Abstract
Leukocytes and leukocyte-derived microparticles contain low levels of tissue factor (TF) and incorporate into forming thrombi. Although this circulating pool of TF has been proposed to play a key role in thrombosis, its functional significance relative to that of vascular wall TF is poorly defined. We tested the hypothesis that leukocyte-derived TF contributes to thrombus formation in vivo. Compared to wild-type mice, mice with severe TF deficiency (ie, TF–/–, hTF-Tg+, or “low-TF”) demonstrated markedly impaired thrombus formation after carotid artery injury or inferior vena cava ligation. A bone marrow transplantation strategy was used to modulate levels of leukocyte-derived TF. Transplantation of low-TF marrow into wild-type mice did not suppress arterial or venous thrombus formation. Similarly, transplantation of wild-type marrow into low-TF mice did not accelerate thrombosis. In vitro analyses revealed that TF activity in the blood was very low and was markedly exceeded by that present in the vessel wall. Therefore, our results suggest that thrombus formation in the arterial and venous macrovasculature is driven primarily by TF derived from the blood vessel wall as opposed to leukocytes.
Introduction
Tissue factor (TF) is a membrane-bound protein that forms a complex with blood coagulation factor VII to activate the coagulation protease cascade.1,2 In a normal artery, TF is expressed primarily in the adventitia and is inaccessible to flowing blood. Hemostasis after vascular injury is initiated when TF within the vessel wall is exposed to factor VII in plasma. TF expression is markedly increased within arterial atherosclerotic plaques, where it is thought to play a key role in triggering intravascular clotting after spontaneous plaque rupture.3-5 Extension of the thrombus into the blood vessel lumen can obstruct blood flow, causing unstable angina and acute myocardial infarction. The TF/factor VIIa complex also plays a major role in the pathogenesis of venous thrombosis, a leading cause of morbidity and mortality.6 In distinction to its role in thrombus initiation, the role of vessel wall–derived TF in thrombus propagation is unclear; it may become inaccessible to circulating clotting factors due to rapid deposition of platelets and fibrin on the exposed vessel surface that results in physical separation of vessel wall TF from circulating blood.7,8
Recent studies indicate that a small amount of TF is detectable in the blood and is capable of supporting clot formation in vitro.7 Plasma TF levels are elevated in patients with unstable angina and acute myocardial infarction and correlate with adverse outcomes.9-11 Hence, it has been proposed that a circulating pool of TF may play an important role in thrombus propagation by sustaining thrombin production on the clot surface, ultimately leading to the development of an occlusive thrombus.8,12 Although the cellular origin of circulating TF is unknown, evidence suggests that leukocytes and leukocyte-derived microparticles are a major source. Leukocytes are the only blood cells known to synthesize TF.12,13 Leukocyte-associated TF is present in clots formed in vitro and in human thrombi in situ.7,14 Furthermore, TF can be transferred from leukocytes to platelets in vitro via an interaction involving CD15-expressing membrane microparticles and P-selectin.15 Thus, based on in vitro studies, coupled with the observation of increased levels of circulating TF+ leukocyte-derived microparticles in various thrombotic diseases,16-19 a growing body of evidence favors a role for leukocyte-derived TF in thrombosis. However, no existing data clearly define the functional role of leukocyte-derived TF, particularly its contribution to thrombus formation relative to that of TF derived from the blood vessel wall.
We have conducted a series of in vivo experiments to examine the role of TF originating from leukocytes or leukocyte-derived microparticles in thrombosis after vascular injury. We used a bone marrow transplantation (BMT) strategy to alter TF expression within leukocytes to determine whether TF synthesized by the leukocyte is an important contributor to thrombosis. Because targeted disruption of the TF gene in mice yields an embryonically lethal phenotype,20-22 we used transgenic mice with severe TF deficiency as a source of TF-deficient hematopoietic stem cells.23 We hypothesized that if circulating leukocytes are an important source of thrombogenic TF, then reduction of TF expression within this pool would limit thrombus growth. Similarly, we hypothesized that restoration of wild-type leukocyte TF expression in mice with very low levels of vessel wall TF would augment thrombus formation. However, our results obtained using models of macrovascular arterial and venous thrombosis suggest that the TF driving thrombus formation is not of leukocyte origin.
Materials and methods
Animals
C57BL/6J, 129 × 1/SvJ, and low-density lipoprotein receptor-deficient (LDLR–/–) mice (backcrossed 10 generations into the C57BL/6 genetic background) were obtained from Jackson Laboratories (Bar Harbor, ME). Mice heterozygous for a null mutation at the TF locus (TF+ /–) were a generous gift from Dr George Broze (Washington University, St Louis, MO).20 Transgenic mice expressing very low levels of human TF (approximately 1% relative to mouse TF) and completely lacking murine TF (ie, TF–/–, hTF-Tg+, designated “low-TF”) were generated as described previously.23 These mice were backcrossed (F6) into the C57BL/6J genetic background. All animal care and experimental procedures were approved by the University of Michigan Committee on Use and Care of Animals.
Hematopoietic stem cell transplantation
The fetal liver contains hematopoietic stem cells capable of marrow engraftment in irradiated adult mice.24 To obtain hematopoietic stem cells completely devoid of TF, TF+/– mice were mated and females were humanely killed 13 to 14 days after coitus. The polymerase chain reaction (PCR) was used to genotype 104 embryos, but no viable TF–/– offspring were detected at this stage of gestation. Similarly, screening of 81 progeny generated by mating TF+/– mice of a mixed 129 × 1/SvJ-C57BL/6 genetic background revealed no viable TF–/– offspring, results consistent with previous studies demonstrating the near-complete lethality of TF deficiency by embryonic day 10.5.20-22 Therefore, bone marrow (BM) from adult low-TF mice was used as a source of TF-deficient hematopoietic stem cells. BMT was performed as described previously,25 with minor modifications. Recipient mice were fed acidified water for 1 week prior to and 6 weeks following BMT. Recipient mouse BM was ablated by irradiation (2 doses of 650 rad delivered 3 hours apart [13 Gy total]). Each recipient mouse received injections of 4 × 106 cells via the retro-orbital sinus. Mice were allowed to recover at least 6 weeks after transplantation before being studied in experimental protocols. BM reconstitution after transplantation was assessed by performing whole blood cell counts and leukocyte differentials with an automated cell counter (Hemavet, CDC Technologies, Oxford, CT). To assess the degree of eradication of recipient BM, PCR analysis (30 cycles) of DNA isolated from circulating blood cells was performed with DNA primers specific for either the wild-type or null TF allele. Five groups with BM transplants were generated. Wild-type C57BL/6 mice were given transplants with BM from either wild-type or low-TF animals, and low-TF mice received transplants of BM from wild-type mice. LDLR–/– mice (16 weeks of age) were given transplants with BM from either wild-type or low-TF mice. After BMT, LDLR–/– mice were fed high-fat chow (TD96121, 1.25% cholesterol, Harlan Teklad, Madison, WI) for 6 weeks before being studied in the thrombosis protocol. In all groups, mice that were irradiated but not given transplants uniformly died 7 to 10 days after irradiation.
Procoagulant activity of peritoneal macrophages
Macrophages were obtained by flushing the peritoneal cavity with phosphate-buffered saline (PBS) 4 days after intraperitoneal injection with 1 mL of a 3% thioglycollate solution (Difco Laboratories, Sparks, MD). Cells were washed twice with PBS, resuspended at a final concentration of 2 × 107 cells/mL in RPMI 1640 medium (Gibco, Grand island, NY) containing 10% fetal calf serum and incubated in the presence or absence of 1 μg/mL lipopolysaccharide (LPS; Escherichia coli serotype O55:B5; Difco Laboratories) for 6 hours at 37°C in 5% CO2. Macrophage procoagulant activity was determined by mixing 40 μL cell suspension, 40 μL mouse platelet-poor plasma, and 40 μL CaCl2 (final concentration 5 mM), and measuring the time to clot formation with a KC4 Delta Micro-Coagulation Analyzer (Trinity Biotech, Bray, Co Wicklow, Ireland). Clotting times were expressed in relative units by use of a standard curve generated from serially diluted wild-type mouse brain extracts.
Carotid artery TF activity
TF activity in homogenized, uninjured carotid arteries was determined by a previously described clotting-based assay.26 A functional chromogenic assay that measures factor Xa generation also was performed. Carotid artery homogenate (30 μL) was combined with 90 μL of a reaction mixture containing 3 nM human factor VIIa (Haematologic Technologies [Haemtech], Essex Junction, VT), 100 nM human factor X (Haemtech), 8.3 mM CaCl2, and 0.33 mM Spectrozyme fXa (American Diagnostica, Greenwich, CT) in Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS) with 1 mg/mL bovine serum albumin (BSA). After a 45-minute incubation at 37°C, changes in optical density at 405 nM were quantified. TF activity was expressed in arbitrary units by reference to a standard curve constructed from recombinant human lipidated TF (rTF; American Diagnostica).
Factor Xa generation in plasma
Whole blood was collected in 3.8% sodium citrate (9 parts blood to 1 part sodium citrate) from the inferior vena cava (IVC) and centrifuged at 1500g for 10 minutes. Platelet-poor plasma was collected and frozen at –80°C. Citrated plasma (10 μL) was incubated in 96-well microtiter plates with 60 μL TBS containing 1 mg/mL BSA and corn trypsin inhibitor (CTI; Haemtech, final concentration 80 μg/mL) to inactivate the intrinsic coagulation pathway,27 for 10 minutes at room temperature. Thirty microliters of a reaction mixture containing 0.5 mM Spectrozyme factor Xa, 8.3 mM CaCl2, and 1 μg/mL lepirudin (Berlex Laboratories, Seattle, WA) was then added. Factor Xa generation was determined by monitoring the absorbance at 405 nm (A405) of the reaction for 30 minutes at 37°C in a microtiter plate reader. Where indicated, nematode anticoagulant protein c2 (rNAPc2, a generous gift from Dr William Rote, San Diego, CA [final concentration 500 nM]), an inhibitor of the TF/VIIa complex that does not interfere with the amidolytic activity of factor Xa,28 or diluted wild-type mouse brain extract was added to the plasma samples.
Procoagulant activity of plasma microparticles and whole blood cell membranes
Microparticles and whole blood cell membranes were isolated from mouse plasma as described previously.29,30 The total protein concentration of microparticles and blood cell membrane preparations were determined using BCA reagent (Pierce, Rockford, IL). Forty microliters of the microparticle suspension (2.9 mg/mL), blood cell membrane suspension (8 mg/mL), carotid artery homogenate (10 μg/mL), or vehicle was mixed with 40 μL citrated pooled wild-type mouse plasma and 40 μL CaCl2 (final concentration 5 mM), and time to clot formation was measured as for the macrophage assay. Clotting times were expressed in relative units/mg protein based on a standard curve of serially diluted mouse brain extract.
Carotid artery thrombosis model
Photochemical vascular injury to the midportion of the common carotid artery was performed on pentobarbital-anesthetized mice as previously described.31 Flow was monitored continuously from the onset of injury until stable occlusion occurred (defined as no flow for ≥ 10 minutes), or for 120 minutes if occlusion did not occur. Occlusion time was defined as the interval between the initiation of vascular injury and the onset of stable occlusion. For the purpose of calculating mean occlusion times, animals that did not develop an occlusion were assigned an occlusion time of 120 minutes.
Venous thrombosis model
Mice were anesthetized with an inhalation mixture of isoflurane gas (1.5%-2%) in oxygen. A midline laparotomy was performed and the IVC was ligated immediately inferior to the renal veins, as described previously.32 The abdomen was closed and the animal was allowed to recover. After 48 hours, the IVC was harvested and weighed, and the weight was normalized to vein length (mg/cm) to calculate the mass of thrombus that formed during the period of venous stasis.
Statistical analysis
All results are reported as mean ± SE unless noted otherwise. Comparison of continuous variables was performed using one-way analysis of variance with Tukey post hoc between-group comparisons. Paired t tests were used to analyze differences in factor Xa generation between samples incubated with and without rNAPc2. Carotid occlusion in the arterial thrombosis experiments was treated as a time-to-failure analysis and was performed in 2 stages. First, a proportional hazards model including all experiment groups was constructed to determine overall statistical significance of the experimental groups. Because this overall test was highly statistically significant (P = .003), a second stage of individual between-group comparisons was performed using log-rank. Analyses were performed using SAS 8.2 (SAS Institute, Cary, NC) or Sigmastat 3.0 (Systat Software, Point Richmond, CA).
Results
Arterial and venous thrombosis is impaired in low-TF mice
There was a marked effect of reduced total TF expression on thrombosis. After photochemical carotid artery injury, all wild-type mice developed arterial thrombotic occlusion within 75 minutes (mean occlusion time of 43.5 ± 6.0 minutes, n = 9; Figure 1A-B). In comparison, the mean occlusion time of low-TF mice was 98.7 ± 15.1 minutes (n = 7, P = .003 versus wild-type), a value that likely underestimates the difference between the 2 groups, because 5 of 7 low-TF mice failed to develop an occlusion during 120 minutes of monitored flow.
Carotid artery and IVC thrombosis in wild-type and low-TF mice. (A) The percentage of animals with patent arteries in each group are plotted in a Kaplan-Meier graph as a function of time after initiation of photochemical injury. (B) Mean occlusion times following arterial injury. (C) Thrombus mass measured 48 hours after IVC ligation. *P < .01 versus wild-type mice.
Carotid artery and IVC thrombosis in wild-type and low-TF mice. (A) The percentage of animals with patent arteries in each group are plotted in a Kaplan-Meier graph as a function of time after initiation of photochemical injury. (B) Mean occlusion times following arterial injury. (C) Thrombus mass measured 48 hours after IVC ligation. *P < .01 versus wild-type mice.
In an IVC ligation model of venous thrombosis, the mean thrombus mass of low-TF mice (6.9 ± 2.1 mg/cm, n = 9) was markedly reduced compared to that of wild-type animals (20.4 ± 2.7 mg/cm, n = 10, P = .002; Figure 1C). A thrombus was grossly visible in only 1 of 9 low-TF animals compared to 9 of 10 wild-type mice.
BMT successfully reconstitutes recipient BM without altering vascular wall TF activity
Genomic DNA (gDNA) was prepared from peripheral blood cells obtained 6 weeks after transplantation. PCR analysis failed to detect recipient mouse cell DNA, while demonstrating a strong signal attributable to transplanted cells (Figure 2). Control PCRs were performed under identical reaction conditions with template DNA consisting of mixtures of wild-type and low-TF gDNA at various ratios. These experiments demonstrated that PCR detected both the wild-type gene and the low-TF gene when either comprised as little as 0.5% of the DNAmixture (data not shown). These results, which were consistent with previous reports regarding the efficacy of BMT in mice,33,34 suggest that our transplantation strategy effectively replaced recipient BM progenitor cells with those derived from donor mice. Complete blood cell counts and leukocyte differentials performed 6 weeks after transplantation demonstrated reconstitution of all blood cell lines (Table 1). The only significant differences in transplanted groups compared to wild-type mice not receiving transplants were higher total leukocyte counts in wild-type mice given transplants with wild-type BM and low-TF mice given transplants with wild-type BM and higher monocyte counts in low-TF mice receiving transplants with wild-type BM.
PCR analysis of peripheral blood cell DNA. Oligonucleotide primer pairs are specific for (A) the murine wild-type TF allele and (B) the neomycin gene from the TF null allele. Lane 1 in each panel contains molecular weight markers. Results from 2 mice in each group are shown. Wild-type mice given transplants with wild-type marrow are designated as wt BM in wt, wild-type mice given transplants with low-TF marrow as low-TF BM in wt, and low-TF mice given transplants with wild-type marrow as wt BM in low-TF.
PCR analysis of peripheral blood cell DNA. Oligonucleotide primer pairs are specific for (A) the murine wild-type TF allele and (B) the neomycin gene from the TF null allele. Lane 1 in each panel contains molecular weight markers. Results from 2 mice in each group are shown. Wild-type mice given transplants with wild-type marrow are designated as wt BM in wt, wild-type mice given transplants with low-TF marrow as low-TF BM in wt, and low-TF mice given transplants with wild-type marrow as wt BM in low-TF.
Peripheral blood cell counts after BMT
. | Wt (no transplant), n = 10 . | Wt BM in wt, n = 10 . | Low-TF BM in wt, n = 5 . | Wt BM in low TF, n = 7 . |
|---|---|---|---|---|
| WBC count, ×1000/μL | 7.4 ± 0.8 | 16.0 ± 1.1* | 12.3 ± 2.4 | 16.2 ± 1.2* |
| Neutrophils, % | 19.8 ± 3.1 | 14.5 ± 1.3 | 18.1 ± 1.2 | 20.4 ± 1.8 |
| Lymphocytes, % | 77.0 ± 3.2 | 82.2 ± 1.2 | 76.9 ± 1.2 | 71.6 ± 2.4 |
| Monocytes, % | 3.1 ± 0.4 | 2.9 ± 0.3 | 3.1 ± 0.4 | 5.9 ± 0.6* |
| Hemoglobin concentration, g/dL | 12.5 ± 0.4 | 13.4 ± 0.2 | 13.7 ± 1.3 | 11.3 ± 0.4 |
| Platelet count, 109/L | 970 ± 50 | 837 ± 29 | 802 ± 79 | 848 ± 66 |
. | Wt (no transplant), n = 10 . | Wt BM in wt, n = 10 . | Low-TF BM in wt, n = 5 . | Wt BM in low TF, n = 7 . |
|---|---|---|---|---|
| WBC count, ×1000/μL | 7.4 ± 0.8 | 16.0 ± 1.1* | 12.3 ± 2.4 | 16.2 ± 1.2* |
| Neutrophils, % | 19.8 ± 3.1 | 14.5 ± 1.3 | 18.1 ± 1.2 | 20.4 ± 1.8 |
| Lymphocytes, % | 77.0 ± 3.2 | 82.2 ± 1.2 | 76.9 ± 1.2 | 71.6 ± 2.4 |
| Monocytes, % | 3.1 ± 0.4 | 2.9 ± 0.3 | 3.1 ± 0.4 | 5.9 ± 0.6* |
| Hemoglobin concentration, g/dL | 12.5 ± 0.4 | 13.4 ± 0.2 | 13.7 ± 1.3 | 11.3 ± 0.4 |
| Platelet count, 109/L | 970 ± 50 | 837 ± 29 | 802 ± 79 | 848 ± 66 |
Wt indicates wild type; WBC, white blood cell.
P < .05 versus wild-type.
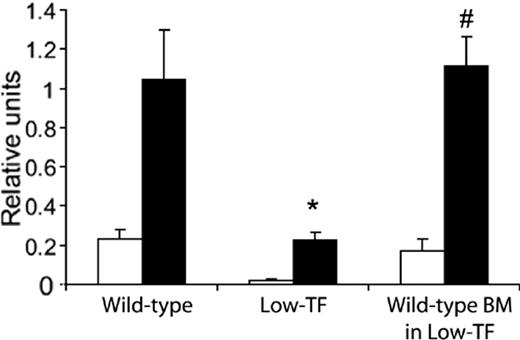
To determine if transplanted BM cells were capable of expressing TF after engraftment, TF activity of peritoneal macrophages was assessed. TF activity was significantly lower in macrophages from low-TF mice compared to wild-type mice (Figure 3). However, TF activity of macrophages from low-TF mice given transplants with wild-type BM was similar to that of wild-type animals (both with and without LPS stimulation), suggesting that leukocyte TF activity was appropriately altered by BMT.
Procoagulant activity of peritoneal macrophages. Macrophages were isolated from wild-type mice, low-TF mice, and low-TF mice given transplants with wild-type BM. Cells were cultured in the presence (▪) or absence (□) of LPS, and procoagulant activity was measured. Macrophages were pooled from 2 mice in each group and samples were run in triplicate for each condition. * P < .01 compared to the other 2 groups. # P > .05 compared to wild-type.
Procoagulant activity of peritoneal macrophages. Macrophages were isolated from wild-type mice, low-TF mice, and low-TF mice given transplants with wild-type BM. Cells were cultured in the presence (▪) or absence (□) of LPS, and procoagulant activity was measured. Macrophages were pooled from 2 mice in each group and samples were run in triplicate for each condition. * P < .01 compared to the other 2 groups. # P > .05 compared to wild-type.
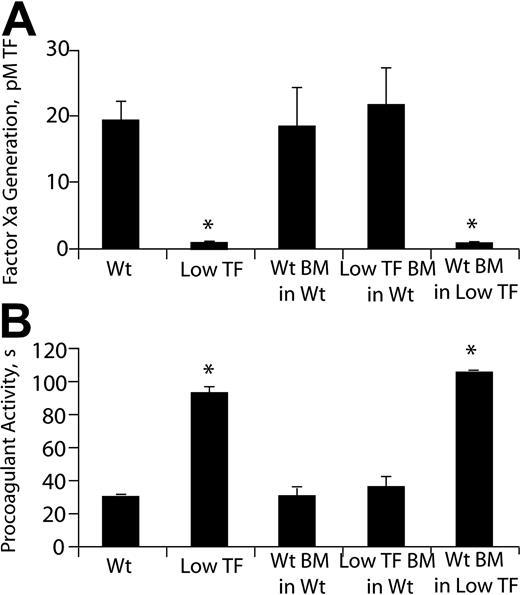
To assess the functional pool of TF in the blood vessel wall, the procoagulant activities of homogenized carotid arteries were analyzed in an amidolytic assay that measures factor Xa generation (Figure 4A). Mean carotid TF activity was markedly lower in low-TF arteries compared to wild-type arteries, but was not significantly different between mice that had undergone BMT and their recipient genotype-matched counterparts not receiving transplants. These results, which were corroborated in a clotting-based assay that used murine plasma (Figure 4B), suggest that BMT did not significantly alter vascular wall TF expression.
Carotid artery TF activity. (A) Chromogenic assay that detects factor Xa generation. (B) Clotting-based assay using mouse plasma. n = 6-8/group; *P < .05 compared to the other 3 groups.
Carotid artery TF activity. (A) Chromogenic assay that detects factor Xa generation. (B) Clotting-based assay using mouse plasma. n = 6-8/group; *P < .05 compared to the other 3 groups.
Modulation of the leukocyte pool of TF does not significantly alter arterial or venous thrombosis
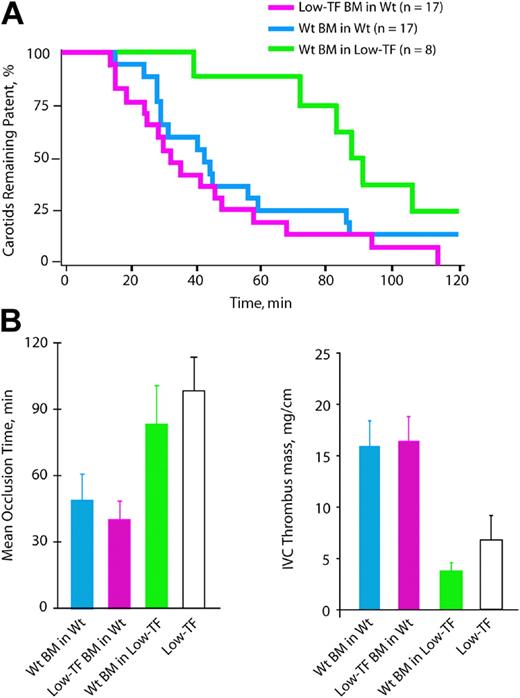
The mean carotid artery occlusion time of wild-type mice given transplants with wild-type BM (51.5 ± 7.9 minutes, n = 17) did not differ significantly from that of wild-type mice not receiving transplants (43.5 ± 6.0 minutes, P = .47). The mean occlusion time of wild-type mice given transplants of low-TF BM (40.9 ± 6.9 minutes, n = 17; Figure 5A-B) was not significantly different from that of wild-type mice (43.5 ± 6.0 minute, P = .88) or from that of wild-type mice given transplants of wild-type BM (51.5 ± 7.9 minutes, P = .30). Therefore, in the presence of normal vessel wall TF expression, a reduction of the leukocyte pool of TF did not affect the development of an occlusive arterial thrombus. In the converse transplantation strategy, low-TF mice were given transplants with wild-type BM. This group had a prolonged mean occlusion time (89.9 ± 9.5 minutes, n = 8) compared to wild-type mice (P < .01) or wild-type mice given transplants with wild-type BM (P = .05), which was not significantly shorter than that of low-TF mice not given transplants (98.7 ± 15.1 minutes, P = .16). As a whole, the BMT experiments described did not support a significant role of the leukocyte pool of TF in determining the rate of occlusive thrombus formation after arterial injury.
Carotid artery and venous thrombosis in mice receiving BM transplants. (A) The percentage of animals with patent arteries in each group are plotted in a Kaplan-Meier graph as a function of time after initiation of injury. (B) Mean occlusion times following arterial injury. (C) Thrombus mass after IVC ligation.
Carotid artery and venous thrombosis in mice receiving BM transplants. (A) The percentage of animals with patent arteries in each group are plotted in a Kaplan-Meier graph as a function of time after initiation of injury. (B) Mean occlusion times following arterial injury. (C) Thrombus mass after IVC ligation.
We also studied carotid artery thrombosis in LDLR–/– mice with hyperlipidemia, a condition associated with increased circulating TF activity in humans.35,36 Mean plasma total cholesterol levels were 862 ± 112 mg/dL and 727 ± 109 mg/dL in LDLR–/– mice given transplants with wild-type versus low-TF marrow, respectively (P = .4). Thrombosis was accelerated in both groups of LDLR–/– mice compared to wild-type mice, consistent with other studies.36 However, the mean occlusion time in LDLR–/– mice receiving transplants of low-TF BM (14.8 ± 2.6 minutes, n = 5) did not differ significantly from that of LDLR–/– mice given transplants with wild-type BM (16 ± 2.6 minutes, n = 5, P = .76).
IVC ligation experiments indicated that TF is important in venous thrombosis and that the major source of TF driving venous thrombus generation is the vessel wall and not leukocytes. Two days after IVC ligation, there was no significant difference in mean thrombus mass between wild-type mice given transplants of low-TF BM (16.4 ± 2.4 mg/cm, n = 10, Figure 5C) or wild-type BM (15.9 ± 2.5 mg/cm, n = 9, P = 1.0). In contrast, low-TF mice receiving transplants of wild-type BM demonstrated a marked reduction in mean thrombus mass (3.8 ± 0.8 mg/cm, n = 7) compared to wild-type mice given transplants with wild-type BM (P = .01), indicating that restoration of normal expression of TF in leukocytes did not enhance thrombus formation in low-TF mice. Overall, these results were concordant with those obtained in the arterial thrombosis model.
Plasma factor Xa generation and procoagulant activities of blood cell membranes and microparticles do not differ between wild-type and low-TF mice
To complement our thrombosis experiments, we performed a series of in vitro studies examining the TF activity present in the blood and blood vessel wall. We used an amidolytic assay that measures factor Xa generation in plasma in the presence of CTI, an inhibitor of the intrinsic coagulation pathway, to examine plasma TF activity. There was no significant difference in factor Xa generation between wild-type and low-TF plasmas (1.88 ± 0.21 and 1.67 ± 0.16 milli-optical density units [mOD]/min, respectively, P = .43) and addition of rNAPc2 had no effect in either group (1.68 ± 0.21, P = .68 and 1.61 ± 0.35 mOD/min, P = .29). Addition of a trace amount (0.2 μL) of wild-type mouse brain extract to the plasma potently accelerated factor Xa generation (to 20.3 ± 1.8 mOD, n = 4), and this signal was almost completely inhibited by rNAPc2 (3.1 ± 0.3 mOD/min, n = 3). Ultracentrifugation of wild-type plasma to deplete it of microparticles did not reduce its low level of factor Xa-generating capacity. These results suggest that the concentration of active TF in mouse plasma, including its microparticle fraction, was very low.
There has been considerable interest in the role of TF in generating the procoagulant activity associated with plasma microparticles and blood cells. We used a clotting-based assay to examine the procoagulant activities of plasma microparticles and peripheral blood cell membranes prepared from wild-type and low-TF mice. The addition of wild-type microparticles to plasma accelerated fibrin formation, yielding a mean clotting time of 60 ± 3 seconds (n = 8) versus 119 ± 13 seconds (n = 4) for control reactions. Similarly, depleting wild-type pooled plasma (prepared from 25 mice) of microparticles by ultracentrifugation prolonged the mean clotting time to 294 seconds, compared to 130 seconds for nonultracentrifuged pooled plasma. However, the mean procoagulant activities (expressed as units of TF/mg protein) of wild-type plasma microparticles (1.33 ± 0.23 U/mg) and blood cell membranes (0.003 ± 0.001 U/mg) did not differ from those of low-TF mice (1.68 ± 0.69 U/mg and 0.003 ± 0.001 U/mg, respectively; P > .3). In contrast, the mean procoagulant activity of carotid artery homogenates prepared from low-TF mice (1.80 ± 1.05 U/mg) was markedly lower than that of wild-type mice (121 ± 31 U/mg; P = .01). These results, coupled with those of the amidolytic assay (Figure 4), indicate that the procoagulant activity of the arterial wall is largely dependent on TF. When compared on a per milligram protein basis, the procoagulant activity of arterial wall homogenates from wild-type mice was 90-fold greater than that of circulating microparticles and over 1000-fold greater than that of blood cell membranes. Together, these in vitro data suggest that under basal conditions circulating TF activity is very low and is markedly less than that present in the blood vessel wall.
Discussion
It is generally accepted that initial thrombus formation after vascular injury is triggered by TF present in the blood vessel wall.1,2,37 However, it has been proposed that vessel wall TF is effectively prevented from contributing to subsequent thrombus growth after it is isolated from flowing blood by the mural thrombus itself.8,38 A better understanding of the factors that determine continued thrombus propagation after the initial wave of fibrin and platelet deposition is of critical importance because thrombi that completely occlude blood flow within medium- and large-sized blood vessels play a major role in the pathogenesis of life-threatening vascular diseases such as acute myocardial infarction and stroke. The demonstration of TF in the blood,7 as well as its deposition in thrombi,14 has led to the hypothesis that a circulating pool of TF plays a significant role in thrombus growth by supporting continued factor X activation and thrombin generation on the clot surface.7,8 Although the cellular origin of circulating TF remains elusive, substantial evidence from in vitro studies favors leukocytes, or microparticles derived from leukocytes, as the major source.7,14,15 Nevertheless, existing data have shown primarily an association of circulating TF with leukocytes, without a clear demonstration of its functional significance. Our data do not support the hypothesis that leukocyte-derived TF is a key determinant of the thrombotic response to macrovascular injury because a selective and marked reduction in the BM-derived pool of TF had no apparent effect on arterial or venous thrombus formation in mice with normal vessel wall TF expression. Similarly, restoration of wild-type TF expression in leukocytes did not enhance thrombus formation in low-TF mice.
Ex vivo perfusion chamber studies using blood from healthy individuals have suggested that leukocyte TF may contribute to thrombus development even under basal conditions.7 Using an arterial injury model that is sensitive to circulating coagulation factors,25,39,40 our experiments suggest that the vascular wall, rather than the BM, is the dominant source of TF that drives occlusive thrombus formation after injury to a medium-sized artery. Consistent with our results, recent intravital microscopy studies of thrombi forming in cremasteric arterioles detected TF predominantly at the thrombus-vessel wall interface, with very little on the luminal surface of the thrombus.41,42 However, a more recent study demonstrated P-selectin–dependent incorporation of TF-bearing microparticles, generated ex vivo by activation of mouse blood cells, into growing thrombi.43 Although these results suggest a role for circulating TF and P-selectin glycoprotein ligand-1–expressing microparticles of leukocyte origin in early thrombus formation in the mouse microcirculation, they do not provide direct evidence that circulating TF synthesized by leukocytes under basal conditions is a critical determinant of the formation of an occlusive thrombus relative to that of TF derived from the blood vessel wall. Differences observed between this study and ours may be attributable to differences in the vascular bed studied, the nature and severity of vessel injury, and the time over which thrombus formation was monitored. The molecular mechanisms governing clot development are likely to differ in different sized blood vessels following varying degrees of vascular injury and therefore, the contribution of leukocyte-derived TF to thrombosis in animal studies may be highly dependent on the model. Further studies will be necessary to investigate the role of leukocyte-derived TF in microvascular thrombosis. Finally, it is important to recognize that there may be fundamental species differences in the contribution of different pools of TF to thrombus development between mice and humans.
There are several potential explanations for our findings. First, it is possible that vascular wall TF may not be entirely walled off by the forming clot. TF derived from cells within the vascular wall could be delivered to the surface of the thrombus by intermittent, flow-induced dislodgement of the upstream edge of the thrombus, allowing exposure and release of vascular wall TF into the blood. It is also possible that other factors, such as shear stress, thrombin-independent platelet activation, or the intrinsic coagulation system may play more important roles than circulating TF in determining thrombus formation in these models.44 For example, factor XI appears to be essential for clot initiation after murine arterial injury, as well as clot propagation after TF-triggered clot formation within synthetic vascular grafts in primates.45,46 Another possibility is that the leukocyte pool of TF may play a role in thrombosis only under conditions in which the synthesis of TF by monocytes, the only blood cells capable of synthesizing TF,12,13 is up-regulated. For example, monocyte TF expression is induced by LPS and may promote microvascular thrombosis associated with sepsis.47 In addition, monocyte TF expression is substantially increased in acute myocardial infarction48 and hyperlipidemia.36 In a preliminary attempt to examine the potential effect of leukocyte-derived TF in hyperlipidemic animals, we studied thrombosis in a nonatherosclerotic portion of the common carotid artery in LDLR–/– mice given transplants of wild-type or low-TF BM. We did not observe significant differences in thrombosis between these 2 groups. However, further studies will be necessary to more rigorously explore the importance of leukocyte TF under pathologic conditions and to define its role in atherogenesis and plaque thrombogenicity.
The pathophysiology of venous thrombosis differs considerably from that of arterial thrombosis. Although endothelial disruption is characteristic, it is often less extensive than that which triggers arterial thrombosis.49 Additionally, leukocyte adhesion and transmigration have been demonstrated as early events in the initiation of venous thrombosis.49,50 Thus, it has been proposed that circulating TF, perhaps derived from leukocytes, could be a key determinant of fibrin generation in this setting.38,51 Interestingly, a recent study demonstrated that inhibition of TF significantly reduced fibrin accumulation on a collagen-coated thread implanted into a rabbit vein.52 Immunostaining revealed TF mostly associated with leukocytes within the thrombi. Although these studies suggest a potential role for circulating leukocyte-associated TF in venous thrombosis, they do not establish a cause-and-effect relationship between leukocyte TF expression and thrombus growth. Our results do not support an essential role for leukocyte-derived TF in the development of stasis-induced venous thrombosis because modulation of leukocyte TF expression by BMT had no significant effect on thrombosis, neither in the presence of normal nor reduced vascular wall TF.
Although our experiments suggest that the vascular wall is the dominant source of thrombogenic TF in medium-sized and large blood vessels, they do not exclude a minor role of leukocyte-derived TF in thrombus formation. Similarly, they do not exclude a potential role for a circulating pool of TF in thrombosis because this pool could originate from sources other than BM. Several recent reports indicate that platelets contain functionally active TF that is released on platelet activation in vitro.12,53,54 However, no TF antigen or mRNA is detectable in megakaryocytes,12,54 suggesting that platelet TF is acquired from elsewhere. Potential cellular sources of circulating TF other than leukocytes include vascular endothelial cells and smooth muscle cells, both of which are capable of releasing TF-bearing membrane microparticles.16,18,55-57 In fact, high levels of procoagulant microparticles of endothelial cell origin have been detected in the blood of patients with acute coronary syndromes.58 Furthermore, TF procoagulant activity measured in whole blood from patients with severe BM aplasia is only reduced by 45%.59
Although there has been intense interest in the biologic function of circulating TF, a major challenge in the field has been the development of assays for measuring TF activity in blood, particularly in the mouse. We found that murine plasma TF activity under basal conditions was very low and did not differ between wild-type mice and low-TF mice. These results are consistent with a recent report suggesting that the concentration of active TF in human plasma is below 20 fM.60 We also were unable to discern differences between the procoagulant activities of plasma microparticles and peripheral blood cell membranes of wild-type mice versus low-TF mice. These results suggest that these components of blood may support clotting by mechanisms other than their TF activity, for example, by providing a phospholipid surface to support assembly of coagulation complexes. Consistent with our data, a recent study concluded that the procoagulant activity of microparticles prepared from healthy humans was independent of their TF content.61 Our in vitro studies also suggest that the TF activity present in the blood vessel wall vastly exceeds that present in blood components, further supporting the hypothesis that the blood vessel wall is the dominant source of thrombogenic TF. However, it must be emphasized that in vitro assessment of TF activity is difficult and that in vitro assays may not provide the conditions necessary to activate encrypted TF within blood components. Therefore, in vivo experiments, which were the main focus of our study, are essential to examine the biologic function of leukocyte-derived TF. We used several functional assays to compare TF activities of tissue and blood samples from wild-type and low-TF mice, some of which used mouse plasma and others purified human coagulation proteins. Human and mouse TF would not be expected to have identical specific activities in all of these assays, and it is therefore conceivable that there is some error in the comparisons of TF activity between experimental groups expressing exclusively mouse versus human TF. Because human TF interacts equally well with human or mouse VIIa62,63 and clots mouse plasma efficiently,62 species specificity should not have been a major issue in the assays using mouse plasma. However, because mouse TF interacts poorly with human VIIa,62,63 the use of human coagulation reagents in the measurement of carotid TF activities may have resulted in an underestimation of the differences between wild-type and low-TF mice. As purified preparations of mouse coagulation factors and inhibitory antibodies to them become available, the development and validation of in vitro assays for mouse TF will become more feasible.
In summary, our results suggest that thrombosis following macrovascular injury is driven primarily by TF derived from the vessel wall as opposed to leukocytes. These data have important therapeutic implications and support the development of an antithrombotic strategy targeting vessel wall-derived TF or its release into the blood.
Prepublished online as Blood First Edition Paper, August 31, 2004; DOI 10.1182/blood-2004-06-2225.
Supported in part by the National Institutes of Health (NIH) T32 Training Grant in Molecular and Cellular Cardiology (HL07853; S.M.D.) and NIH grants HL65226 (N.M.), HL70766 (T.W.W.), and HL57346 (W.P.F.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to John Younger for statistical analysis, to James Ferrara for sharing his expertise in bone marrow transplantation, to David Ginsburg for his editorial assistance, and to Jinghong Sun and Ying Huang for technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal