Abstract
HLA matching between the donor and recipient improves the success of unrelated hematopoietic cell transplantation (HCT). Matched donors are available for only a minority of patients. Further information is needed to evaluate the limits of HLA mismatching. We examined the association of mortality with HLA-A, -B, -C, -DRB1, and -DQB1 mismatching in 948 patients who received a T-replete unrelated HCT for treatment of a marrow disorder. A single HLA allele or antigen mismatch was associated with increased mortality among patients with chronic myeloid leukemia (CML) within 2 years after diagnosis compared to patients with no HLA mismatch, but not among those with more advanced malignancy. In particular, a single HLA-C mismatch conferred increased risk of mortality compared to matches. There was a suggestion for increased mortality with multiple mismatches involving HLA-DQB1 compared to multiple mismatches not involving HLA-DQB1. Donors with a single HLA allele or antigen mismatch may be used for HCT when a fully matched donor is not available for patients with diseases that do not permit time for a lengthy search. Whenever possible, HLA-C mismatches should be avoided for patients with early stage CML, and HLA-DQB1 mismatches should be avoided for patients with multiple mismatches.
Introduction
Unrelated hematopoietic cell transplantation (HCT) can cure malignant and nonmalignant hematologic disorders.1-5 The safety and efficacy of unrelated HCT are optimal when HLA alleles of the donor and recipient are matched and when the burden of malignant cells is low.6-15 Unfortunately, most patients who need HCT do not have HLA-matched donors, and many patients have active or advanced malignancy, which lowers the probability of cure. We have evaluated the relationship between HLA mismatching, disease stage, and outcome after HCT in order to characterize the extent to which HLA mismatching can be tolerated.
Patients and methods
Study population
A total of 1249 patients received a myeloablative conditioning regimen and an unrelated bone marrow or peripheral blood stem cell (PBSC) HCT between 1985 and 2003 for treatment of leukemia or myelodysplastic syndrome. Methotrexate and cyclosporine were administered to prevent graft-versus-host disease, and T cells were not removed from the donor marrow. Results were analyzed for 948 donor-recipient pairs (Table 1) for whom DNA or lymphoblastoid cell lines were available. This study was approved by the institutional review board of the Fred Hutchinson Cancer Research Center, and informed consent was obtained from all patients.
Histocompatibility testing and donor selection criteria
Before 1991, microcytotoxicity assays and cellular assays were used for HLA typing, and donor selection was based on matching for HLA-A, -B, -DR, and -Dw.6 Molecular methods were introduced for HLA-DRB1 and DQB1 typing in 1991 and 1992, respectively.16,17 Serologic typing of HLA-C antigens was introduced in 1996,18 and molecular typing of HLA-C alleles was introduced in 2000.12 As of 2002, patients and donors are typed for HLA-A, -B, -C, -DRB1, and -DQB1 alleles.15 In the present study, sequencing methods were used to type HLA-A, -B, -C, and -DPB1 genes.15 Mismatching was defined as the presence of donor antigens or alleles not shared by the recipient or the presence of recipient antigens or alleles not shared by the donor for the analysis of mortality, relapse, and transplant-related mortality (TRM).
Transplantation procedure
Statistical analysis
Pretransplant risk categories were defined according to established criteria.9,11,19-26(Tab1) Cox regression models were fit to examine the association of mismatching at the various HLA loci with the hazards of mortality, relapse, and TRM. Base models were fit from non-HLA variables known or suspected to be associated with each outcome (Table 1), and variables representing HLA mismatching were then added to these base models. All reported 2-sided P values were estimated from the Wald test, and no adjustments were made for multiple comparisons, therefore P values between .01 and .05 should be considered as suggestive rather than conclusive evidence of a difference.
Results
Importance of pretransplantation risk category for single mismatches and definition of HLA mismatching
The pretransplantation risk category influenced the effect of HLA mismatching on posttransplantation mortality, and the impact of a mismatch at a particular locus depended on the total number of mismatches. Therefore, we first compared single HLA allele or antigen mismatches to matches.
Mortality was statistically significantly increased among mismatched patients compared to matched patients in the low-risk category (hazard ratio [HR] 2.27; 95% CI, 1.47-3.51; Figure 1A), but there was no such difference among those in the intermediaterisk category (HR 1.0; 95% CI, 0.71-1.39; Figure 1B) or those in the high-risk category (HR 1.19; 95% CI, 0.84-1.68; Figure 1C). The effect of a single mismatch was not statistically different among patients in the intermediate-risk group compared to those in the high-risk group (a statistical test of interaction yielded P = .38). There was, however, evidence that the effect of a mismatch was different in the low-risk group compared to both the intermediate- and high-risk groups (test of interaction yielded P = .003 for comparison of low-risk versus intermediate-risk, and P = .04 for low-risk versus high-risk). For these reasons, analyses focused on single mismatches in combined intermediate-risk and high-risk patients.
Kaplan-Meier estimates of survival for patients in the low-risk, intermediate-risk, and high-risk disease categories according to the presence or absence of a single mismatch. (A) No deaths occurred beyond 12 years in any group. Among low-risk patients, 12 in the matched group and 4 in the mismatched group have follow-up beyond 12 years and are indicated as censored observations at 12 years. (B) In the intermediate-risk group, 9 patients in the matched group and 8 in the mismatched group have follow-up beyond 12 years and are indicated as censored observations at 12 years. (C) In the high-risk group, 3 patients in the matched group and 1 in the mismatched group have follow-up beyond 12 years and are indicated as censored observations at 12 years.
Kaplan-Meier estimates of survival for patients in the low-risk, intermediate-risk, and high-risk disease categories according to the presence or absence of a single mismatch. (A) No deaths occurred beyond 12 years in any group. Among low-risk patients, 12 in the matched group and 4 in the mismatched group have follow-up beyond 12 years and are indicated as censored observations at 12 years. (B) In the intermediate-risk group, 9 patients in the matched group and 8 in the mismatched group have follow-up beyond 12 years and are indicated as censored observations at 12 years. (C) In the high-risk group, 3 patients in the matched group and 1 in the mismatched group have follow-up beyond 12 years and are indicated as censored observations at 12 years.
There was no statistically significant association between mismatching and outcome in the intermediate- or high-risk groups for either relapse or transplant-related mortality (TRM) (Table 2). For patients in the low-risk category, however, a single HLA mismatch conferred a statistically significantly increased risk of TRM compared to matches (HR 2.13; 95% CI, 1.34-3.39; Table 2). The number of mismatches was correlated positively with the hazard of mortality in both the low-risk (P < .0001) and higher risk categories (P = .002) when the number of mismatched alleles or antigens was modeled as a continuous linear variable (data not shown).
Allele and antigen mismatches confer different risks to graft failure,12 but the effect of allele and antigen mismatches on mortality has not been defined. To address this question, we examined 81 low-risk and 167 higher-risk patients with a single HLA mismatch (Table 3). With the matched low-risk patients as the reference group, the HR of mortality was 2.44 (95% CI, 1.41-4.22) among patients with a single allele mismatch and 2.15 (95% CI, 1.28-3.60) among those with a single antigen mismatch. With the matched higher-risk patients as the reference group, the HR of mortality was 1.02 (95% CI, 0.70-1.48) among patients with a single allele mismatch and 1.12 (95% CI, 0.86-1.47) among those with a single antigen mismatch. These results suggest that allele and antigen disparities have similar effects on mortality, regardless of the pretransplantation risk category.
In the above analyses, the mismatches were not categorized according to locus. There were insufficient numbers of patients with a single HLA-A, -B, -C, -DRB1, or -DQB1 disparity to draw definitive conclusions as to whether mismatching at any individual locus was associated with a distinctively increased risk of mortality. Among higher-risk patients, none of the locus-specific single disparities showed a statistically significant association with increased mortality (Table 4, bottom). Among low-risk patients, HR point estimates ranged from 1.64 to 3.21 (Table 4, top). The HR for a single HLA-C disparity was 3.18 (95% CI, 1.74-5.82). The increased mortality among low-risk patients with a single disparity at HLA-C did not appear to be due to NK-KIR ligand matching.27 The HR was 3.06 (95% CI, 1.56-6.01) for single HLA-C-mismatched patients who were matched for NK-KIR ligands and 3.55 (95% CI, 1.25-10.10) for those who were mismatched.
Multiple mismatches and locus-specific effects
For some patients, the only available donors have 2 or more mismatches. For these patients, data are needed to determine whether combinations of 2 or more mismatches involving certain HLA loci should be avoided. Of the 948 pairs in this study, 219 had 2 or more mismatches. Of these 219 patients, 160 (73%) received transplants after July 1, 1992, when standard of care included the use of prophylactic fluconazole and gancyclovir, and hence year of transplantation was included in the model. The association of locus-specific mismatching with mortality did not depend on pretransplantation risk category among patients with multiple mismatches, and there was no suggestion of an interaction between mismatching at a specific locus and the total number of disparities (data not shown). To evaluate the effect of mismatching at a specific locus, the mortality among all patients with 2 or more mismatches that included the locus of interest was compared to the mortality among all patients with 2 or more mismatches that did not include the locus of interest (Table 5). Patients with multiple disparities involving HLA-A had similar outcomes to those whose disparities did not involve HLA-A; the same conclusions held for HLA-B, -C, and -DRB1. In contrast, there was a suggestion for multiple mismatches that included HLA-DQB1 to be associated with a higher risk of mortality as compared to multiple mismatches that did not involve HLA-DQB1. Whether this association is real or a result of multiple comparisons will require further examination in an independent data set addressing this same question.
Effect of HLA mismatching and timing of transplantation among patients with chronic myeloid leukemia
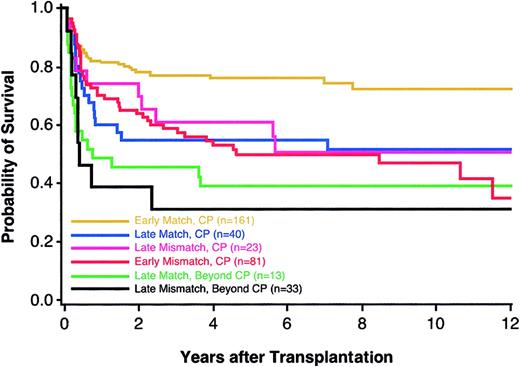
Previous studies have demonstrated that a longer time interval from diagnosis to transplantation is associated with an increased risk of mortality among patients with CML.9 For this reason, we examined the potential gain of extending the search for a fully compatible donor against the potential harm of lengthening the time from diagnosis of CML to transplantation, a decision faced most acutely by patients who have mismatched donors and who carry the diagnosis of CML in chronic phase (CP) for fewer than 2 years. For patients who elect to extend their donor search, 3 potential outcomes could arise: a matched donor might be successfully identified, thereby enabling transplantation during early CP; a matched donor might be identified after the disease advances to accelerated phase (AP) or blast crisis (BC); or, a matched donor might not be identified as the disease advances. Among 242 patients who received transplantations fewer than 2 years from diagnosis, we defined 161 matched (“early matches”) and 81 single mismatched pairs (“early mismatches”); among 63 CML patients who had received transplantations more than 2 years from diagnosis, 40 were matched (“late matches”) and 23 had a single mismatch (“late mismatches”). Compared to late matches, the HR of mortality among early mismatches was 1.16 (95% CI, 0.65-2.08). The overall survival of matched patients with CML that progressed beyond CP (AP or BC) before a transplantation was lower than the survival of patients with CML in early CP who had either matched or single-mismatched donors (Figure 2). The numbers of patients with a single mismatch do not allow for conclusive analyses of locus-specific effects.
Kaplan-Meier estimates of survival among CML patients according to disease phase and presence or absence of a single HLA mismatch. Groups shown are early CP matches (n = 161; yellow), late CP matches (n = 40; blue), late CP mismatches (n = 23; pink), early CP mismatches (n = 81; red), late matches beyond CP (n = 13; green), and late mismatches beyond CP (n = 33; black).
Kaplan-Meier estimates of survival among CML patients according to disease phase and presence or absence of a single HLA mismatch. Groups shown are early CP matches (n = 161; yellow), late CP matches (n = 40; blue), late CP mismatches (n = 23; pink), early CP mismatches (n = 81; red), late matches beyond CP (n = 13; green), and late mismatches beyond CP (n = 33; black).
Discussion
Increasing availability and improving safety and efficacy remain important goals of unrelated HCT. The outcome of unrelated HCT is influenced by the degree of donor HLA compatibility and by toxicities associated with the pretransplantation conditioning regimen and posttransplantation immunosuppressive regimen.6-12 For these reasons, patients derive the greatest benefit from unrelated HCT when they have HLA-matched donors and when they can tolerate the transplantation procedure. The efficacy of unrelated HCT in treating hematologic malignancies increases when the disease is well controlled.9,11,22,26
We found that the tolerability of a given HLA mismatch depends on the pretransplantation risk category in patients undergoing T-replete HCT following myeloablative conditioning and who receive cyclosporine and methotrexate for postgrafting immunosuppression. Single mismatches in low-risk patients were associated with a statistically significantly increased risk of mortality compared to matches. A single mismatch among higher-risk patients did not show such an association. These results suggest that when a fully compatible donor is not available, a transplant for higher risk patients from a donor with a single mismatch may permit early treatment, thereby avoiding the risk of disease progression during a prolonged search for a fully matched donor. The tolerability of disparity at a particular locus among patients with multiple mismatches did not show a strong dependence on the pretransplantation risk category but was dependent on the presence or absence of an HLA-DQB1 mismatch. These data suggest that both genetic and nongenetic factors influence the effects of single and multiple mismatches.
We previously showed that the risk of transplantation complications increases according to the total number of HLA mismatches.15 In the current analysis, the total number of HLA mismatches was a significant risk factor for mortality in both low-risk and higher-risk patients, and single allele and antigen mismatches both conferred an increased risk of mortality among low-risk patients. For these reasons, molecular typing of HLA alleles for donor selection is essential.
Since many patients in need of an unrelated transplant have only donors with multiple mismatches, we sought to determine whether certain combinations of mismatches should be avoided. Multiple mismatches that involved HLA-DQB1 were associated with increased mortality. These results suggest that when fully compatible or single mismatched donors are not available, mismatches that involve HLA-DQB1 should be avoided. Several investigators have evaluated the significance of HLA-DQ mismatching.10,13,17,26 Most recently, the National Marrow Donor Program (NMDP) found that HLA-DQ mismatching did not increase posttransplantation complications.28 This analysis was performed among all patients, however, regardless of number of mismatches at other loci, and the HLA-DQ effect was estimated after adjustment for mismatches elsewhere. We observed a suggestion of a deleterious effect of mismatching at HLA-DQ, but only among patients who had multiple mismatches. Direct comparison of our results with those of other studies, in particular the NMDP study, cannot be performed because the study questions and comparison groups were not the same. The apparent association of HLA-DQB1 mismatching with mortality in the current study could be a result of multiple comparisons, and further analysis of mismatches involving HLA-DQB1 among larger numbers of patients with multiple disparities will be needed to confirm our results. Furthermore, the results from our study are pertinent to patients whose transplantation procedure included myeloablative conditioning regimens, no manipulation of the graft, and cyclosporine and methotrexate as graft-versus-host disease prophylaxis. If our results are confirmed in a large independent analysis of patients receiving the same or different transplantation regimens, then the mechanism(s) potentially accounting for the increased mortality with HLA-DQB1 mismatches but not HLA-DRB1 mismatches would remain to be defined. It is possible that HLA-DRB1 and HLA-DQB1 are expressed by different subsets of antigen-presenting cells or that the interactions of T-cell receptors with HLA-DQB1 and HLA-DRB1 molecules on antigen-presenting cells lead to qualitatively different immune responses.
We previously found that single antigen mismatches conferred a higher risk of graft failure than a single allele mismatch.12 In the current study, we did not find any apparent differences between allele and antigen mismatches with respect to the risk of death, indicating that the number of potential candidate donors can be broadened to include either kind of single mismatch for higher-risk patients who otherwise lack matched donors. Differences between host-versus-donor and donor-versus-host immune responses may help to explain the differences between allele and antigen mismatches on the risks of graft failure and mortality. In graft rejection, host-versus-donor recognition involves a small number of recipient T cells that survive the transplantation conditioning regimen, while the donor-versus-host recognition that contributes most to increased mortality involves large numbers of alloreactive donor T cells. The assessment of allele and antigen mismatching on the risk of mortality could have been confounded by variation in the distribution of allele and antigen mismatches among the different HLA loci in our study population. The small numbers of single allele and antigen mismatches precluded a formal analysis of locus-specific effects.
The increased mortality associated with a longer time interval from diagnosis of CML to HCT has been reported previously for patients with related and unrelated donors and has provided a strong rationale for HCT within the first 1 or 2 years after the diagnosis in order to derive maximal benefit from transplantation. The management of chronic phase CML patients has changed dramatically with the availability of the tyrosine kinase inhibitor imatinib, which can produce complete cytogenetic remissions in more than 70% of chronic phase patients.29 The durability of imatinib-induced remissions is unknown. Long-term treatment with imatinib will increase the time from diagnosis to transplantation. Although the full impact of pretransplantation imatinib therapy on transplantation outcome remains to be defined, lengthened time from diagnosis to transplantation, disease progression, and other factors may lead to inferior results after HCT for patients who have received long-term treatment with imatinib.30 When a donor search is highly unlikely to yield matched donors for newly diagnosed CML patients, the increased mortality associated with a longer time interval from diagnosis to HCT must be weighed carefully against the increased mortality associated with earlier transplantation with a mismatched donor and against the chance of disease progression to advanced phase CML during a prolonged donor search.
In conclusion, the effect of donor HLA incompatibility on mortality after unrelated HCT is a complex product of both genetic and nongenetic factors. The number of HLA mismatches, the involvement of HLA-DQB1 in multiple mismatches, and the pretransplantation risk category all influence the limits of HLA incompatibility. The availability of unrelated HCT can be increased through the judicious selection of donors with HLA mismatches that do not substantially lower survival in certain patient populations.
Supported by grants CA18029, CA72978, and AI33484 from the National Institutes of Health, Bethesda, MD.
Prepublished online as Blood First Edition Paper, July 13, 2004; DOI 10.1182/blood-2004-04-1674.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.