Abstract
We have established human B-lineage (BLIN) acute lymphoblastic leukemia cell lines that retain a dependency on fibroblast monolayers for survival and proliferation. Eight hours following removal from adherent cell contact BLIN cells undergo a decrease in mitochondrial transmembrane potential and an increase in annexin V binding. Unexpectedly, the caspase-9 inhibitor (C9i) benzyloxycarbonyl-Leu-Glu-His-Asp-fluoromethylketone enhanced the appearance of apoptotic cells within 8 hours following removal of BLIN cells from fibroblast monolayers. C9i enhancement of apoptosis was dose dependent and did not occur with irreversible inhibitors of caspases-2, -3, -6, and -8. C9i also enhanced apoptosis in cord blood-derived CD19+ B-lineage cells (but not myeloid cells) removed from murine stromal cells. Longer exposure (> 18 hours) to C9i culminated in apoptosis in a panel of B-lineage acute lymphoblastic leukemia (ALL) cell lines in the presence or absence of fibroblast monolayers, as well as in 2 proliferating leukemic cell lines (RAMOS and CEM). BLIN-4L cells made deficient in caspase-9 by RNA interference exhibited no resistance to apoptotic signals and actually showed increased apoptotic sensitivity to staurosporine. These collective results suggest that a 4-amino acid caspase inhibitor of caspase-9 can promote apoptosis and that at least some types of apoptotic pathways in B-lineage ALL do not require caspase-9.
Introduction
Normal lymphoid cell development in the bone marrow and thymus is autonomously regulated by transcription factors that modify patterns of gene expression1 and externally regulated by the extracellular matrix, stromal cells, and cytokines/chemokines/colony-stimulating factors.2,3 The fate of developing lymphoid cells is further influenced by the functional balance of proapoptotic and antiapoptotic proteins expressed at specific points in development and the survival signals that are transduced by external cues such as cytokines.4,5
Induction of apoptosis proceeds through 2 main pathways. The first initiates at cell surface death receptors such as CD95/Fas or the tumor necrosis receptor (the extrinsic pathway).6,7 The second is triggered by stress, genotoxic agents, or cytokine deprivation (the intrinsic or mitochondrial pathway).8,9 The biochemical coupling of proapoptotic molecules of the Bcl-2 homology region 3 (BH3) death domain and B-cell lymphoma 2 (Bcl-2) family members to caspase activation and downstream dismantling of cellular structure has been extensively studied in all metazoans. However, caspase activation by itself does not guarantee that apoptotic signals proceed unchecked. Members of the inhibitor of apoptosis (IAP) family of proteins can suppress or delay apoptosis by inhibiting the enzymatic activity of initiator and executioner caspases.10,11 Whereas activation of initiator (eg, caspases-8, -9, and -10) and executioner (eg, caspases-3, -6, and -7) caspases generally occur by oligomerization of monomeric zymogens and caspase-mediated cleavage, respectively,12-14 the consequences of inhibition of activated caspases are not completely understood. Although activation of executioner caspases leads to irreversible commitment to death when IAP function is neutralized by second mitochondria-derived activator of caspase (SMAC)/direct IAP binding protein with low PI (DIABLO), loss of mitochondrial function does not. Outer mitochondrial membrane permeabilization (and concomitant translocation of cytochrome C) is not an irreversible event, since caspase inhibition at this juncture can lead to restoration of mitochondrial transmembrane potential (ΔΨm).15 This result might be explained by the recent finding that caspase translocation into the mitochondria leads to cleavage of electron transport complexes I and II and further dissolution of mitochondrial integrity.16
Mammalian B-cell development is an excellent model to study cell fate decisions that culminate in survival or death, since functional immunoglobulin gene rearrangement and appropriate cues from the bone marrow (BM) microenvironment are essential for survival and differentiation.17 Perturbations in the balance between proapoptotic and antiapoptotic signals can contribute to development of lymphohematopoietic malignancies.18 During the course of characterizing the role of the mitochondrial pathway in the demise of bone marrow stromal cell-dependent B-lineage acute lymphoblastic leukemia (ALL), we made the unexpected observation that a selective inhibitor of caspase-9 (Z-LEHD-fmk) can enhance (rather than retard) apoptosis to selected stimuli. We go on to demonstrate that this inhibitor can also induce apoptosis in healthy B-lineage ALL cells and several other leukemia/lymphoma cell lines. Thus, a 4-amino acid peptide previously identified by its capacity to inhibit caspase-9 activity can also act as a proapoptotic peptide.
Materials and methods
Origin and maintenance of cell lines
Establishment and characterization of the BM stromal cell-dependent B-lineage ALL cell lines BLIN-2, BLIN-3, and BLIN-4L have been previously described.19-21 The 3 cell lines were maintained on nonirradiated human foreskin fibroblasts in X-VIVO 10 serum-free medium (Bio-Whittaker, Walkersville, MD). Proliferation of BLIN-3 and BLIN-4L cells was enhanced by addition of 1 ng/mL recombinant human interleukin 7 (IL-7; PeproTech, Rocky Hill, NJ). The leukemic cells were removed by gentle washing of the fibroblast monolayers and were more than 90% viable prior to being used in individual experiments. All other leukemia and lymphoma cell lines (Figure 5) were maintained in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and were tested for Mycoplasma infectivity bimonthly.
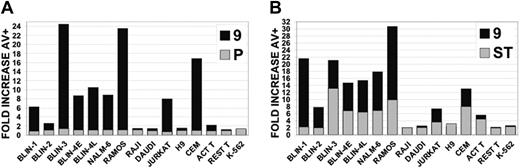
Leukemic cell lines vary in their sensitivity to C9i. The leukemic cell lines shown were cultured for 18 hours in DMSO, 50 μM C9i, or 50 μM PCi (A); or DMSO, staurosporine, or staurosporine + 50 μM C9i (B). Staurosporine was used at 0.2 μM in responsive cells and 2.0 μM in nonresponsive cells. The cells were harvested, stained with annexin V, and analyzed by flow cytometry. Data (y-axis) are plotted as fold increase in annexin V+ cells. In panel A, ▦ represents the fold increase in cells incubated with PCi (P) compared with DMSO, and ▪ represents the fold increase in cells incubated with C9i (9) compared with DMSO. In panel B, ▦ represents the fold increase in cells incubated with staurosporine (ST) compared with DMSO, and ▪ represents the fold increase in cells incubated in staurosporine + C9i (9) compared with DMSO.
Leukemic cell lines vary in their sensitivity to C9i. The leukemic cell lines shown were cultured for 18 hours in DMSO, 50 μM C9i, or 50 μM PCi (A); or DMSO, staurosporine, or staurosporine + 50 μM C9i (B). Staurosporine was used at 0.2 μM in responsive cells and 2.0 μM in nonresponsive cells. The cells were harvested, stained with annexin V, and analyzed by flow cytometry. Data (y-axis) are plotted as fold increase in annexin V+ cells. In panel A, ▦ represents the fold increase in cells incubated with PCi (P) compared with DMSO, and ▪ represents the fold increase in cells incubated with C9i (9) compared with DMSO. In panel B, ▦ represents the fold increase in cells incubated with staurosporine (ST) compared with DMSO, and ▪ represents the fold increase in cells incubated in staurosporine + C9i (9) compared with DMSO.
Normal B-lineage cells
CD19+ B-lineage cells were derived from CD34+ human cord blood stem cells plated on the murine stromal cell line MS-5.22 MS-5 stromal cells were kindly provided by Dr Paul Kincade (University of Oklahoma Medical Research Foundation, Oklahoma City, OK), following permission from Professor K. John Mori (Niigata University, Niigata, Japan). Fluorescence-activated cell sorter (FACS)-purified CD34+/CD38+/lineage- cord blood hematopoietic stem cells were plated onto confluent, nonirradiated MS-5 stromal cells in 96-well flat-bottom plates (200 cells/well) containing minimal essential medium supplemented with 10% FBS. Stem cell factor and granulocyte colony-stimulating factor (G-CSF) were added at 10 ng/mL (twice weekly) to enhance the emergence of CD19+ B-lineage cells.23,24 Analysis of cultures at approximately 4 weeks revealed that 10% to 30% of cells with lymphoid light scatter characteristics were CD19+. Wells were then pooled, stained with phycoerythrin (PE)-conjugated anti-CD19+ propidium iodide (PI), and sorted on a FACSVantage (BD Immunocytometry Systems, San Jose, CA). Cord blood CD34+/CD38+/lineage- cells were provided by Valerie McCullar and Dr Jeff Miller of the University of Minnesota Cancer Center (Minneapolis, MN) in accordance with the University of Minnesota Institutional Review Board on Human Subjects.
Chemicals and reagents
Caspase inhibitors were purchased from R&D Systems (Minneapolis, MN) and Calbiochem (La Jolla, CA). Stock solutions were prepared to 20 mM in dimethyl sulfoxide (DMSO) and stored at -20°C. The following irreversible inhibitors were used: caspase-2 (benzyloxycarbonyl-Val-Asp-Val-Ala-Asp-fluoromethylketone), caspase-3 (benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethylketone and benzyloxycarbonyl-Asp-Gln-Met-Asp-fluoromethylketone), caspase-6 (benzyloxycarbonyl-Val-Glu-Ile-Asp-fluoromethylketone), caspase-8 (benzyloxycarbonyl-Ile-Glu-Thr-Asp-fluoromethylketone); caspase-9 (Z-LEHD-fmk), and pancaspase (benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone). We also used the reversible caspase-9 inhibitor Leu-Glu-His-Asp-aldehyde (catalog no. P446; Biomol Research Laboratories, Plymouth Meeting, PA). The protein kinase C/serine-threonine kinase inhibitor staurosporine was purchased from Alexis Biochemicals (San Diego, CA).
Flow cytometry
Quantitation of apoptotic events was conducted using a FACS Calibur (BD Immunocytometry Systems). All analyses were conducted on cells with lymphoid light scatter characteristics (ie, low to medium forward scatter and low 90° side scatter).
Mitochondrial transmembrane potential (ΔΨm). The cationic lipophilic fluorescent dye tetramethylrhodamine, ethyl ester, perchlorate (TMRE) was purchased from Molecular Probes, Inc (Eugene, OR). TMRE has a high degree of membrane permeability and accumulates into mitochondria in accordance with the Nernst equation.25 Cells were washed once and resuspended to 0.5 × 106/mL to 1.0 × 106/mL in X-VIVO 10. TMRE was added to a final concentration of 50 nM and the cells were incubated in the dark at 37°C for 15 minutes. Cells were then washed twice in phosphate-buffered saline (PBS), resuspended in PBS containing 2
μg/mL PI, and immediately analyzed. The protonophore carbonylphenylhydrazone (50 μM, carbonyl cyanide-m-chlorophenyl-hydrazone [CCCP]; Sigma, St Louis, MO) was used as a positive control to effect loss of ΔΨm in 100% of cells.
YO-PRO-1. The green fluorescent DNA intercalant dye YO-PRO-126 was purchased from Molecular Probes as the Vybrant Apoptosis Assay Kit no. 4. Dual staining with YO-PRO and PI allows for the detection of early apoptotic cells (YO-PRO-1+/PI-) that have undergone initial changes in permeability to small molecules. Cells were stained according to the manufacturer's recommendations.
Annexin V. Fluorescein isothiocyanate (FITC)-labeled annexin V (BD Pharmingen, San Diego, CA) was used to detect external phosphatidylserine residues appearing on the external surface of early apoptotic cells.27 Cells were stained according to the manufacturer's recommendations.
General experimental strategy
BLIN cell lines were removed from fibroblast monolayers, washed once, and resuspended to 7.5 × 105/mL in X-VIVO 10. Two hundred microliters of resuspended cells were added to 96-well flat-bottom microtiter plates (ISC Bioexpress, Kaysville, VT) followed by 0.5 to 1.0 μL of caspase inhibitors in DMSO. An identical volume of DMSO alone was added to separate wells as a vehicle control. This final DMSO concentration of 0.25% to 0.5% vol/vol had no adverse affect on any of the BLIN cell lines. At the indicated times, the entire content of individual wells was harvested and stained in the fluorescent protocols described in “Flow cytometry.”
RNA interference using small interfering RNA (siRNA)
Specific suppression of caspase-9 expression was accomplished using siRNAs.28 The Dharmacon Inc (Lafayette, CO) SMARTpool design technology was used to target human caspase-9. This technology employs a double algorithm. The first is designated SMARTselection and uses 33 criteria and parameters that effectively eliminate nonfunctional siRNAs. The second identifies 4 siRNA duplexes from the original SMARTselected pool. In preliminary studies using FITC-conjugated control siRNA (Dharmacon), we determined that the BLIN cell lines could be transfected to more than 80% efficiency by electroporation. Based on these results, the following protocol was developed. BLIN-4L cells were washed and adjusted to 2.5 × 107 cells/mL in Opti-MEM I (catalog no. 31985-070; Invitrogen, Carlsbad, CA). Two hundred microliters of target cells were then added to Gene Pulsar cuvettes (catalog no. 165-2088; BioRad, Hercules, CA) and electroporation was conducted using a BTX Electroporation System/ElectroSquare Porator T820. Cells received a single pulse of 240 volts and were rested for 30 minutes at room temperature. BLIN-4L cells were then diluted into 4.8 mL of X-VIVO 10 supplemented with 10% FBS and 5 ng/mL of IL-7. The 5-mL suspension was then cultured for 3 days in 25-cm2 flasks to effect reduction of caspase-9 protein prior to testing in apoptotic assays. This was sufficient to promote recovery (ie, maintain survival) of the cells in the absence of significant proliferation (< 2-fold increase in cell number in 3 days).
Western blotting
This method was conducted essentially as previously described.20 The antibodies used were rabbit anti-caspase-2 (catalog no. sc-625; Santa Cruz Biotechnology Inc, Santa Cruz, CA), rabbit anti-caspase-3 (catalog no. 9661; Upstate Cell Signaling Solutions, Waltham, MA), rabbit anticaspase-6 (catalog no. 9761; Upstate Cell Signaling Solutions), rabbit anti-caspase-9 (catalog no. 9502; Upstate Cell Signaling Solutions), mouse anti-poly(adenosine diphosphate-ribose) polymerase (anti-PARP; catalog no. 556494; BD Pharmingen), and rabbit anti-BID (catalog no. AF846; R&D Systems). The antibodies to caspase-2 and caspase-9 were generated to the procaspase isoforms, whereas the antibodies to caspase-3 and caspase-6 were generated to cleavage products.
Results
Apoptosis of B-lineage ALL cells is enhanced by an irreversible inhibitor of caspase-9
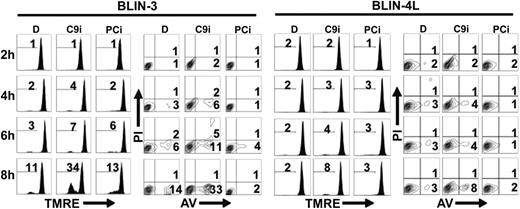
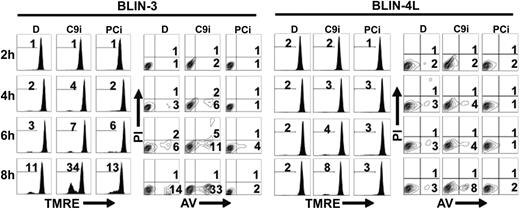
The apoptotic pathway activated in B-lineage ALL cell lines deprived of their adherent cell microenvironment includes loss of ΔΨm (detected by TMRE staining), appearance of phosphatidylserine residues on the outer membrane (detected by annexin V staining), and cleavage of procaspases-2, -3, -6, and -9 (Shah et al,19 Bertrand et al,20 and Shah et al21 ; data not shown). Since these results implicate the mitochondrial/apoptosome pathway in the demise of BLIN cell lines, we determined whether the caspase-9 inhibitor (C9i) Z-LEHD-fmk would delay cell death. Remarkably, the opposite result was obtained. A typical experiment is shown in Figure 1. BLIN-3 and BLIN-4L cells were removed from adherent fibroblasts, incubated with 50 μM C9i, and assayed by annexin V and TMRE staining at 2-hour time points. Incubation of BLIN-3 cells with C9i led to pronounced enhancement of apoptosis in BLIN-3 cells beyond that which occurred in control cells incubated with the DMSO vehicle alone. Inclusion of 50 μM pan-caspase inhibitor (PCi) did not prevent loss of ΔΨm in BLIN-3 cells (compare results with DMSO), suggesting that the initial apoptotic events leading to loss of mitochondrial function are minimally caspase dependent. However, PCi completely blocked the appearance of annexin V+ cells, consistent with the effector caspase dependency of this characteristic of apoptosis. C9i also promoted apoptosis in BLIN-4L cells, albeit not as pronounced as in BLIN-3 cells. In other experiments with BLIN-4L cells, the level of apoptotic enhancement by C9i was twice that shown in Figure 1. BLIN-3 cells and BLIN-4L cells cultured in medium alone or in medium supplemented with DMSO (the latter shown in Figure 1) gave identical results. This precluded any contribution of DMSO to the induction of apoptosis by C9i.
C9i enhances apoptosis within 4 hours following removal of BLIN-3 and BLIN-4L cells from fibroblast monolayers. Cells were cultured in DMSO (D), 50 μM C9i, or 50 μM PCi. Cells were harvested at 2-hour time points and stained with TMRE or annexin V (AV). Numbers in histograms indicate percentage of PI- cells that underwent a loss of ΔΨm. Numbers in contour plots indicate percentage of annexin V+/PI- cells.
C9i enhances apoptosis within 4 hours following removal of BLIN-3 and BLIN-4L cells from fibroblast monolayers. Cells were cultured in DMSO (D), 50 μM C9i, or 50 μM PCi. Cells were harvested at 2-hour time points and stained with TMRE or annexin V (AV). Numbers in histograms indicate percentage of PI- cells that underwent a loss of ΔΨm. Numbers in contour plots indicate percentage of annexin V+/PI- cells.
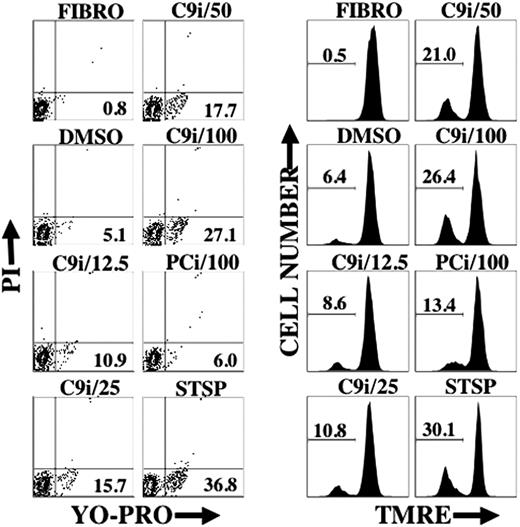
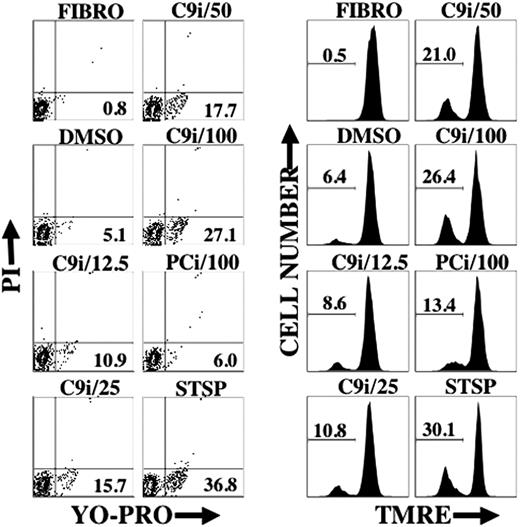
As shown in Figure 2, enhancement of apoptosis by C9i was dose dependent. The percentage of cells in a given condition that were YO-PRO+/PI- was similar to the percentage of cells with reduced TMRE staining. This result probably reflects a concomitant change in cell membrane permeability and loss of ΔΨm.
C9i induces changes in cell permeability and loss of ΔΨm in a dose-dependent manner. BLIN-3 cells were cultured in the absence of adherent fibroblasts for 8 hours under the conditions shown and analyzed for changes in cell permeability (YO-PRO) or ΔΨm (TMRE). Designations at the top of each contour plot or histogram indicate caspase inhibitor concentration in μM. FIBRO- refers to BLIN-3 cells removed from fibroblasts and tested immediately. DMSO indicates vehicle control; and STSP, positive control for induction of apoptosis. Numbers in contour plots indicate percentage of YO-PRO+/PI- cells. Numbers in histograms indicate percentage of PI- cells that underwent a loss of ΔΨm. Similar results were obtained in 2 other experiments.
C9i induces changes in cell permeability and loss of ΔΨm in a dose-dependent manner. BLIN-3 cells were cultured in the absence of adherent fibroblasts for 8 hours under the conditions shown and analyzed for changes in cell permeability (YO-PRO) or ΔΨm (TMRE). Designations at the top of each contour plot or histogram indicate caspase inhibitor concentration in μM. FIBRO- refers to BLIN-3 cells removed from fibroblasts and tested immediately. DMSO indicates vehicle control; and STSP, positive control for induction of apoptosis. Numbers in contour plots indicate percentage of YO-PRO+/PI- cells. Numbers in histograms indicate percentage of PI- cells that underwent a loss of ΔΨm. Similar results were obtained in 2 other experiments.
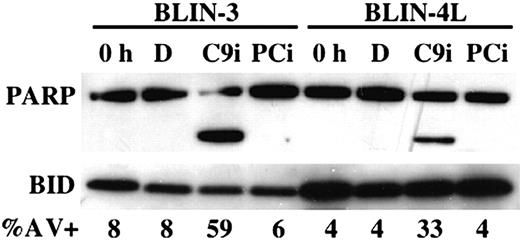
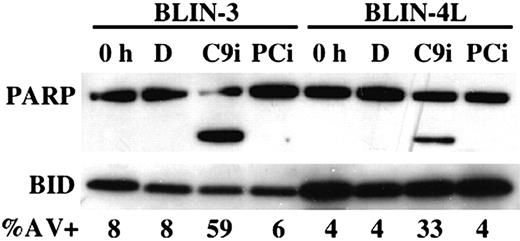
Figure 3 shows that overnight incubation of BLIN-3 and BLIN-4L cells with 50 μM C9i led to cleavage of the caspase-3/caspase-7 substrate PARP. As expected, inclusion of 50 μM PCi had no effect. The degree of PARP cleavage in the presence of C9i (greater in BLIN-3 cells than BLIN-4L cells) correlated with the percentage of annexin V+ cells (Figure 3). In other experiments we demonstrated that (a) irreversible inhibitors of caspases-2, -3, -6, and -8 did not enhance apoptosis; (b) C9i enhancement of apoptosis occurred at concentrations as low as 6.25 μM; (c) identical results were obtained using C9i from 2 separate vendors; and (d) we could confirm the capacity of C9i to block apoptosis at some level since 50 μM C9i blocked the Fas response of JURKAT T-lymphoma cells.
PARP cleavage occurs following treatment of BLIN-3 and BLIN-4L with C9i. BLIN-3 and BLIN-4L cells were removed from fibroblast monolayers and cultured for 18 hours in DMSO (D), 50 μM C9i, or 50 μM PCi. Cells were then lysed and analyzed by Western blotting. BID was used as loading control. Lanes labeled 0 h represent lysates from freshly isolated cells. The percentage of annexin V+ cells is shown for each condition.
PARP cleavage occurs following treatment of BLIN-3 and BLIN-4L with C9i. BLIN-3 and BLIN-4L cells were removed from fibroblast monolayers and cultured for 18 hours in DMSO (D), 50 μM C9i, or 50 μM PCi. Cells were then lysed and analyzed by Western blotting. BID was used as loading control. Lanes labeled 0 h represent lysates from freshly isolated cells. The percentage of annexin V+ cells is shown for each condition.
Effect of C9i on normal B-cell precursors
Normal CD19+ B-cell precursors and CD33+ myeloid cells were derived by plating of CD34+/CD38+/lin- cord blood hematopoietic stem cells onto murine MS-5 stromal cells. After 4 weeks, the CD19+ and CD33+ cells were purified by cell sorting and tested for their sensitivity to C9i. Table 1 shows 1 of 3 separate experiments that gave similar results. Consistent with the results obtained using the stromal cell-dependent BLIN cell lines, C9i significantly enhanced the death of CD19+ cells removed from their MS-5 microenvironment, whereas PCi partially inhibited death. In contrast, CD33+ myeloid cell death was partially inhibited by C9i and PCi.
Differential effect of C9i on other leukemic cells
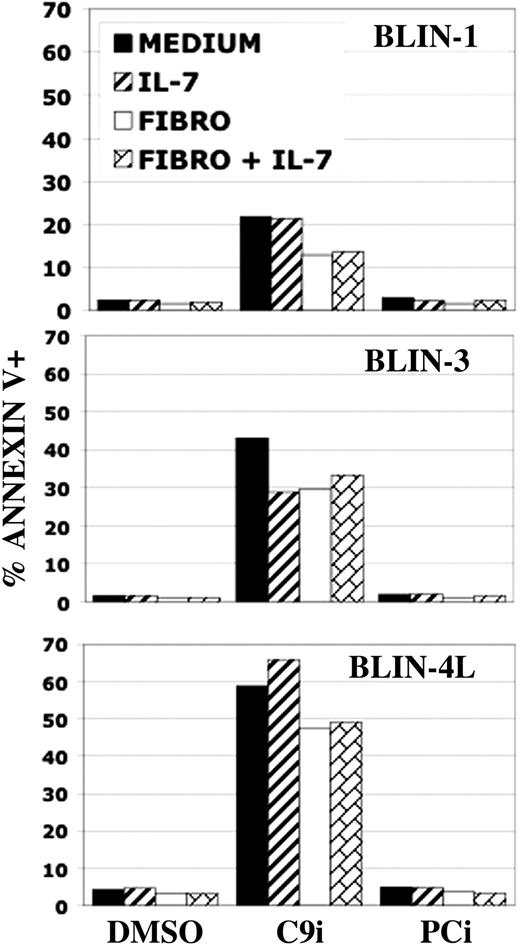
In preliminary experiments we found that C9i did not promote apoptosis in BLIN-3 and BLIN-4L cells when the cells were maintained on adherent fibroblasts and/or IL-7 for 8 hours. However, when the incubation period was extended to 18 hours, C9i promoted apoptosis in BLIN-3 and BLIN-4L cells maintained on adherent fibroblasts and/or IL-7 (Figure 4). This result suggested that C9i could also promote apoptosis in nonstressed, healthy cells.
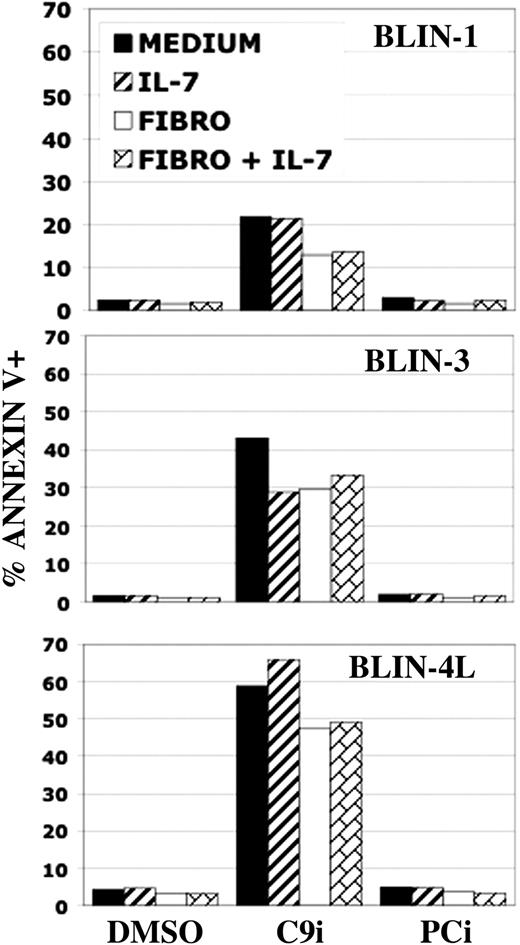
BLIN leukemic cells are sensitive to C9i independent of culture conditions. BLIN-1, BLIN-3, and BLIN-4L were plated in medium alone, in medium supplemented with 10 ng/mL IL-7, on adherent fibroblasts, or on adherent fibroblasts supplemented with 10 ng/mL IL-7. All wells had either DMSO, 50 μM C9i, or 50 μM PCi. Following 18-hour incubation, the wells were harvested and the cells were stained with annexin V and analyzed by flow cytometry. Annexin V percentages include PI+ and PI- events. Each bar represents the mean of duplicate values.
