Comment on Rosenbloom et al, page 4569
In this issue, Rosenbloom and colleagues studied whether patients with Gaucher disease have an increased risk of malignancies. Their results, based on an international patient registry, suggest that the cancer rate—with the exception of multiple myeloma—is not elevated.
As both the life expectancy and spectrum of phenotypes associated with Gaucher disease are extended, there is increased concern among patients and physicians regarding the possible association of glucocerebrosidase deficiency with other life-threatening or debilitating illnesses. Examples include small subsets of adult patients who develop either pulmonary hypertension1 or parkinsonism.2 Another concern, based on information in earlier publications, is of an increased risk for various cancers, especially hematologic malignancies.3 In each of these cases, the associated disorder may be more frightening than type 1 Gaucher disease itself.FIG1
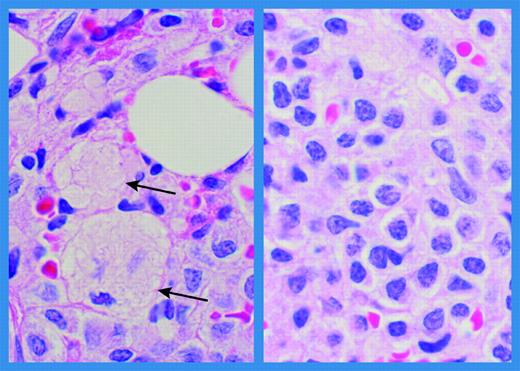
Bone marrow histology in a patient with Gaucher disease and multiple myeloma. Bone marrow biospsy obtained at the time of diagnosis of multiple myeloma in a 56-year-old male patient with Gaucher disease. (Left panel) Small areas containing Gaucher cells (arrows). (Right panel) Extensive infiltration with abnormal plasmacytoid lymphocytes and plasma cells (hematoxylin and eosin; original magnification ×1000; Olympus BH-2). Immunostaining was positive for CD38, immunoglobulin G, and lambda. Karyotyping revealed no chromosomal rearrangements. (Courtesy of Dr Margaret E. Rick, Hematology Service, Department of Laboratory Medicine, Clinical Center, National Institutes of Health, Bethesda, MD.)
Bone marrow histology in a patient with Gaucher disease and multiple myeloma. Bone marrow biospsy obtained at the time of diagnosis of multiple myeloma in a 56-year-old male patient with Gaucher disease. (Left panel) Small areas containing Gaucher cells (arrows). (Right panel) Extensive infiltration with abnormal plasmacytoid lymphocytes and plasma cells (hematoxylin and eosin; original magnification ×1000; Olympus BH-2). Immunostaining was positive for CD38, immunoglobulin G, and lambda. Karyotyping revealed no chromosomal rearrangements. (Courtesy of Dr Margaret E. Rick, Hematology Service, Department of Laboratory Medicine, Clinical Center, National Institutes of Health, Bethesda, MD.)
In this issue of Blood, Rosenbloom and colleagues study a larger cohort of patients with Gaucher disease, albeit one with inherent biases, and conclude that multiple myeloma is the only malignancy observed at an increased frequency, with an estimated relative risk of 5.9. This leads to a mixed “good news/bad news” message: we can stop worrying excessively about most cancers, but the association with myeloma is reinforced.
Are these associated adult-onset disorders part of the natural history of Gaucher disease, or does abnormal glucocerebrosidase serve as an additional risk factor in individuals who are otherwise prone to developing these illnesses? The answer is not so straightforward. Among individuals who develop parkinsonism, mutations in glucocerebrosidase appear to be only one contributing risk factor. This is supported by the evidence that, whereas the vast majority of patients with Gaucher disease never develop parkinsonian manifestations, parkinsonism in some families is more prevalent among Gaucher carriers and patients, although there is incomplete penetrance. Conversely, there is an increased frequency of heterozygosity for glucocerebrosidase mutations in cohorts of patients with sporadic Parkinson disease.2
What about multiple myeloma? The existence of an association between Gaucher disease and this malignancy, a plasma cell neoplasm characterized by production of monoclonal immunoglobulin, has been known for some time. The important role of macrophages in the pathogenesis of Gaucher disease and reports of abnormalities of both B and T cells have led to speculation about the contribution of immune activation. In Gaucher disease, elevated levels of proinflammatory cytokines, particularly interleukin 6 (IL-6), may correlate with clonal expansion of B cells.4 IL-6 is the major cytokine involved in the growth and survival of myeloma cells, and its secretion is up-regulated by CD40 activation.5 Regulation of this system involves complex interactions between many genetically determined cofactors.
The biologic and clinical behavior of myeloma cells is not exclusively determined by their genetic background; it is also influenced by the bidirectional relationship with the bone marrow, which provides a microenvironment in which myeloma cells can survive and induce bone marrow resorption through osteoclast activation.5 In Gaucher disease, the skeletal lesions reflect an imbalance in this system and are part of the disease course.
Thus, the increased frequency of multiple myeloma found by Rosenbloom et al may reflect the interplay between the natural history of Gaucher disease and as-yet unknown genetic risk factors for the development of gammopathies in some patients. The difficulties in determining the genetic and mechanistic bases for this association highlight the complexities of this “simple” Mendelian disorder. ▪