkat cell lysates. Representative data from 1 of 4 blots are shown.
In addition to detecting α4β1 integrins in the high-affinity conformation, VCAM-1/Fc was used to selectively precipitate high-affinity integrins from cell lysates. VCAM-1/Fc precipitated lower levels of α4 integrin from Jurkat cells when compared to an anti-α4 antibody (Figure 2), consistent with only a fraction of the integrins being in the high-affinity conformation. The phosphorylation of Ser988 in the cytoplasmic tail of α4 was determined by blotting with a phospho-specific antibody.18 Ser988 was dephosphorylated in high-affinity α4 integrins that were precipitated by VCAM-1/Fc (Figure 2). In contrast, Ser988 was phosphorylated in low-affinity α4 integrins, which were immunoprecipitated from lysates depleted of high-affinity α4β1. Furthermore, paxillin selectively coprecipitated with high-affinity but not low-affinity α4 integrins (Figure 2). The amount of paxillin remaining in the cell lysate after precipitation with VCAM-1/Fc was similar to that in a control lysate (data not shown). Similarly, coprecipitation of talin was found predominantly in the VCAM-1/Fc precipitate.
High-affinity but not low-affinity α4 integrins in Jurkat cells are dephosphorylated on Ser988 and are associated with paxillin. A representative experiment (1 of 4) of sequentially probed Western blots is shown. In lane 1, both high- and low-affinity α4 integrins in 1 mg Jurkat cell lysate were precipitated by HP2/1 antibody. Phosphorylation of Ser988 and association of paxillin and talin with α4 is seen. Selective precipitation of high-affinity α4 integrins with VCAM-1/Fc in lane 2 (4 mg lysate) reveals that these integrins are not phosphorylated but are associated with paxillin and talin. In contrast, in lane 3 low-affinity integrins precipitated by HP2/1 from 1 mg lysate depleted of high-affinity integrins were phosphorylated on Ser988 and were not associated with paxillin. Lane 4 shows 20 μg total Jurkat cell lysate.
High-affinity but not low-affinity α4 integrins in Jurkat cells are dephosphorylated on Ser988 and are associated with paxillin. A representative experiment (1 of 4) of sequentially probed Western blots is shown. In lane 1, both high- and low-affinity α4 integrins in 1 mg Jurkat cell lysate were precipitated by HP2/1 antibody. Phosphorylation of Ser988 and association of paxillin and talin with α4 is seen. Selective precipitation of high-affinity α4 integrins with VCAM-1/Fc in lane 2 (4 mg lysate) reveals that these integrins are not phosphorylated but are associated with paxillin and talin. In contrast, in lane 3 low-affinity integrins precipitated by HP2/1 from 1 mg lysate depleted of high-affinity integrins were phosphorylated on Ser988 and were not associated with paxillin. Lane 4 shows 20 μg total Jurkat cell lysate.
Chemoattractant stimulation induces paxillin association with α4 integrins
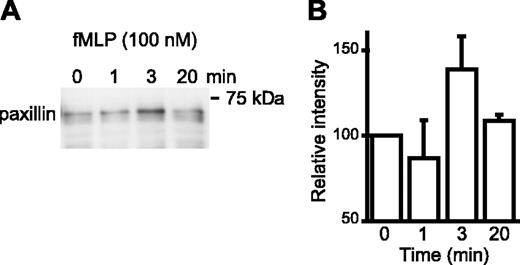
The α4 integrins are expressed predominantly in a low-affinity conformation by most circulating leukocytes3,19 and by U937 cells (Figure 1) and stimulation of these cells by chemoattractants or chemokines up-regulates integrin affinity rapidly and transiently.3 To investigate if transient up-regulation of α4 integrin affinity is temporally associated with paxillin binding, U937 cells, which were transfected with the formyl peptide receptor, were stimulated with N-formyl-methyonyl-leucyl-phenylalanine (fMLP). Paxillin coprecipitation with high-affinity α4 integrins increased at 3 minutes and declined to basal levels by 20 minutes (Figure 3). This suggests that a short-lived up-regulation of α4 integrin affinity by G protein-coupled receptor signaling results in transient association of paxillin with high-affinity α4 integrins. Immunodetection of paxillin in Western blots is much more sensitive than α4 integrins; therefore, we were not able to detect α4 integrins in VCAM-1/Fc precipitates of fMLP-stimulated U937 cell lysates.