BLIN leukemic cells are sensitive to C9i independent of culture conditions. BLIN-1, BLIN-3, and BLIN-4L were plated in medium alone, in medium supplemented with 10 ng/mL IL-7, on adherent fibroblasts, or on adherent fibroblasts supplemented with 10 ng/mL IL-7. All wells had either DMSO, 50 μM C9i, or 50 μM PCi. Following 18-hour incubation, the wells were harvested and the cells were stained with annexin V and analyzed by flow cytometry. Annexin V percentages include PI+ and PI- events. Each bar represents the mean of duplicate values.
We expanded our analysis of C9i to other leukemic cell lines. As shown in Figure 5, incubation of a panel of leukemic cell lines with 50 μM C9i for 18 hours revealed a wide range of sensitivities. All 6 B-cell precursor ALL cell lines (BLIN-1, -2, -3, -4E, -4L, and NALM-6) were C9i sensitive, although BLIN-2 was less sensitive than the others. In addition, the RAMOS Burkitt lymphoma and the CCRF-CEM T-lymphoma cell lines were extremely sensitive. The JURKAT T-lymphoma cell line was also sensitive. In contrast, the RAJI and DAUDI Burkitt lymphoma cell lines, the H9 T-lymphoma cell line, and the K-562 erythroleukemia cell line were insensitive to C9i. Resting and activated (ie, CD3/CD28 crosslinked) normal human T cells were also analyzed, with activated T cells being marginally sensitive. These collective results indicate that leukemic (and normal; Table 1) B-cell precursors are uniformly sensitive to C9i, with other leukemia/lymphoma cell lines showing a wide variation in sensitivity. We also tested whether C9i would potentiate the apoptotic effect of the widely used serine/threonine kinase inhibitor staurosporine. As shown in Figure 5, C9i enhanced the staurosporine response of those leukemic cells that responded to C9i alone. Thus, the leukemic cell lines were generally responsive to both C9i and staurosporine, or responsive to neither.
Apoptotic sensitivity of cells made deficient in caspase-9 by siRNA
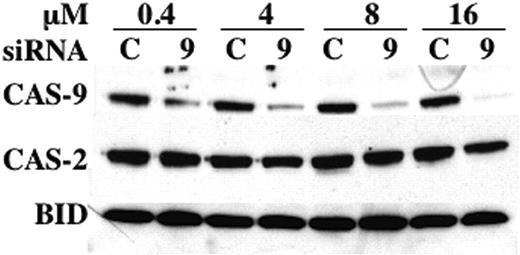
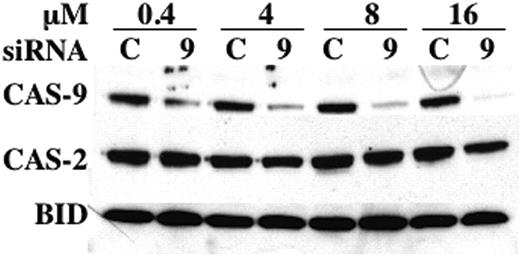
To determine whether the results obtained with C9i could be corroborated by another method, we turned to RNA interference using siRNA. As shown in Figure 6, a SMARTpool of siRNA specific for human caspase-9 effectively and specifically suppressed BLIN-4L caspase-9 protein in a dose-dependent manner. In contrast, an identical amount of control siRNA had no effect on caspase-9. This degree of caspase-9 inhibition was reproducibly achieved 48 to 72 hours after electroporation.
Reduction of caspase-9 in BLIN-4L. Cells were electroporated with the indicated concentrations of control (C) or caspase-9 (9) siRNA, allowed to recover for 3 days, and harvested for Western blotting as described in “Materials and methods.” Densitometric scanning showed that electroporation with 0.4, 4, 8, and 16 μM caspase-9 siRNA led to a reduction in caspase-9 protein of 71%, 78%, 86%, and 91%, respectively, when normalized to BID or caspase-2 as loading controls.
Reduction of caspase-9 in BLIN-4L. Cells were electroporated with the indicated concentrations of control (C) or caspase-9 (9) siRNA, allowed to recover for 3 days, and harvested for Western blotting as described in “Materials and methods.” Densitometric scanning showed that electroporation with 0.4, 4, 8, and 16 μM caspase-9 siRNA led to a reduction in caspase-9 protein of 71%, 78%, 86%, and 91%, respectively, when normalized to BID or caspase-2 as loading controls.
We then tested whether caspase-9-deficient BLIN-4L cells would undergo a heightened response to apoptotic stimuli. As shown in Figure 7, caspase-9-deficient BLIN-4L cells were significantly more sensitive to staurosporine than control siRNA-treated cells. In contrast, there was no difference in the response to etoposide. It is noteworthy that caspase-9-deficient cells incubated in medium alone also underwent a significantly greater degree of apoptosis than control siRNA-treated cells.
Caspase-9-deficient BLIN-4L cells show increased apoptotic sensitivity to staurosporine. Cells were electroporated with control or caspase-9 siRNA and allowed to recover as described in “Materials and methods.” After 3 days, the 2 treated populations were removed from recovery medium and assayed immediately for annexin V binding (0 h value). The remaining cells were cultured for 6 hours in X-VIVO 10 containing DMSO (vehicle control [CONT]), 1.0 μM etoposide (ETOP), or 0.02 μM staurosporine (STSP). The etoposide and staurosporine values represent the percentage of total annexin V+ events (PI+ and PI-) minus the number present in DMSO, in order to eliminate the inherent difference in the apoptotic sensitivity of the 2 populations. The results represent the mean ± SD from 3 separate experiments. *P < .008 compared with control siRNA treatment (Wilcoxon rank sum method).
Caspase-9-deficient BLIN-4L cells show increased apoptotic sensitivity to staurosporine. Cells were electroporated with control or caspase-9 siRNA and allowed to recover as described in “Materials and methods.” After 3 days, the 2 treated populations were removed from recovery medium and assayed immediately for annexin V binding (0 h value). The remaining cells were cultured for 6 hours in X-VIVO 10 containing DMSO (vehicle control [CONT]), 1.0 μM etoposide (ETOP), or 0.02 μM staurosporine (STSP). The etoposide and staurosporine values represent the percentage of total annexin V+ events (PI+ and PI-) minus the number present in DMSO, in order to eliminate the inherent difference in the apoptotic sensitivity of the 2 populations. The results represent the mean ± SD from 3 separate experiments. *P < .008 compared with control siRNA treatment (Wilcoxon rank sum method).
Discussion
The interactions between normal and leukemic B-lineage cells and BM stromal cells are bidirectional. BM stromal cells transduce signals to B-lineage cells,29 and contact of human B-lineage cells with BM stromal cells induces phosphorylation of several substrates and synthesis of IL-6 in stromal cells.30,31 Apoptotic pathways activated in B-lineage ALL following loss of stromal cell contact can be considered a stress response or death by neglect. However, apoptotic pathways activated in this example of heterotypic cellular dependency are not necessarily identical to the pathways activated by DNA-damaging agents (eg, x-irradiation or chemotherapeutic drugs) or single-cytokine deprivation (eg, IL-3 or IL-7). Death by neglect in vivo may be initiated when leukemic cell crowding around a stromal cell niche leads to an increase in leukemic cells lacking direct contact with stromal cell membranes. Failure of a leukemic cell to have access to a stromal cell niche could then activate an apoptotic cascade or trigger further mutations that lead to the emergence of stromal cell-independent subclones.
BLIN cell lines deprived of fibroblast monolayers undergo a gradual accumulation of apoptotic cells culminating in death within 24 to 48 hours.19-21 Since caspase-9 activation generally occurs after a loss in mitochondrial function, C9i would not be expected to inhibit loss of ΔΨm. However, it was unexpected to discover that C9i enhanced the loss of ΔΨm (Figures 1-2) and concomitant or subsequent events downstream of mitochondrial function, including annexin V binding (Figure 1) and membrane permeability changes (Figure 2). The C9i effect was dose dependent (Figure 2) and could be detected at concentrations as low as 6.25 μM. Enhancement of apoptosis was unique to treatment with C9i. Inhibitors of caspase-2, caspase-3, caspase-6, caspase-8, and the pan-caspase inhibitor Z-VAD-fmk did not enhance apoptosis. Western blot results in Figure 3 corroborated the flow cytometry data in Figures 1 and 2 and demonstrated that C9i treatment led to PARP cleavage, an essentially universal response to caspase-3/caspase-7 activation. We also detected activation of several caspases (ie, caspases-2, -3, -6, and -9) in C9i-treated cells by Western blotting (data not shown). Thus, independent of mechanism, simple treatment of BLIN-3 and BLIN-4L cells with 50 μM C9i culminates in the activation of a caspase cascade.
The C9i enhancement of apoptosis occurred in all of our adherent cell-dependent BLIN cell lines and to a lesser degree in normal adherent cell-dependent CD19+ B-lineage cells derived from cord blood CD34+ hematopoietic stem cells (Table 1). Thus, the enhanced apoptotic pathway is not unique to leukemic B-lineage cells. Moreover, C9i did not enhance apoptosis of normal stromal cell-dependent CD33+ myeloid cells and actually had a minor inhibitory effect (Table 1). The latter is an important control since it confirms the capacity of C9i to inhibit apoptosis of normal lymphohematopoietic (largely myeloid) cells that emerge under the same conditions as the CD19+ B-lineage cells.
Experiments examining the sensitivity of leukemic cell lines under the conditions shown in Figure 1 indicated that C9i did not induce apoptosis (data not shown). Further analysis revealed that cells incubated for 18 to 24 hours with 50 μM C9i underwent varying degrees of apoptosis (Figures 4-5). Notably, BLIN-3 and BLIN-4L cells treated with C9i and incubated on fibroblast monolayers and/or IL-7 were still C9i sensitive (Figure 4). Thus, C9i is not simply promoting stress-induced apoptosis. Figure 5 shows that not all cells were sensitive, and there was an excellent correlation between C9i and staurosporine sensitivity at the level of individual leukemic cell lines. The fact that some proliferating leukemic cell lines are C9i sensitive (eg, RAMOS and CEM) indicates that C9i is exerting a proapoptotic effect that is functionally the opposite of its simple inhibition of activated caspase-9. It is important to emphasize that irreversible inhibitors for caspase-2, caspase-3, caspase-6, and caspase-8 did not promote apoptosis, as would be expected. Since all inhibitors are synthesized with a benzyloxycarbonyl group (the Z group) at the N-terminus and a fluoromethyl ketone group (fmk) at the C-terminus, these chemical modifications could not confer apoptotic properties on C9i. Rather, there must be something unique about the LEHD sequence that activates an apoptotic pathway. One clue may lie in the fact that loss of ΔΨm is an early consequence of C9i treatment. Thus, C9i could be acting at the level of the outer mitochondrial membrane or could be exerting an effect comparable to activation of a BH3 death domain.
Given the limitations of using a short peptide inhibitor such as C9i, we used RNA interference to more directly assess the role of caspase-9. We developed a protocol that consistently led to more than 90% reduction in caspase-9 protein expression (Figure 6). Given that reduced caspase-9 expression correlated with increased apoptotic sensitivity (Figure 7), it is conceivable that the cells recovered after caspase-9 siRNA treatment in Figure 6 are those with the least efficient reduction of caspase-9. If so, this would suggest even greater efficacy of siRNA-mediated suppression of caspase-9 than the values shown in Figure 6. We went on to demonstrate that BLIN-4L cells deficient in caspase-9 exhibited an enhanced response to staurosporine (Figure 7), consistent with the results obtained with C9i (Figure 5). Caspase-9-deficient BLIN-4L cells also exhibited a significantly enhanced level of apoptosis following 6-hour removal from adherent fibroblasts. It is possible that the failure to reduce apoptotic sensitivity in caspase-9-deficient BLIN-4L cells reflects the functional efficacy of the remaining caspase-9 to form an apoptosome. However, this could not explain why caspase-9-deficient BLIN-4L cells show increased sensitivity. It is conceivable that loss of caspase-9 triggers a compensatory pathway involving another caspase (eg, caspase-2). It is noteworthy that mice deficient in caspase-9 undergo a rapid activation of alternative caspases (notably caspase-2) following Fas cross-linking in vivo.32 Conversely, studies with nerve growth factor-dependent caspase-2 null neurons have revealed elevated levels of caspase-9 and SMAC/DIABLO.33
In conclusion, we have shown through use of the caspase inhibitor Z-LEHD-fmk and targeting of caspase-9 by RNA interference that B-lineage ALL cell lines undergo heightened spontaneous and drug-induced apoptosis. The sensitivity of B-lineage ALL cell lines and some other leukemia/lymphoma cell lines to C9i alone suggests that it transduces a proapoptotic signal in some cells. The mechanism whereby reduction of caspase-9 increases sensitivity to apoptotic stimuli is unknown. It is unclear whether the apoptotic enhancement observed by either approach is part of an endogenous pathway that regulates apoptosis. However, the apoptotic enhancement induced by C9i and/or reduction in caspase-9 could form the foundation for a new approach to enhancing apoptosis in leukemic lymphoid cells.
Prepublished online as Blood First Edition Paper, July 8, 2004 DOI 10.1182/blood-2003-10-3720.
Supported by R01 CA31685 from the National Institutes of Health, the Leukemia Research Fund, and the Apogee Enterprises Chair in Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Robin Bliss (University of Minnesota Cancer Center Biostatistics Core) for assistance with statistical analysis; Greg Veltri (University of Minnesota Cancer Center Flow Cytometry Core) and Ameeta Kelekar for comments on the manuscript; Sandi Sherman for assistance with the manuscript; and Karen Dallas, Lisa Jasperson, and Beth Thielen for laboratory assistance.

![Figure 7. Caspase-9-deficient BLIN-4L cells show increased apoptotic sensitivity to staurosporine. Cells were electroporated with control or caspase-9 siRNA and allowed to recover as described in “Materials and methods.” After 3 days, the 2 treated populations were removed from recovery medium and assayed immediately for annexin V binding (0 h value). The remaining cells were cultured for 6 hours in X-VIVO 10 containing DMSO (vehicle control [CONT]), 1.0 μM etoposide (ETOP), or 0.02 μM staurosporine (STSP). The etoposide and staurosporine values represent the percentage of total annexin V+ events (PI+ and PI-) minus the number present in DMSO, in order to eliminate the inherent difference in the apoptotic sensitivity of the 2 populations. The results represent the mean ± SD from 3 separate experiments. *P < .008 compared with control siRNA treatment (Wilcoxon rank sum method).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/9/10.1182_blood-2003-10-3720/6/m_zh80210468700007.jpeg?Expires=1768248621&Signature=AHZGJ0-ELAI6cjpfNeSIt6S~eradWUrorqamYSs1QIRKmB5re~k06lj21~5wHkD71SRdp8daXT4HLJOHQzvoaO5wXFbf2Fqaqq4M0u7OdOemwuw2mJS6i~xoStp-mcvIx01l3hO4Oipp7lIm12ChNAHfEed9BsbNURgEJ96rjlHssMauTnj5DzhO0rLPZNQY78b9z6QE97w8oanq7KFdTLWv4EKohszt~sIc1KK4UQzlkaKD3uAP3iHqteXDdNoZV76QOuKyKK365INXt2yCrVjlNsckgJO3NmVz4iRv87YiDIUvwSppt7x-p6REplnnUXVeeYl3FPt1isEnEQUQ3w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 7. Caspase-9-deficient BLIN-4L cells show increased apoptotic sensitivity to staurosporine. Cells were electroporated with control or caspase-9 siRNA and allowed to recover as described in “Materials and methods.” After 3 days, the 2 treated populations were removed from recovery medium and assayed immediately for annexin V binding (0 h value). The remaining cells were cultured for 6 hours in X-VIVO 10 containing DMSO (vehicle control [CONT]), 1.0 μM etoposide (ETOP), or 0.02 μM staurosporine (STSP). The etoposide and staurosporine values represent the percentage of total annexin V+ events (PI+ and PI-) minus the number present in DMSO, in order to eliminate the inherent difference in the apoptotic sensitivity of the 2 populations. The results represent the mean ± SD from 3 separate experiments. *P < .008 compared with control siRNA treatment (Wilcoxon rank sum method).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/9/10.1182_blood-2003-10-3720/6/m_zh80210468700007.jpeg?Expires=1768248622&Signature=lrbF0zlOsok8mCk03JXvxnQNxaUv0bf~68gj5U9krudHuhaNsf5-cK6HPwruSC3J0AMkr3f1CRbzlzH~aGXDf7FXydf1uu8sSHfgJme3rWTuwdfviqnLRqyiv-FgFziysAEfohWEi2mQ7cKmSSkv1QeCypoUexSqTyYG7qWTBi7mLTdFp7ABzRS9Rh1cUdeh5d8UREiy-vlHYLpu1Cgk8jEanQgu3JZj-kCZ8mOgcQrTHWazmFECSVYkzmzyCtyf3kZB~2bvID4JlD0BqLLbhtGtbiaYFNLYEJv67c52xZ~eEUl4YaWovCkdsLyUEI4M1mJRxKXOdJ3qkC5j-R2rEQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)