Comment on Day et al, page 192
Recent studies have suggested that blood-borne tissue factor (in leukocyte-derived microparticles) plays an essential role in thrombus growth. This study uses bone marrow transplantation of genetically modified mice to show that macrovascular thrombosis is driven by tissue factor in the vessel wall, not leukocyte-derived tissue factor.
In the traditional view, blood clotting is triggered when tissue factor—an integral membrane protein found on the surface of a variety of nonvascular cell types—is exposed to blood following vascular injury. But a number of researchers have reported the presence of tissue factor antigen in plasma, albeit at very low pg/mL levels. The functional significance, as well as the provenance, of circulating tissue factor antigen was unclear.1 Blood-borne tissue factor moved into the limelight in 1999 when Giesen et al2 reported that tissue-factor antigen accumulated in ex vivo thrombi within minutes, too short a time scale for blood cells to synthesize tissue factor de novo. This could only mean that the trace amounts of tissue factor in blood were somehow concentrated within the growing thrombi, which were initiated by flowing freshly drawn blood over collagen-coated surfaces (and which were therefore initially devoid of tissue factor). Experiments with specific inhibitors of tissue factor function showed that the propagation of these ex vivo thrombi was dependent on tissue factor. These studies were followed by others using various artificial surfaces, underscoring the contribution of blood-borne tissue factor to thrombus growth.3 This has led to an alternative view of blood clotting in which thrombus formation is initiated by tissue factor exposed in the vessel wall following injury, but thrombus propagation is due to incorporation of active, blood-borne tissue factor into the growing clot.4 The origin of this blood-borne tissue factor is somewhat uncertain, although many investigators consider membrane microparticles shed from leukocytes a likely source.FIG1
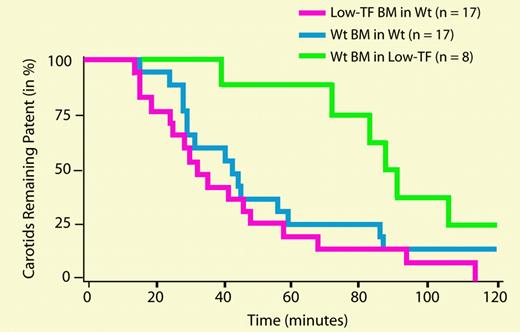
Carotid artery and venous thrombosis in mice receiving BM transplants. See the complete figure in the article beginning on page 192.
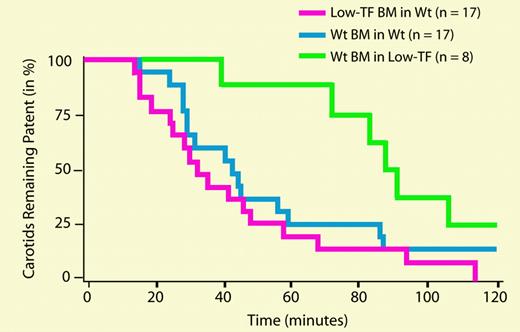
Carotid artery and venous thrombosis in mice receiving BM transplants. See the complete figure in the article beginning on page 192.
In this issue of Blood, Day and colleagues cleverly use genetically modified mice to evaluate the relative contributions of leukocyte-derived and vessel wall tissue factor to macrovascular thrombosis. Tissue factor–null mice die as early embryos, precluding their use in such experiments. Instead, Day et al employed mice genetically modified to express only 1% of the normal level of tissue factor. These low-tissue-factor mice are highly resistant to the development of both arterial and venous thrombosis (following photochemical injury of the carotid artery or ligation of the inferior vena cava). But the reduction in thrombosis could be due either to lower expression of tissue factor in the vessel wall, or to lower levels of blood-borne tissue factor. To address this question, they used bone marrow transplantation between wild-type and low-tissue-factor mice to create animals which were deficient only in leukocyte-derived tissue factor, or alternatively, which had wild-type leukocytes but low tissue factor throughout the rest of their bodies. Significantly, the low-tissue-factor mice that had wild-type leukocytes were fully protected against arterial and venous thrombosis, whereas the wild-type mice with low-tissue-factor leukocytes were not. These results suggest that macrovascular thrombosis is dependent solely on the level of tissue factor present in the vessel wall and not at all on the expression of blood-borne tissue factor derived from leukocytes. Interestingly, another study recently published in Blood5 (from some of the same authors of the present study) used the same bone marrow transplantation strategy with the same type of mice to examine this question in a mouse model of microvascular thrombosis (using laser-induced arterial injury). In contrast to the findings of Day et al, they found that leukocyte-derived tissue factor contributed significantly to thrombus propagation. There are many differences between these 2 studies in terms of the site and manner of injury, the size of the arteries under study, whether or not occlusive thrombi were studied, and the technologies used to study thrombus formation, which could explain the differences in conclusions. It is tempting to speculate that the relative contributions of blood-borne and vessel wall tissue factor to thrombosis may depend on how much tissue-factor activity is initially exposed following vascular injury. This innovative mouse model will be enormously useful in examining these questions in detail.