Inside-out signaling induces paxillin association with high-affinity α4 integrins. (A) Formyl peptide receptor-transfected U937 cells were stimulated with fMLP (100 nM) for the indicated time prior to lysis and precipitation of cell lysates with VCAM-1/Fc (7 mg/lane). A representative Western blot probed for paxillin (1 of 4 experiments) is shown. (B) Blots from all 4 experiments were analyzed by densitometry and normalized to the 0 time point. The mean ± SEM is shown.
Inside-out signaling induces paxillin association with high-affinity α4 integrins. (A) Formyl peptide receptor-transfected U937 cells were stimulated with fMLP (100 nM) for the indicated time prior to lysis and precipitation of cell lysates with VCAM-1/Fc (7 mg/lane). A representative Western blot probed for paxillin (1 of 4 experiments) is shown. (B) Blots from all 4 experiments were analyzed by densitometry and normalized to the 0 time point. The mean ± SEM is shown.
Subpopulations of murine mononuclear leukocytes express high-affinity α4 integrins constitutively
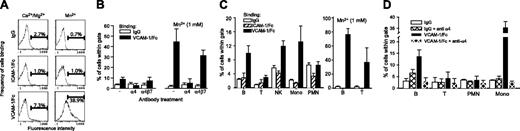
In human blood, α4 integrins are expressed in a low-affinity conformation by most mononuclear leukocytes19 ; however, the affinity state of murine leukocyte α4 integrins remains unknown. VCAM-1/Fc binding was used to evaluate the affinity of α4 integrins expressed by murine leukocytes in various compartments, including the blood, bone marrow, and lymphoid tissues. VCAM-1/Fc binding to peripheral blood leukocytes was performed in whole blood (anticoagulated and diluted 1:1 with PBS) to minimize potential activation of integrins, which can occur during isolation of cells. Relative to human IgG or human ICAM-1/Fc, which does not bind to murine β2 integrins,20 increased VCAM-1/Fc binding was observed to only a small number of peripheral blood leukocytes (Figure 4A). Treatment of leukocytes with 1 mM Mn2+, which binds directly to integrins and induces a high-affinity conformation, markedly increased VCAM-1/Fc, but not IgG or ICAM-1/Fc binding, indicating that VCAM-1/Fc binding was specific and that binding by Fc receptors was not significant. The relatively small number of leukocytes that did not bind VCAM-1/Fc in the presence of MnCl2 likely represents cells that express α4 integrins at low levels. Function-blocking antibodies to α4 were used to further demonstrate the specificity of VCAM-1/Fc binding and specific blockade of α4β7 demonstrated that most of the VCAM-1/Fc binding was mediated by α4β1 (Figure 4B). Subtyping of peripheral blood leukocytes revealed increased binding of VCAM-1/Fc (relative to IgG and ICAM-1/Fc) to B cells (10%), NK cells (10%), and monocytes (10%-15%), but not to T cells and neutrophils (Figure 4C), irrespective of whether leukocytes were obtained from C57BL/6 or BALB/c mice. Incubating cells with MnCl2 increased VCAM-1/Fc binding on 70% to 80% of B cells and 30% to 40% of T cells (Figure 4C). Blockade of α4 integrins with function-blocking antibody inhibited VCAM-1/Fc binding to B cells, T cells, and monocytes (Figure 4D), but did not diminish binding of IgG to any cell type, nor did it reduce VCAM-1/Fc binding to neutrophils, which is presumably mediated by Fc receptors. Further analysis of blood B cells revealed that a higher percentage of IgD-, CD11b+, and CD5+ B cells bound VCAM-1/Fc at levels above IgG or ICAM-1/Fc (data not shown), which suggests that immature B cells and B1 cells preferentially express high-affinity α4 integrins.
VCAM-1/Fc binds to murine high-affinity α4 integrins and identifies subtype-specific regulation of integrin affinity. (A) Representative flow cytometry profiles of human IgG, ICAM-1/Fc, and VCAM-1/Fc binding to mouse peripheral blood leukocytes. Binding was carried out in diluted whole blood with 1 mM Ca2+ and Mg2+ with or without 1 mM Mn2+. (B) Blockade of VCAM-1/Fc binding with function-blocking anti-α4 integrin and anti-α4β7 antibodies (30 minutes of pretreatment) in Ca2+/Mg2+ containing buffers with or without Mn2+. Data are expressed as mean ± SEM, n = 4 mice. (C) IgG, ICAM-1/Fc, and VCAM-1/Fc binding to different leukocyte subtypes, identified by immunostaining for specific cell surface markers. MnCl2 (1 mM) was included in some assays (right panel). Data pooled from C57BL/6 and BALB/c mice are expressed as the mean ± SEM, n = 10 mice. (D) Blockade of VCAM-1/Fc binding to B cells, T cells, and monocytes (Mono) with anti-α4 integrin (mean ± SEM, n = 4 mice).
VCAM-1/Fc binds to murine high-affinity α4 integrins and identifies subtype-specific regulation of integrin affinity. (A) Representative flow cytometry profiles of human IgG, ICAM-1/Fc, and VCAM-1/Fc binding to mouse peripheral blood leukocytes. Binding was carried out in diluted whole blood with 1 mM Ca2+ and Mg2+ with or without 1 mM Mn2+. (B) Blockade of VCAM-1/Fc binding with function-blocking anti-α4 integrin and anti-α4β7 antibodies (30 minutes of pretreatment) in Ca2+/Mg2+ containing buffers with or without Mn2+. Data are expressed as mean ± SEM, n = 4 mice. (C) IgG, ICAM-1/Fc, and VCAM-1/Fc binding to different leukocyte subtypes, identified by immunostaining for specific cell surface markers. MnCl2 (1 mM) was included in some assays (right panel). Data pooled from C57BL/6 and BALB/c mice are expressed as the mean ± SEM, n = 10 mice. (D) Blockade of VCAM-1/Fc binding to B cells, T cells, and monocytes (Mono) with anti-α4 integrin (mean ± SEM, n = 4 mice).
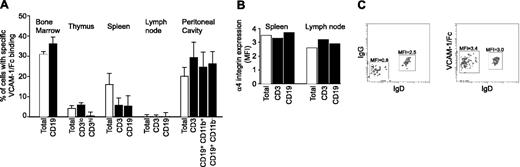
Analysis of leukocytes isolated from different tissues revealed differences in specific VCAM-1/Fc binding (binding levels that exceeded binding of IgG; Figure 5A). Elevated binding was found in the bone marrow, thymus, spleen, and peritoneal cavity, but not peripheral lymph nodes. In the bone marrow, 35% of cells bound VCAM-1/Fc, including CD19+ and CD19- populations. VCAM-1/Fc binding to immature bone marrow B cells (CD19+, IgM+, IgD-) was significantly higher compared to mature IgD+ B cells (Figure 5C). Similar observations were obtained with blood B cells (data not shown). Only a small percentage of thymocytes bound VCAM-1/Fc (Figure 5A), and binding was associated with cells expressing a low level of CD3, indicative of immature T cells. T cells in the thymus with high levels of CD3 expression did not bind VCAM-1/Fc and higher VCAM-1/Fc binding was observed to double-positive T cells than single-positive cells (not shown).
Differential expression of high-affinity α4 integrins in murine tissues. (A) The percent of leukocytes exhibiting specific VCAM-1/Fc binding, indicative of expression of high-affinity α4 integrin, was determined by subtracting IgG from VCAM-1/Fc binding. Specific VCAM-1/Fc binding to leukocytes and leukocyte subtypes isolated from various tissues is shown. Data are expressed as mean ± SEM of values obtained from at least 4 mice. (B) The expression of α4 integrins on the cell surface of spleen and lymph node leukocytes was determined by flow cytometry. The MFI of immunostaining from a representative experiment is shown. (C) IgG and VCAM-1/Fc binding (left and right panels, respectively) to IgD+ and IgD- populations of IgM+ bone marrow B cells. The MFIs determined by flow cytometry are indicated. Representative data from 1 of 4 experiments are shown.
Differential expression of high-affinity α4 integrins in murine tissues. (A) The percent of leukocytes exhibiting specific VCAM-1/Fc binding, indicative of expression of high-affinity α4 integrin, was determined by subtracting IgG from VCAM-1/Fc binding. Specific VCAM-1/Fc binding to leukocytes and leukocyte subtypes isolated from various tissues is shown. Data are expressed as mean ± SEM of values obtained from at least 4 mice. (B) The expression of α4 integrins on the cell surface of spleen and lymph node leukocytes was determined by flow cytometry. The MFI of immunostaining from a representative experiment is shown. (C) IgG and VCAM-1/Fc binding (left and right panels, respectively) to IgD+ and IgD- populations of IgM+ bone marrow B cells. The MFIs determined by flow cytometry are indicated. Representative data from 1 of 4 experiments are shown.
Lymphocytes isolated from secondary lymphoid organs showed striking differences in specific VCAM-1/Fc binding (Figure 5A). VCAM-1/Fc bound to 15% of spleen cells, with a similar level of binding to B and T lymphocytes. In contrast, VCAM-1/Fc binding to either B or T cells from lymph nodes was not observed although expression of α4 integrin by lymph node and spleen B and T cells was similar (Figure 5B). VCAM-1/Fc bound to 30% to 50% of T and B lymphocytes lavaged from the unstimulated peritoneal cavity and purified by depleting monocytes and macrophages through adherence to plastic. B1 (CD19+, CD5+, CD11b+) and B2 (CD19+, CD5-, CD11b-) cells had comparable binding.
Paxillin preferentially associates with high-affinity α4 integrins that are constitutively expressed by murine leukocytes
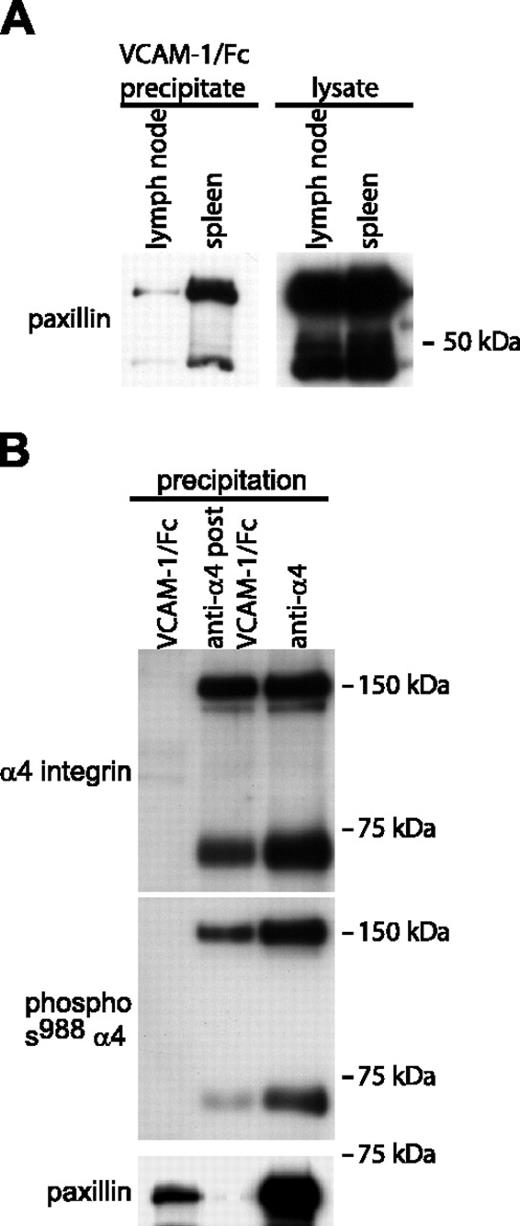
Because the experiments demonstrated that certain murine mononuclear leukocytes express α4 integrins that are constitutively or spontaneously in the high-affinity conformation, we investigated whether paxillin associates with high-affinity α4 integrins on these cells. Paxillin association with α4 integrins was detected in VCAM-1/Fc precipitates of mouse spleen (Figure 6A) and peritoneal leukocyte lysates (data not shown). In contrast, only relatively small quantities of paxillin were detected in VCAM-1/Fc precipitates from lymph node lysates. As with Jurkat cells, in mouse spleen cell lysates, paxillin was associated preferentially with high-affinity α4 integrins that were dephosphorylated on Ser988 (Figure 6B). Lysates depleted of high-affinity integrins by VCAM-1/Fc precipitation still contained abundant α4 integrins that were phosphorylated on Ser988 (Figure 6B).
Paxillin associates with high-affinity α4 integrins isolated from murine spleen cells. (A) The left Western blot compares paxillin coprecipitation with high-affinity α4 integrins isolated from mouse lymph nodes and spleens (1 mg of each lysate was incubated with VCAM-1/Fc). The right blot shows paxillin expression in total cell lysates (20 μg/lane). (B) High-affinity, low-affinity, and both high- and low-affinity α4 integrins were precipitated from murine spleen cell lysates (2 mg total cell lysate per lane) as described in Figure 2. Blots were sequentially probed for phosphorylated Ser988 α4 integrin, paxillin, and α4 integrins. Representative data from 1 of 3 experiments are shown.
Paxillin associates with high-affinity α4 integrins isolated from murine spleen cells. (A) The left Western blot compares paxillin coprecipitation with high-affinity α4 integrins isolated from mouse lymph nodes and spleens (1 mg of each lysate was incubated with VCAM-1/Fc). The right blot shows paxillin expression in total cell lysates (20 μg/lane). (B) High-affinity, low-affinity, and both high- and low-affinity α4 integrins were precipitated from murine spleen cell lysates (2 mg total cell lysate per lane) as described in Figure 2. Blots were sequentially probed for phosphorylated Ser988 α4 integrin, paxillin, and α4 integrins. Representative data from 1 of 3 experiments are shown.
The affinity of α4 integrins is up-regulated in activated/proliferating T cells
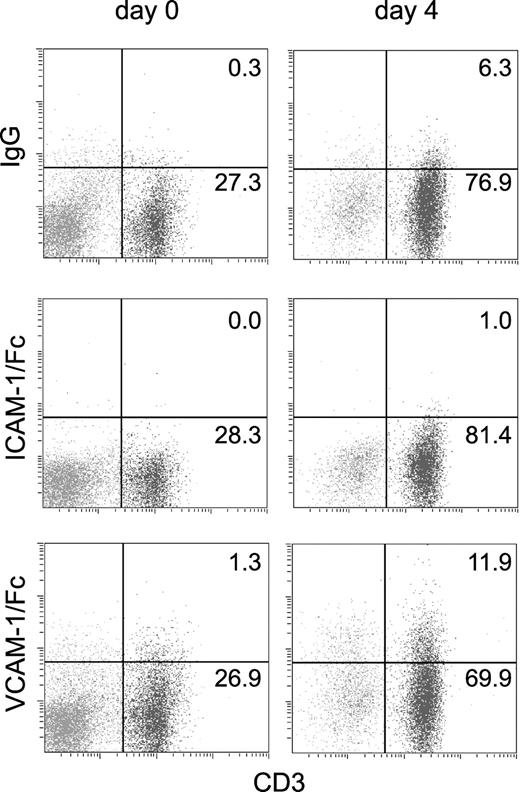
We investigated whether activation of T cells by specific antigen increased the affinity of α4 integrin for VCAM-1. A low percentage of freshly isolated splenic T cells from (2Cxdm2)F1 mice, which express a transgenic TCR reactive against Ld class I MHC, bound VCAM-1/Fc, similar to the level of VCAM-1/Fc binding observed for C57BL/6 or BALB/c splenocytes. VCAM-1/Fc binding increased following T-cell stimulation with irradiated Ld splenocytes for 96 hours (Figure 7). In contrast, α4β1 integrin expression was unchanged by T-cell stimulation. The MFI of staining for α4 integrin was 3.2 before stimulation versus 3.5 after stimulation (compared to 0.3 and 0.6 for an isotype control antibody). This increase in VCAM-1/Fc binding was comparable to that previously observed on encephalitogenic T-cell blasts cultured with protein lipid-protein.21
T-cell activation with antigen induces a small increase in VCAM-1/Fc binding. VCAM-1/Fc, ICAM-1/Fc, and human IgG binding to CD3+ and CD3- splenocytes isolated from (2Cxdm2)F1 mice that express a transgenic TCR specific for Ld was determined by flow cytometry. Binding was measured prior to (day 0) and after 4 days of culturing with irradiated Ld splenocytes (day 4). The percent of cells in CD3+ quadrants is indicated. Representative data from 1 of 3 experiments are shown. The cell surface expression of α4 integrins was unchanged in antigen-challenged cells.
T-cell activation with antigen induces a small increase in VCAM-1/Fc binding. VCAM-1/Fc, ICAM-1/Fc, and human IgG binding to CD3+ and CD3- splenocytes isolated from (2Cxdm2)F1 mice that express a transgenic TCR specific for Ld was determined by flow cytometry. Binding was measured prior to (day 0) and after 4 days of culturing with irradiated Ld splenocytes (day 4). The percent of cells in CD3+ quadrants is indicated. Representative data from 1 of 3 experiments are shown. The cell surface expression of α4 integrins was unchanged in antigen-challenged cells.
Discussion
On most circulating leukocytes, α4 integrins have a low binding affinity for VCAM-1,2 yet inside-out signaling can rapidly upregulate their affinity, and thus contributes to various stages of leukocyte emigration from blood into tissues.3,4 The α4 integrins can also initiate intracellular or outside-in signals on binding of ligands, which regulate leukocyte adhesion, cytoskeletal rearrangement, migration, survival, and hematopoiesis.2,7-10 Integrins signal through association with cytoplasmic adaptor molecules, and when clustered, as in focal adhesions, they are associated with multiple cytoskeletal and signaling molecules.22,23 However, integrins may also initiate outside-in signaling when they are not in large clusters.24 The α4β1 integrin is unique in that its α chain can directly bind to paxillin when Ser988 is dephosphorylated12 and initiate signaling through binding of kinases such as FAK and Pyk2. Previously, paxillin association with α4β1 was demonstrated in Jurkat cells, but it was not known whether it binds to high-affinity or low-affinity α4 integrins. Preferential association with a particular affinity state of α4 integrins is important because the regulation of affinity would then influence not only ligand binding but also outside-in signaling.
Paxillin associates specifically with the α4 integrin cytoplasmic tail and the related α9 tail25 but not with other integrin cytoplasmic tails.13 Consequently, these integrins may regulate signal transduction differently from other integrins. Using cytoplasmic domain deletions and substitutions, Hemler et al have shown that the α4 cytoplasmic domain supports weaker cytoskeletal interactions than other integrins, which leads to reduced cell spreading and adhesion strengthening and augmentation of rolling interactions and cell migration.26 The ability of both α4 and α9 integrin to inhibit cell spreading is a consequence of their interactions with paxillin.13,25,27
Signals from α4 integrins regulate the functions of other integrins (eg, β2 integrin-mediated leukocyte adhesion and migration on ICAM-1), protrusion of filopodia, and cytoskeletal rearrangement on α4 integrin ligands.8-10,28 High-affinity α4 integrin has been shown to play a role in these signaling events.8,28 Paxillin binding to the α4 integrin cytoplasmic domain augments phosphorylation (and activation) of focal adhesion kinase family proteins FAK and Pyk2 following integrin engagement.2 Paxillin association with α4 integrin and activation of FAK and Pyk2 are essential for α4β1 integrin-mediated β2 integrin-dependent migration.2 Previous studies have suggested that the ability of α4β1 integrin to stimulate β2 integrin-mediated adhesion and migration is dependent on α4β1 integrin affinity state.8,28 This is consistent with our data illustrating that high-affinity but not low-affinity α4 integrins associate with paxillin and can consequently activate FAK and Pyk2.
Paxillin association with α4 integrins is not required for integrin activation. Neither disruption of the association between α4 integrin and paxillin nor forced association altered the affinity of α4 integrins for VCAM-1.2,29 In contrast, talin association with the integrin β chain appears to be necessary for up-regulation of integrin affinity. Overexpression of the talin head domain leads to alterations in integrin conformation5 and activation,30 and siRNA-knockdown of talin expression inhibits integrin activation.6 High-affinity α4 integrins are capable of stable binding of ligand, which may lead to outside-in signaling via paxillin, whereas ligand binding by low-affinity α4 integrins may be incapable of signal transduction.
Only a small fraction of human peripheral blood leukocytes express α4 integrins with constitutive high affinity.19 In this report, our experiments using mouse blood demonstrated that 10% to 20% of NK cells, but not T cells, bind VCAM-1/Fc, which is consistent with previous studies of human blood.19 We also demonstrated high-affinity α4 integrin expression by murine peripheral blood B cells and monocytes. Although peripheral blood T cells did not constitutively bind VCAM-1/Fc, they express α4 integrins and we were able to induce VCAM-1/Fc binding by directly modulating integrin conformation with MnCl2. In a previous study, we demonstrated that stimulation of human peripheral blood CD3+ cells with stromal cell-derived factor 1α (SDF1α) led to a rapid and transient increase in α4 integrin affinity for VCAM-1/Fc.3 Similarly, Rose et al demonstrated an increase in VCAM-1/Fc binding to memory (CD45RO) but not naive (CD45RA) T cells on stimulation with phorbol ester.19 Together, these data suggest that under basal conditions α4 integrins on circulating memory T cells are in a low-affinity state, but on stimulation with the appropriate chemokine, affinity for VCAM-1 is increased, which may lead to increased adhesion and transmigration at a site of inflammation.
Our data suggest that T cells exhibit developmental regulation of α4 integrin affinity. The ability to bind soluble VCAM-1/Fc was highest in thymocytes that exhibited low to medium expression of CD3, and in double-positive thymocytes when compared to single-positive cells. This confirms previous reports that double-positive thymocytes express functionally active α4β1 integrin that facilitates adhesion to VCAM-1 expressed in the cortical thymus.31 In this manner, a suppression of α4 integrin affinity on thymocytes as they mature may permit detachment from the thymic stroma and allow T cells to recirculate and respond to chemotactic stimuli at other sites. Similarly, developmental regulation of α4 integrin affinity may play an important role in hematopoiesis in the bone marrow. Hematopoietic stem cells are mobilized from the bone marrow by antibodies against the α4 integrin.32 A complete absence of α4 integrins produced major defects in the development of the lymphoid and myeloid lineages in mice.33 These results underscore the importance of α4 integrin adhesive interactions in lymphocyte development. High-affinity α4 integrin may not only regulate the adhesive interactions that maintain hematopoietic cells in the bone marrow, but may also provide some of the signals that regulate development and differentiation.
In contrast to the unstimulated peritoneal cavity and the spleen, neither B nor T cells isolated from normal peripheral lymph nodes expressed high-affinity α4 integrins constitutively. This is consistent with the lymph node as a site for recirculation of T and B cells in search of a specific antigen. Cells that do not recognize antigen must maintain a highly motile state to exit via the lymphatics and reenter the circulation. Because 15% of circulating B cells express high-affinity α4β1, these cells either cannot be recruited into peripheral lymph nodes or their integrins rapidly revert to a low-affinity state on entering a lymph node.
In summary, our data demonstrate that the regulation of leukocyte α4 integrin affinity in mouse tissues is complex and appears to be dependent on tissue distribution, activation state, and maturation. The preferential association of paxillin with high-affinity α4 integrins implies that high-affinity α4 integrins are capable of outside-in signaling on binding of ligand.
Prepublished online as Blood First Edition Paper, July 8, 2004; DOI 10.1182/blood-2003-12-4402.
Supported by Canadian Institutes of Health Research grant MOP-14151 (M.I.C.) and Astra Zeneca-MRC-PMAC-RFP (S.J.H.; PFE-36617). M.I.C is a recipient of a Career Investigator award from the Heart and Stroke Society of Canada. S.J.H. was a recipient of a Postdoctoral Fellowship from the Multiple Sclerosis Society of Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Drs Gregory Downey (University of Toronto), Li Zhang (University of Toronto), Don Staunton (ICOS Corporation), and Mark Ginsberg (Scripps Research Institute) for providing reagents.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal