Abstract
Glycoprotein (GP) VI, the primary collagen receptor on platelets, has been shown to have variable expression, possibly as a consequence of immune modulation. The present study was designed to determine the mechanism by which GP VI clearance occurs. We found that direct activation of GP VI both by a GP VI–specific antibody and by GP VI ligands (collagen and convulxin) reduced binding of biotinylated convulxin to the stimulated platelets. Analysis of immunoblots of platelets and supernatants showed that the stimulated platelets contained less GP VI, while the soluble fraction contained a 57-kDa cleavage product. Stimulation of platelets with PAR-1 agonists (TRAP peptide and thrombin) also caused GP VI cleavage, although the amount of GP VI loss was less than that observed with direct GP VI ligands. The metalloproteinase (MMP) inhibitors GM6001 and TAPI prevented both the clearance of GP VI from the platelet surface and the appearance of the soluble cleavage product. Induction of GP VI cleavage caused specific down-regulation of collagen-induced platelet aggregation, providing a mechanism for the modulation of platelet responsiveness to this important platelet agonist.
Introduction
Activation of platelets at sites of arterial injury is the first step in a chain of molecular and cellular events that lead to thrombosis. One of the primary activating ligands at sites of vessel wall injury is collagen, which mediates both platelet adhesion and activation.1 Platelets have 2 primary receptors that directly bind collagen, the integrin α2β1 and the immunoglobulin domain-containing glycoprotein (GP) VI. Platelet activation following adhesion of collagen to GP VI leads to the conversion of the α2β1 and GP IIb-IIIa integrins into high-affinity receptors, allowing firm adhesion and platelet aggregation, respectively. GP VI signaling also leads to α-granule and dense body secretion and to the release of TxA2, all of which serve to propagate thrombus growth (reviewed by Nieswandt and Watson2 ).
The levels of GP VI appear to be tightly regulated, with a 2-fold variation reported in a population of healthy donors measured by antibody titration.3,4 However, antibodies to GP VI appear to cause specific depletion of this receptor from the platelet surface. For example, in mice infused with GP VI monoclonal antibodies JAQ 1, 2, and 3 (directed to different epitopes of GP VI), irreversible down-regulation of the receptor has been observed,5 providing long-term antithrombotic protection.6 A patient with autoantibodies to GP VI, deficient in platelet and soluble plasma GP VI, recently has been shown to have normal levels of GP VI mRNA.7 In addition, defects resulting in reduced levels of GP VI have been described in other “GP VI–deficient patients,” and these have mostly been associated with the presence of autoantibodies.8,9
Matrix metalloproteinases (MMPs) are a family of proteases traditionally believed to be involved in the physiologic and pathologic remodeling of the extracellular matrix.10 Several MMPs are expressed in platelets including MMP-1,11 MMP-2,12 and MMP-9.13 Recent data suggest that platelet MMPs affect specific receptors in platelets. For example, while platelet activation causes the expression of CD40L14 and P-selectin15 on the platelet surface, platelet MMPs cleave these proteins generating soluble forms. MMP-induced cleavage of GP Ibα also has been reported.16 Although treatment of platelets with the MMP inhibitor GM6001 is reported to improve their adhesion to collagen under flow during aging in vitro,16 the mechanism and function of MMP-mediated receptor shedding remains poorly understood.
In the present study, we found that GP VI antibody and GP VI agonists induce efficient MMP-dependent cleavage of GP VI, leading to the release of a GP VI proteolytic fragment, termed sGP VI. We show also that platelets with cleaved GP VI have decreased responsiveness to collagen, providing a mechanism for the modulation of platelet responsiveness to this important platelet agonist.
Materials and methods
Reagents
Collagen was from Biodata Corporation (Horsham, PA), and convulxin was from Pentapharm (Basel, Switzerland). Thrombin receptor–activating peptide (TRAP-SFLLRN) was from Peninsula Labs (Belmont, CA), and thrombin was from Haematologic Technologies (Essex Junction, VT). Horseradish peroxidase (HRP)–conjugated anti–rabbit IgG, anti–mouse IgG, and ECL Plus were from Amersham Life Science (Arlington Heights, IL). Western Lightning ECL detection kits were from Perkin Elmer (MA). Protease inhibitors, Complete Mini Protease Inhibitor Cocktail, was from Roche Diagnostics (Indianapolis, IN). TAPI (N-(R)-[2-(hydroxyaminocarbonyl)methyl]-4-methylpentanoyl-l-naphthylalanyl-l-alanine, 2-aminoethyl amide) and GM6001 (N-[(2R)-2-(hydroxamidocarbonylmethyl)-4-methylpentanoyl]-l-tryptophan methylamide) were from Calbiochem (San Diego, CA). Dimethyl sulfoxide (DMSO) was from Sigma-Aldrich (St Louis, MO).
Antibodies
Anti–GP VI monoclonal antibody 9012.2,17 anti–human GP IIIa monoclonal antibody C3a.19.5 (which recognizes the cytoplasmic tail of the GP IIIa subunit18 ), and polyclonal anti–GP VI antibodies19 have been described previously. Monoclonal antibody IV.3 hybridoma was from American Type Culture Collection (ATCC) (Manassas, VA), and the antibody was produced according to the manufacturer's instructions.
Platelet preparation
Blood was drawn from healthy, aspirin-free donors on the day of use, and washed platelets prepared as described previously20 except that 50 ng/mL prostaglandin I2 was present in the collecting solution. Approval was obtained from Western Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki. Before stimulation the platelets (3 × 108/mL) were incubated at room temperature for 45 minutes in a Tyrode/HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, 10 mM HEPES pH 7.4). Platelets were supplemented with 1 mM CaCl2 and MgCl2 before use.
All studies using MMP inhibitors used washed platelets (3 × 108/mL) pre-incubated with metalloproteinase inhibitors TAPI-121 or GM600122 for 10 minutes at 37°C prior to agonist stimulation. Stock solutions of TAPI-1 and GM6001 were prepared in DMSO. Control DMSO-treated platelets received equivalent concentrations of DMSO. Final concentrations of DMSO did not exceed 0.2% (vol/vol).
Flow cytometry of surface GP VI
Convulxin was biotinylated as described previously.23 Briefly, purified convulxin was mixed with a 1/100 volume of 10-fold concentrated Sulfo-NHS-LC biotin (Pierce, Rockford, IL) dissolved in DMSO at room temperature for 2 hours. Following labeling, the mixture was extensively dialyzed against phosphate-buffered saline (PBS), pH 7.4 supplemented with 0.05% sodium azide. Washed platelets were left unstimulated or stimulated with agonist before fixation in 1% paraformaldehyde for 15 minutes at room temperature. Platelets were diluted to 1 × 107/mL. Samples were labeled for 1 hour with biotinylated convulxin (50 ng/mL) and stained with fluorescein streptavidin in the dark for 30 minutes before analysis. Data were collected and analyzed using a FACScan flow cytometer and CELLQuest software (BD Biosciences, San Jose, CA).
Immunoblotting
Washed platelets (3 × 108/mL) were left unstimulated or stimulated with agonist for 45 minutes at 37°C under static conditions. Platelets were lysed in 3 × sodium dodecyl sulfate (SDS) sample buffer in the absence of β-mercaptoethanol. The amount of GP VI loss was measured after separation by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions and immunoblotting with 9012.2 (recognizing human GP VI) and anti–human GP IIIa monoclonal antibody C3a.19.5 for normalization of lysate loading. Platelet supernatants were prepared following platelet activation and cleared of microparticles by ultracentrifugation (TLX Ultracentrifuge TLA 100 Rotor, Beckman Instruments, Fullerton, CA) at 100 000g for 2 hours. Supernatants and platelet lysates were loaded for platelet number equivalency and separated by SDS-PAGE under reducing conditions and transferred to nitrocellulose. Membranes were immunoblotted using polyclonal GP VI antibodies,19 and bound antibodies were detected by peroxidase-coupled antirabbit antibodies and chemiluminescence using ECL Plus.
Densitometric analysis of GP VI and sGP VI
Each gel was loaded with a resting platelet lysate sample that remained on ice throughout the experiment. This sample was solubilized immediately prior to gel loading and was used to determine the total amount of GP VI present in the platelet sample under normal conditions.
Percentage of GP VI reduction. The densities of bands corresponding to GP IIIa at 98 kDa and GP VI at 63 kDa were optically scanned from immunoblots using a Model GS-700 Imaging Densitometer from BioRad Laboratories (Hercules, CA). Protein levels were normalized to GP IIIa levels using Biorad Multi-Analyst Software and the relative values subtracted from the control platelet lysate value. Results were expressed as the percentage of GP VI lost from each sample relative to the total found in the control lysate.
Percentage of sGP VI. Equal volumes of sample supernatant and control platelet lysate were loaded for direct comparison of the amount of sGP VI released from the total pool of GP VI under control conditions. The densities of bands corresponding to GP VI at 63 kDa and sGP VI at 57 kDa were optically scanned and results expressed as the sGP VI released relative to the total found in the control platelet lysate. Mean and SEM values were calculated using PRISM software.
Molecular mass determination
Molecular mass was calculated using Multi-Analyst Software. The difference in molecular mass from intact GP VI following cleavage of the extracellular domain was estimated as 7.8 kDa, with the compute Pi/Mr tool,24 using the amino acid sequence of the combined intracellular and transmembrane spanning domains of GP VI published by Clemetson et al.19 The difference in molecular mass of the product following cleavage in the membrane-spanning region of GP VI is 5.9 kDa estimated from the amino acid sequence of the intracellular domain of GP VI.
Platelet aggregation
Aggregation of washed platelets (3 × 108/mL) was carried out in a lumi-aggregometer (Chrono-Log) with stirring (1000-1200 rpm) at 37°C for 5 minutes. Pre-incubation with antibodies was carried out under static conditions at 37°C for 2 minutes.
Results
GP VI–specific antibody induces cleavage of GP VI
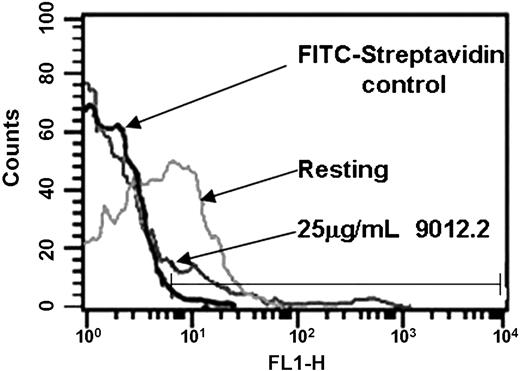
Previous studies have established that some GP VI–deficient patients7-9 have antibodies against GP VI. To determine whether antibodies against GP VI induce its proteolysis, we examined the effect of a GP VI monoclonal antibody on GP VI. As shown in Figure 1, platelets treated with 9012.2 (a GP VI monoclonal antibody known to induce platelet activation17 ) had a marked reduction in the surface expression of GP VI relative to the amount of GP VI on unstimulated (2.2% positive events with fluorescein isothiocyanate (FITC)–streptavidin label, 37% on resting platelets, and 8.4% following 9012.2 activation). When 9012.2-treated platelets were permeabilized with Triton X-100, no increased staining with GP VI was observed (data not shown).
Treatment of platelets with 9012.2 causes loss of platelet surface GP VI. Washed platelets (3 × 108/mL) were labeled with FITC-conjugated streptavidin alone (black lines) or biotinylated-convulxin and FITC-streptavidin (gray lines) as indicated. Platelets were resting (light gray) or treated with 25 μg/mL mAb 9012.2 (dark gray) at 37°C for 45 minutes. Samples were fixed in 1% paraformaldehyde, diluted to 1 × 107/mL, labeled for 1 hour with biotinylated-convulxin (50 ng/mL), and stained with fluorescein-streptavidin before analysis. Data were collected and analyzed using a FACScan flow cytometer and CELLQuest software. Results shown are representative of 3 independent experiments.
Treatment of platelets with 9012.2 causes loss of platelet surface GP VI. Washed platelets (3 × 108/mL) were labeled with FITC-conjugated streptavidin alone (black lines) or biotinylated-convulxin and FITC-streptavidin (gray lines) as indicated. Platelets were resting (light gray) or treated with 25 μg/mL mAb 9012.2 (dark gray) at 37°C for 45 minutes. Samples were fixed in 1% paraformaldehyde, diluted to 1 × 107/mL, labeled for 1 hour with biotinylated-convulxin (50 ng/mL), and stained with fluorescein-streptavidin before analysis. Data were collected and analyzed using a FACScan flow cytometer and CELLQuest software. Results shown are representative of 3 independent experiments.
Platelet activation caused the loss of GP VI and the release of a GP VI proteolytic fragment
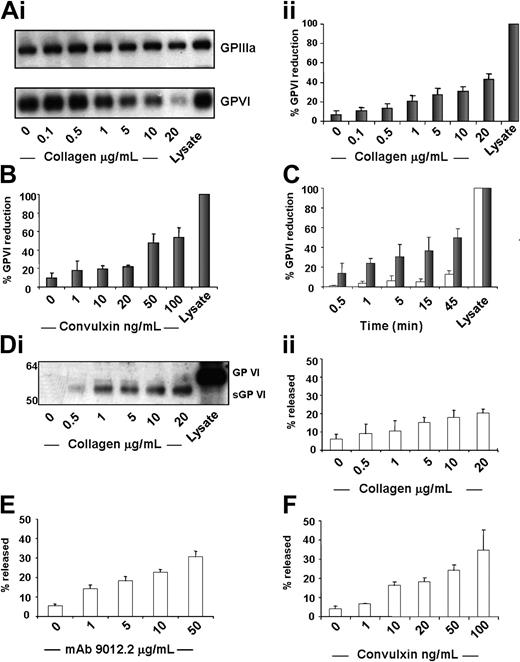
We next analyzed GP VI protein levels in platelet lysates (Figure 2A-I) by Western blotting, following stimulation with collagen, convulxin, and mAb 9012.2. Platelet activation with collagen (20 μg/mL, Figure 2A) or convulxin (100 ng/mL, Figure 2B) for 45 minutes induced 42% ± 6% and 53% ± 11% reduction in GP VI, respectively, as measured by 9012.2 binding. We next examined the time-course of receptor loss. Following 5 minutes of collagen activation (20 μg/mL), 30% ± 13% loss of GP VI protein was evident with 49% ± 9% lost after 45 minutes. Control incubations resulted in a loss of 12% ± 4% GP VI (Figure 2C). The loss of GP VI following activation by thrombin or PAR-1 with TRAP was less profound but still detectable and slower than that found for GP VI–specific agonists (data not shown). The soluble fractions were analyzed to determine whether the GP VI antibody or platelet stimulation caused the release of intact or cleaved GP VI. Using an antibody to purified GP VI, we found that the supernatants of both antibody-activated and collagen-stimulated platelets contained an immunoreactive protein of Mr = 57 kDa, somewhat lower than the Mr = 63 kDa for intact GP VI (Figure 2D-I). Increasing concentrations of antibody or collagen caused the release of increased amounts of this immunoreactive band. Since this protein appeared in parallel with the loss of GP VI protein (Figure 2A-C) with respect to both agonist concentration and kinetics and it was detected by GP VI antibodies, we conclude that it is a proteolytic product of GP VI. We term this product sGP VI (soluble GP VI). Platelet activation with 20 μg/mL collagen (Figure 2D-II), 50 μM mAb 9012.2 (Figure 2E), or 100 ng/mL convulxin (Figure 2F) induced 20% ± 2%, 31% ± 3%, and 40% ± 10% sGP VI release, respectively. The levels of sGP VI in the supernatants provide further evidence for the increased potency of GP VI–specific reagents in the cleavage of GP VI, with collagen and convulxin inducing maximal release of sGP VI, with markedly less found in response to TRAP or thrombin (data not shown). Proteolysis occurs at low levels in resting platelets, with a range of 3% to 6% sGP VI release from samples in the absence of agonist. Proteolysis also appears to be independent of GPIIb-IIIa function, with no reduction of released sGP VI following treatment with the GP IIb-IIIa antagonist Integrilin (data not shown).
Platelet agonist–induced decrease of GP VI. Washed platelets were resting or stimulated with (A-I) collagen for 45 minutes at 37°C under static conditions. Whole platelet proteins were separated by SDS-PAGE under nonreducing conditions and immunoblotted with 9012.2 and C3a.19.5, followed by detection by ECL. The 98-kDa band corresponding to GP IIIa and the 63-kDa band corresponding to GP VI were optically scanned, and the density of the GP VI band relative to the GP IIIa band was calculated. This value was subtracted from the value found in control platelet lysate and expressed as the percent reduction from control. (A) Results are shown as percent reduction ± SEM, n = 5. Platelets were activated using (B) convulxin. (C) Platelets were resting (open bars) or stimulated with collagen 20 μg/mL (solid bars) for 30 seconds and 1, 5, 15, 25, and 45 minutes at 37°C under static conditions. Results are expressed as described above, n = 4 for each condition. (D-I) Washed platelets (3 × 108/mL) were incubated at 37°C for 45 minutes in the presence of agonist, protease inhibitors were added, and the platelets were separated in an ultracentrifuge to remove microparticles. Supernatants from platelets activated in the presence of collagen and resting control lysate were loaded in equivalent amounts on SDS-PAGE under reducing conditions and electroblotted to membranes. Membranes were treated with polyclonal GP VI antibodies19 followed by anti–rabbit-HRP and detection by ECL. (D) The 63-kDa band corresponding to GP VI and the 57-kDa band corresponding to sGP VI were optically scanned, and the density of the sGP VI band relative to the GP VI band was calculated and expressed as percent released sGP VI. Results are shown as percent released ± SEM, n = 3. Platelets were activated using (E) mAb 9012.2 and (F) convulxin.
Platelet agonist–induced decrease of GP VI. Washed platelets were resting or stimulated with (A-I) collagen for 45 minutes at 37°C under static conditions. Whole platelet proteins were separated by SDS-PAGE under nonreducing conditions and immunoblotted with 9012.2 and C3a.19.5, followed by detection by ECL. The 98-kDa band corresponding to GP IIIa and the 63-kDa band corresponding to GP VI were optically scanned, and the density of the GP VI band relative to the GP IIIa band was calculated. This value was subtracted from the value found in control platelet lysate and expressed as the percent reduction from control. (A) Results are shown as percent reduction ± SEM, n = 5. Platelets were activated using (B) convulxin. (C) Platelets were resting (open bars) or stimulated with collagen 20 μg/mL (solid bars) for 30 seconds and 1, 5, 15, 25, and 45 minutes at 37°C under static conditions. Results are expressed as described above, n = 4 for each condition. (D-I) Washed platelets (3 × 108/mL) were incubated at 37°C for 45 minutes in the presence of agonist, protease inhibitors were added, and the platelets were separated in an ultracentrifuge to remove microparticles. Supernatants from platelets activated in the presence of collagen and resting control lysate were loaded in equivalent amounts on SDS-PAGE under reducing conditions and electroblotted to membranes. Membranes were treated with polyclonal GP VI antibodies19 followed by anti–rabbit-HRP and detection by ECL. (D) The 63-kDa band corresponding to GP VI and the 57-kDa band corresponding to sGP VI were optically scanned, and the density of the sGP VI band relative to the GP VI band was calculated and expressed as percent released sGP VI. Results are shown as percent released ± SEM, n = 3. Platelets were activated using (E) mAb 9012.2 and (F) convulxin.
Reduction of collagen-induced platelet aggregation following GP VI cleavage
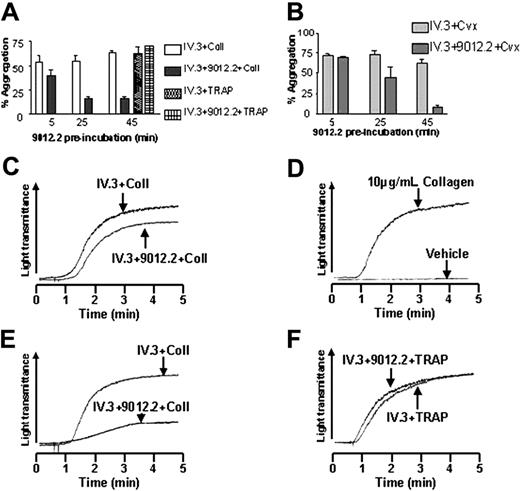
To determine whether GP VI cleavage alters platelet function, platelets were first treated with 9012.2 to induce GP VI cleavage and subsequently challenged with GP VI agonists. As shown in Figure 3, platelets pretreated with 9012.2 under conditions that gave significant proteolysis of GP VI (Figure 2E) showed substantially reduced aggregation to subsequent stimulation by collagen or convulxin with apparently normal responses to TRAP peptide (Figure 3A-B). Since GP VI proteolysis was induced by intact 9012.2, an inhibitor of FcγRIIA also was included (antibody IV.3) to prevent platelet stimulation through this receptor.25 In separate experiments it was established that antibody IV.3 neither induced the cleavage of GP VI nor affected its cleavage by 9012.2 (data not shown) and had no effect on collagen-induced platelet aggregation (Figure 3A). Pre-incubation with IV.3 alone or a combination of IV.3 + 9012.2 for 5 minutes did not change platelet reactivity to collagen (Figure 3C-D) or convulxin (Figure 3B) compared to control (Figure 3D). In contrast, treatment with IV.3 + 9012.2 for 45 minutes to induce maximal GP VI cleavage caused substantially reduced aggregation responses to collagen (Figure 3A,E) and convulxin (Figure 3B) but did not affect reactivity to TRAP peptide (Figure 3A,F).
GP VI cleavage down-regulates receptor platelet aggregation to collagen and convulxin. Washed platelets (3 × 108/mL) were aggregated following preincubation with monoclonal IV.3 (25 μg/mL) in the presence or absence of 9012.2 (25 μg/mL) using collagen (10 μg/mL), convulxin (10 ng/mL), or TRAP (10 μM). (A) Mean aggregation to collagen and TRAP in the presence of IV.3 and IV.3 + 9012.2. (B) Mean aggregation to convulxin in the presence of IV.3 and IV.3 + 9012.2. Data are expressed as mean ± SEM. (C) Platelet aggregations in response to collagen following preincubation at 37°C for 5 minutes in the presence of IV.3 and IV.3 + 9012.2. (D) Platelet aggregations under control and collagen-stimulated conditions. Platelet aggregation to E, collagen, and F, TRAP following 45 minutes' preincubation with antibodies. Results shown are representative of 3 independent experiments.
GP VI cleavage down-regulates receptor platelet aggregation to collagen and convulxin. Washed platelets (3 × 108/mL) were aggregated following preincubation with monoclonal IV.3 (25 μg/mL) in the presence or absence of 9012.2 (25 μg/mL) using collagen (10 μg/mL), convulxin (10 ng/mL), or TRAP (10 μM). (A) Mean aggregation to collagen and TRAP in the presence of IV.3 and IV.3 + 9012.2. (B) Mean aggregation to convulxin in the presence of IV.3 and IV.3 + 9012.2. Data are expressed as mean ± SEM. (C) Platelet aggregations in response to collagen following preincubation at 37°C for 5 minutes in the presence of IV.3 and IV.3 + 9012.2. (D) Platelet aggregations under control and collagen-stimulated conditions. Platelet aggregation to E, collagen, and F, TRAP following 45 minutes' preincubation with antibodies. Results shown are representative of 3 independent experiments.
Inhibition of immune-mediated GP VI loss by metalloproteinase inhibitors
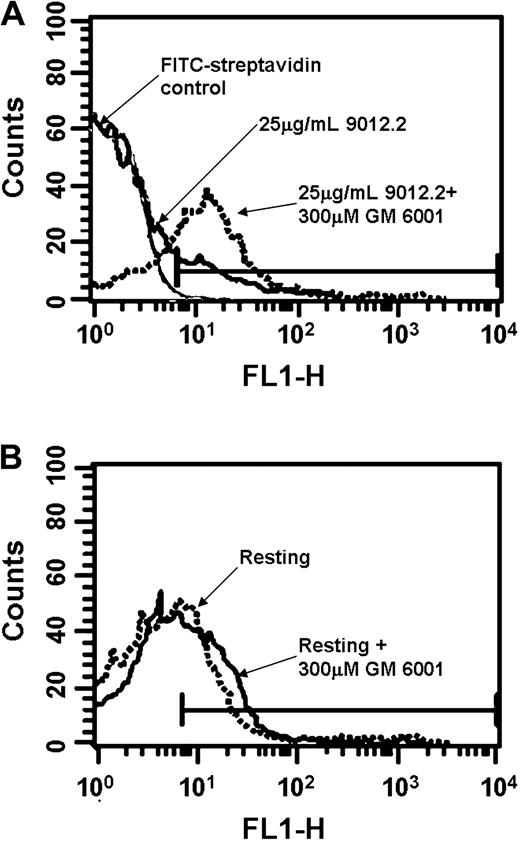
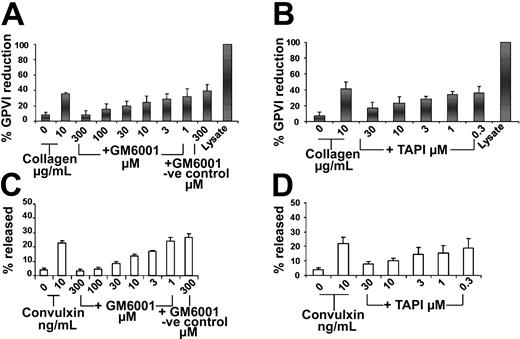
A common mechanism for proteolysis and shedding of cell surface proteins is proteolysis by metalloproteinases. To determine whether GP VI proteolysis was affected by MMP inhibition, surface levels of GP VI were measured in platelets activated in the presence of the broad-spectrum MMP inhibitor GM6001. Pretreatment of activated platelets with GM6001 prevented the loss of GP VI from the platelet surface following activation (8.8% positive events following 9012.2 activation, compared with 23.6% stimulated in the presence of GM6001, Figure 4A). The amount of GP VI on resting platelets was unaffected by the presence of GM6001 (Figure 4B). These findings suggest that specific suppression of MMP activity inhibits activation-induced GP VI proteolysis.
GP VI down-regulation is inhibited by metalloproteinase inhibitors. Washed platelets (3 × 108/mL) were pre-incubated in the presence (broken lines) or absence (solid lines) of 300 μM GM6001. Platelets were stimulated with 25 μg/mL mAb 9012.2 (A) or left resting (B) at 37°C for 45 minutes. Samples were fixed in 1% paraformaldehyde and diluted, labeled for 1 hour with biotinylated-convulxin (50 ng/mL), and stained with fluorescein-streptavidin before analysis. Data were collected and analyzed using a FACScan flow cytometer and CELLQuest software. Results shown are representative of 3 independent experiments.
GP VI down-regulation is inhibited by metalloproteinase inhibitors. Washed platelets (3 × 108/mL) were pre-incubated in the presence (broken lines) or absence (solid lines) of 300 μM GM6001. Platelets were stimulated with 25 μg/mL mAb 9012.2 (A) or left resting (B) at 37°C for 45 minutes. Samples were fixed in 1% paraformaldehyde and diluted, labeled for 1 hour with biotinylated-convulxin (50 ng/mL), and stained with fluorescein-streptavidin before analysis. Data were collected and analyzed using a FACScan flow cytometer and CELLQuest software. Results shown are representative of 3 independent experiments.
Metalloproteinase inhibitors blocked the cleavage of GP VI and release of sGP VI
As shown in Figure 5A, the collagen-induced proteolysis of GP VI is blocked by GM 6001. The negative control compound for GM6001, lacking the hydroxamic acid active moiety, had no effect on GP VI proteolysis (Figure 5A, 8% ± 6% at 300 μM to 32% ± 10% at 1 μM). Pretreatment with TAPI-1 also inhibited collagen-induced loss of GP VI (Figure 5B, 17% ± 8% at 30 μM to 39% ± 8% at 300 nM). In order to determine if sGP VI was released by metalloproteinase cleavage of GP VI, sGP VI levels were measured in the presence of MMP inhibitors. Increasing concentrations of TAPI and GM6001 blocked sGP VI release with the retention of intact GP VI on platelets (Figure 5). sGP VI release following 45 minutes of convulxin activation (10 ng/mL-21% ± 4%) also was inhibited by pretreatment with TAPI (Figure 5C, 8% ± 2% at 30 μM to 19% ± 7% at 300 nM) or GM6001 (Figure 5D, 3% ± 1% at 300 μM to 24% ± 3% at 1 μM). Similarly, the release of sGP VI in response to TRAP also was inhibited in a dose-dependent way by GM6001 (data not shown).
GP VI proteolysis is inhibited by metalloproteinase inhibitors. Washed platelets (3 × 108/mL) were pre-incubated with metalloproteinase inhibitors TAPI-1 or GM6001 for 10 minutes at 37°C prior to agonist stimulation. Platelets were activated for 45 minutes at 37°C with collagen, 10 μg/mL, in the absence or presence of GM6001 or the GM6001 negative control compound (A) or TAPI-1 (B). Platelet proteins were separated by SDS-PAGE under nonreducing conditions, immunoblotted with 9012.2 (GP VI) and C3a (IIIa), visualized by ECL, and expressed as percent reduction ± SEM described previously, n = 3-4. Platelet supernatants were prepared as described previously. Supernatants from platelets activated with convulxin, 10 ng/mL, in the presence and absence of GM6001 or the GM6001 negative control compound (C) or TAPI-1 (D) as well as resting control lysate were loaded in equivalent amounts on SDS-PAGE under reducing conditions and electroblotted to membranes. Membranes were treated with polyclonal GP VI antibodies,19 followed by anti–rabbit-HRP and detection by ECL Results are expressed as percent released sGP VI ± SEM, n = 3.
GP VI proteolysis is inhibited by metalloproteinase inhibitors. Washed platelets (3 × 108/mL) were pre-incubated with metalloproteinase inhibitors TAPI-1 or GM6001 for 10 minutes at 37°C prior to agonist stimulation. Platelets were activated for 45 minutes at 37°C with collagen, 10 μg/mL, in the absence or presence of GM6001 or the GM6001 negative control compound (A) or TAPI-1 (B). Platelet proteins were separated by SDS-PAGE under nonreducing conditions, immunoblotted with 9012.2 (GP VI) and C3a (IIIa), visualized by ECL, and expressed as percent reduction ± SEM described previously, n = 3-4. Platelet supernatants were prepared as described previously. Supernatants from platelets activated with convulxin, 10 ng/mL, in the presence and absence of GM6001 or the GM6001 negative control compound (C) or TAPI-1 (D) as well as resting control lysate were loaded in equivalent amounts on SDS-PAGE under reducing conditions and electroblotted to membranes. Membranes were treated with polyclonal GP VI antibodies,19 followed by anti–rabbit-HRP and detection by ECL Results are expressed as percent released sGP VI ± SEM, n = 3.
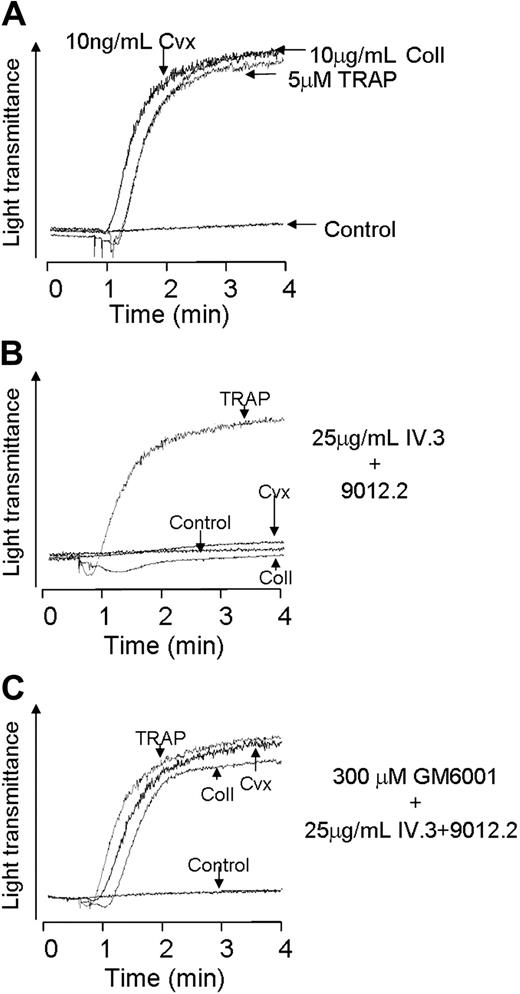
Inhibition of immune-mediated GP VI cleavage prevents loss of platelet function via the receptor
We next asked whether the loss of aggregation response of platelets treated with a GP VI antibody was MMP dependent. As shown in Figure 6B, treatment of platelets with 9012.2 caused a loss of platelet aggregation responsiveness to collagen and convulxin while not affecting the aggregation response to TRAP (compare to Figure 6A). However when GM 6001 was included with the antibody treatment, the aggregation response to collagen and convulxin was preserved (Figure 6C).
Platelet aggregation to collagen and convulxin following immune-mediated cleavage is restored by MMP inhibitor pretreatment. Washed platelets (3 × 108/mL) were aggregated using collagen (10 μg/mL), convulxin (10 ng/mL), or TRAP (10 μM) alone (A), following preincubation with the combination of monoclonal IV.3 (25 μg/mL) and 9012.2 (25 μg/mL) (B-C), following 10 minutes preincubation with GM6001 (300 μM), followed by 45 minutes' incubation with antibodies. Results shown are representative of 3 independent experiments.
Platelet aggregation to collagen and convulxin following immune-mediated cleavage is restored by MMP inhibitor pretreatment. Washed platelets (3 × 108/mL) were aggregated using collagen (10 μg/mL), convulxin (10 ng/mL), or TRAP (10 μM) alone (A), following preincubation with the combination of monoclonal IV.3 (25 μg/mL) and 9012.2 (25 μg/mL) (B-C), following 10 minutes preincubation with GM6001 (300 μM), followed by 45 minutes' incubation with antibodies. Results shown are representative of 3 independent experiments.
Discussion
The present study establishes that exposure of platelets to antibodies directed to GP VI or agonists, in particular GP VI agonists, causes loss of GP VI, generating a soluble, proteolytic fragment termed sGP VI. The calculated change in molecular mass of 7 kDa following GP VI cleavage indicates that GP VI is cleaved at or near the extracellular face of the membrane. Cleavage appears to be mediated by a platelet MMP and is time dependent. Platelet activation through other pathways (PAR-1) also induces cleavage, but less than specific activation through GP VI. Platelets with decreased levels of GP VI are less responsive to collagen. The data provide evidence that MMP-mediated GP VI cleavage provides a physiologic mechanism to reduce platelet responsiveness to collagen.
Previous studies demonstrated that MMPs are present in platelets with MMP-2 released during platelet activation26 and MMP-9 to counteract the aggregation response.13 MMP-1 also has been implicated in the priming of platelet signal transduction events and the targeting of β3 integrins to cell contact areas.11 A potential role for surface-expressed MMPs in platelets is the catalysis of specific shedding reactions and the regulation of platelet function. One example is the poststimulation shedding of CD40L generating soluble CD40L (sCD40L),14 a protein with established roles in both inflammation and thrombosis.27,28 Other studies have established that P-selectin is shed from the surface of activated platelets,15 generating a soluble product with procoagulant activity.29 More recently, endogenous MMP activity has been implicated in platelet senescence via the shedding of GP Ibα and GP VI from aging platelets.16,30 The present studies expand the activities of MMP-catalyzed shedding reactions to include both agonist- and immune-induced cleavage of GP VI and down-regulation of platelet responsiveness to collagen. The calcium-dependent platelet protease calpain does not appear to have any role in the cleavage of GP VI, and calpain inhibitor I does not prevent convulxin or TRAP-induced GP VI cleavage (G. S. and D. R. P., unpublished observations, November 2002). It remains to be determined whether MMP-2, MMP-9, or another MMP is responsible for the cleavage of GP VI.
Previous studies have established that activated platelets remain in circulation.15,31,32 Michelson et al31 showed that thrombin-activated platelets, re-infused into baboons, not only remained in circulation but also were functional in that they generated normal PAC-1 binding following stimulation by adenosine diphosphate and epinephrine. It is interesting that these authors found that that the re-infused platelets had lost collagen-induced PAC-1 binding, suggesting a loss of collagen responsiveness induced by platelet activation. Aged canine platelets also displayed a functional decline in reactivity toward collagen and convulxin, but not to thrombin33 with cleavage of GP VI in response to both activation and platelet aging, resulting in the progressive loss of collagen responsiveness in vivo. As platelets continue to circulate and age, the levels of GP VI on the surface decline, rendering them less sensitive to collagen-induced activation until they are removed from circulation. Indeed, treatment of platelets with the MMP inhibitor GM6001 is reported to improve their adhesion to collagen under flow during aging in vitro.16
The present study provides a mechanism both for the existence of patients with GP VI deficiency and the receptor clearance following anti–GP VI antibody infusion. GP VI–deficient patients have presented with reduced collagen responsiveness and immune disorders. Anti–GP VI antibodies were found in these patients despite the lack of the receptor.8,9 A recently published report from the Newman group described an immune thrombocytopenia purpura (ITP) patient with normal mRNA levels for GP VI yet lacking both the cellular receptor and circulating GP VI fragment, caused by the presence of a GP VI autoantibody.7 Cleavage of GP VI following exposure to autoantibodies could occur on either megakaryocytes or platelets, thus providing a mechanism for the GP VI deficiencies described. GP VI receptor clustering is probably sufficient to induce cleavage of the receptor, supported by the observation that all the JAQ antibodies directed to GP VI can induce specific immunodepletion of GP VI in mice.5,34
Cleavage of platelet receptors in response to activation may act as a physiologic mechanism for thrombosis. Under normal conditions, hemostasis is probably unaffected by GP VI proteolysis, supported by the lack of bleeding phenotype in the GP VI knockout mice.35 However, under pathologic conditions, involving injury or exposure to circulating agonists, GP VI cleavage could protect from thrombosis. Reduction in GP VI levels not only affects thrombosis on collagen, but also on von Willebrand factor (VWF), as GP VI–deficient platelets adhere less to VWF under flow conditions.36 Released sGP VI also may be antithrombotic as a soluble, dimeric form of the extracellular domain of GP VI specifically inhibited platelet adhesion to collagen and abolished the stable arrest and aggregation of murine platelets following vascular injury in vivo.37
This study presents evidence in support of a role for MMPs in the regulation of platelet receptors following activation with the specific example of the down-regulation of platelet responsiveness to collagen. Given the recent data, the platelet receptors that are shed, and the importance of collagen in the initiation of thrombosis, the characterization of the mechanisms involved in GP VI cleavage may serve not only as a prototype for shedding of additional receptors but also as a mechanism for the regulation of thrombosis.
Prepublished online as Blood First Edition Paper, August 31, 2004; DOI 10.1182/blood-2004-07-2842.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal