Abstract
In this report we have investigated the role of the homeobox gene Hex in the development and differentiation of the blast colony-forming cell (BL-CFC), a progenitor with hemangioblast characteristics generated in embryonic stem (ES) cell-derived embryoid bodies (EBs). Molecular analysis showed that Hex is expressed in mesoderm, in populations that contain BL-CFCs, and in blast cell colonies, the progeny of the BL-CFCs. Hex-/- EBs displayed a defect in macrophage development but generated higher numbers of BL-CFCs than did wild-type EBs. In addition to differences in these progenitor populations, we also found that endothelial cells from the Hex-/- EBs showed enhanced proliferative potential compared with those from wild-type EBs. Forced expression of Hex at the onset of ES cell differentiation resulted in reduced EB cellularity, fetal liver kinase-1 (Flk-1) expression, and BL-CFC development. Taken together, these findings demonstrate that Hex functions at multiple stages of development within the differentiating EBs and uncover a novel role for this transcription factor as a negative regulator of the hemangioblast and the endothelial lineage. (Blood. 2005;105: 4590-4597)
Introduction
The first hematopoietic and vascular cells develop from extraembryonic mesoderm in the yolk sac of the gastrulating mouse embryo at approximately day 7.5 of gestation.1,2 Once formed, these cells rapidly organize into blood islands that consist of primitive erythroblasts surrounded by a layer of endothelial cells.3 This close developmental association of the hematopoietic and endothelial lineages within the blood islands has led to the hypothesis that they arise from a common precursor, a cell known as the hemangioblast.4,5 Detailed insights into origins of the hematopoietic and vascular lineages have come from studies using the model system based on the in vitro differentiation potential of embryonic stem (ES) cells.6,7 These experiments have identified a blast colony-forming cell (BL-CFC) in developing embryoid bodies (EBs) that is able to generate blast cell colonies consisting of hematopoietic and vascular progenitors.8 The characteristics of the EB-derived BL-CFCs are identical to the recently identified hemangioblast in the early embryo,9 indicating that this in vitro progenitor is indicative of the earliest stage of hematopoietic and vascular development.
A number of critical molecular pathways have been identified that regulate distinct stages in the developmental progression from mesoderm to the formation of the hematopoietic and vascular lineages. The receptor fetal liver kinase-1 (Flk-1) is required early and appears to play a pivotal role in the migration of mesoderm from the primitive streak to the extraembryonic region of the embryo that will form the yolk sac. In the absence of a functional Flk-1 receptor, the cells accumulate in the amniotic region of the embryo, and, as a consequence, blood islands do not form.10,11 Once the mesodermal cells are positioned in the presumptive yolk sac region, commitment to the hematopoietic lineages is dependent on the function of different transcription factors, including the helix-loop-helix factor Scl/Tal-112 and the core binding factor Runx1.13,14 Scl is essential for the establishment of both the primitive and definitive hematopoietic programs in the yolk sac and EBs, as well as for the development of blast cell colonies from EB-derived progenitors.15-19 Runx1-/--negative embryos and EBs progress further than those lacking Scl and generate the primitive erythroid lineage, but they lack definitive hematopoietic potential.20-22
While Scl and Runx1 play central roles in the establishment of the hematopoietic system, other factors are clearly required in these early developmental decisions. The homeobox gene Hex (hematopoietically expressed homeobox) is of particular interest in this regard, as it is expressed in the developing blood islands of the mouse embryo, in a pattern similar to that of Flk-1.23-26 In zebra fish, Hex is expressed in the posterior lateral plate in the bilateral stripes of the nascent intermediate cell mass, which contains both endothelial and blood precursors.27 Targeting studies revealed that Hex is important for monocyte development both in the mouse embryo and in the ES/EB system28,29 and that this defect is early, possibly at the level of the BL-CFC. In addition to functioning as a positive regulator of the monocyte lineage, other studies have demonstrated that Hex can function as a negative regulator of the eukaryotic translation initiation factor 4E that is essential for cell proliferation and survival.30 These observations suggest that Hex may also function as a suppressor of proliferation and that its mode of action may be cell lineage specific.
Expression analysis and gene targeting studies have indicated that the role of Hex extends beyond that of the hematopoietic system. Hex is first expressed in the anterior visceral endoderm of the embryo prior to the onset of gastrulation and is considered to be one of the earliest markers of anterior and posterior asymmetry.26 It is thought to function as a transcriptional repressor at this stage and to contribute to anterior identity by suppressing signals that promote dorsal mesoderm formation.31 Following induction of definitive endoderm at approximately 8.5 days of gestation, Hex expression is up-regulated in the hepatocyte and thyroid lineages. Analysis of the Hex-/- embryos reveals severe defects in liver and thyroid development,28,32 indicating that Hex is essential for the development of these tissues. Taken together, these findings demonstrate that Hex functions at multiple stages of development, including early germ layer induction, hematopoietic development, and definitive endoderm specification.
Given these diverse roles in early development and its expression in the yolk sac blood islands, we were interested in further defining the role of Hex in hematopoietic and vascular development in the ES/EB system. In this report we show that Hex is expressed in mesoderm, in transitional colonies, and in blast cell colonies, representing the earliest stages of hematopoietic and endothelial commitment.17 Analysis of Hex-/- EBs revealed increased numbers of BL-CFCs, reduced numbers of macrophage and multipotential hematopoietic progenitors, and increased proliferation potential of CD31+ endothelial cells. Forced expression of Hex in wild-type EBs suppressed cell proliferation as well as development of Flk-1+ cells and BL-CFCs. These findings demonstrate that Hex does play a role at different stages of development during EB differentiation and functions as a negative regulator of the hemangioblast and the endothelial lineages.
Materials and methods
Growth and differentiation of ES cells
The development and characterization of the Hex+/+, Hex+/-, and Hex-/- ES cell lines have been described.32 The Hex-plox targeting plasmid was electroporated into the Ainv 15 ES cell line (tet-Hex ES cells) and selected by G418 as described.33 ES cells were maintained on irradiated mouse embryo fibroblast feeder cells as previously described.34
To generate EBs, ES cells were dissociated to a single-cell suspension with trypsin and cultured at various concentrations (0.1-8 × 104 cells/mL) in 60-mm Petri-grade dishes in differentiation media. Serum differentiation media consisted of Iscoves modified Dulbecco medium (IMDM) supplemented with penicillin and streptomycin, 2 mM glutamine (Gibco/BRL, Grand Island, NY), 0.5 mM ascorbic acid (Sigma, St Louis, MO), 4.5 × 10-4 M MTG (monothioglycerol), 15% fetal calf serum (FCS; Summit, Ft Collins, CO), 5% protein-free hybridoma medium (PFHM-II; Gibco/BRL), and 200 μg/mL transferrin (Boehringer Mannheim, Indianapolis, IN). The serum used was selected as optimal for hematopoietic differentiation. All experiments in this study were repeated at least 2 times. Cultures were maintained in a humidified chamber in a 5% CO2-air mixture at 37°C.
Hematopoietic progenitor and blast colony assays
Hematopoietic progenitors were assay as described.34 Primitive erythroid colonies were counted at day 4, whereas macrophage and multilineage (mix) colonies were scored at day 7 of culture. Mix colonies are large colonies that consist of macrophages, definitive erythroid cells, and neutrophils. For blast colony assays, EBs were trypsinized to single-cell suspension and plated (2.5 × 104 cells/mL) in 1% methylcellulose containing 10% FCS, vascular endothelial growth factor (VEGF; 5 ng/mL), c-kit ligand (KL; 1% conditioned medium), interleukin-6 (IL-6; 5 ng/mL), and 25% D4T endothelial cell-conditioned medium. Blast colonies were counted at day 4 of culture. c-kit ligand was derived from media conditioned by Chinese hamster ovary cells transfected with a KL expression vector (kindly provided by Genetics Institute, Cambridge, MA). VEGF and IL-6 were purchased from R&D Systems (Minneapolis, MN).
Monolayer culture of endothelial cells
For monolayer cultures of endothelial cells and smooth muscle cells, 1 to 2 × 104 Flk-1-positive cells were replated on matrigel-coated 24-well plates in IMDM containing 10% FCS, VEGF (5 ng/mL), basic fibroblast growth factor (bFGF; 10 ng/mL), and 25% D4T endothelial cell-conditioned medium. Cells were counted and then harvested for RNA isolation at days 3 and 5 of culture. bFGF was purchased from R&D Systems.
Neuronal assay
For neuroectoderm induction, EB differentiation was initiated in Stem Pro 34 medium (Gibco/BRL) supplemented with 2 mM glutamine, 0.5 mM ascorbic acid, 4.5 × 10-4 M MTG, and c-kit ligand (1% conditioned medium) at a concentration of 2 × 103 ES cells/mL for 2 days. At this stage, the EBs were harvested and transferred to new cultures containing IMDM supplemented with 15% serum replacement media (Gibco/BRL), 2 mM glutamine, 0.5 mM ascorbic acid, 4.5 × 10-4 M MTG. Day 6 EBs cultured in the indicated conditions were replated on matrigel-coated 6-well plate in IMDM with 15% FCS. The number of EBs with the neurite outgrowths was counted 4 days following replating.
Gene expression analysis
The polyA+ global amplification polymerase chain reaction (PCR) was carried out as previously described.17,35 Amplified products from PCR were separated on agarose gels and transferred to a Zeta-probe GT membrane (Bio-Rad, Hercules, CA). The resulting blots were hybridized with 32P randomly primed cDNA fragments (Ready-to-Go Labeling; Pharmacia, Piscataway, NJ) corresponding to the 3′ untranslated region of the genes of interest as previously reported.17,36 For the 3′ untranslated region of Hex, 5′-AGGAGGCTGATCTTGACTGA-3′ as the sense primer and 5′-GTTCGTGTGAGATCAGTCTC-3′ as the antisense primer were used for cDNA fragment amplification by reverse transcription (RT)-PCR.
For gene-specific RT-PCR, total RNA was extracted using RNeasy mini-kits and treated with RNase-free DNase (Qiagen, Valencia, CA). Total RNA (2 μg) was reverse-transcribed into cDNA with random hexamers using Omniscript RT kit (Qiagen). PCR was performed with Taq polymerase (Promega, Madison, WI) in PCR buffer, 2.5 mM, 0.2 mM deoxynucleoside triphosphates (dNTPs). Cycling conditions were as follows: 94°C for 5 minutes followed by 25 to 35 cycles of amplification (94°C denaturation for 1 minute, annealing for 30 seconds, 72°C elongation for 1 minute), with a final incubation at 72°C for 7 minutes. PCR was carried out using the oligonucleotides described in a previous paper.34 For c-fms, 5′-GCGATGTGTGAGCAATGGCAGT-3′ as the sense primer and 5′-AGACCGTTTTGCGTAAGACCTG-3′ as the antisense primer were used for PCR.
FACS analysis and cell sorting
EB-derived cells were stained with a biotinylated antibody to Flk-1.37 Cells visualized by Cy5-streptavidin were analyzed using a FACSCalibur (Becton Dickinson, San Jose, CA) or sorted on a MoFlo cell sorter (Cytomation Systems, Fort Collins, CO).
Immunostaining
For the CD31 and smooth muscle actin (SMA) staining, day-4 Flk-1-positive cells were cultured on HCl-treated, fibronectin-coated glass cover slips for 5 days. Following culture, the cells were fixed in 2% paraformaldehyde for 20 minutes, washed 2 times in phosphate-buffered saline (PBS), permeabilized in PBS with 0.2% Triton X-100, washed in PBS with 10% FCS and 0.2% Tween 20, and then blocked with PBS with 10% horse serum for 10 minutes. Cells were incubated for 1 hour with primary antibodies: biotin-CD31 (Pharmingen, San Diego, CA), SMA (Neomarker, Fremont, CA). Expression of these proteins was visualized using a Cy3-conjugated streptavidin or a fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G (IgG) secondary antibody (Jackson Immunoresearch, West Grove, PA). Images were captured using an Axioskop fluorescent microscope (Zeiss, Thornwood, NJ) with 5×, 10×, 20×, 40×, and 100× Plan NEOFLUAR objectives. CD31+ areas were evaluated with an image analyzer (Axiovision 4.3).
Results
Definitive hematopoietic progenitors are suppressed in Hex-/- EBs
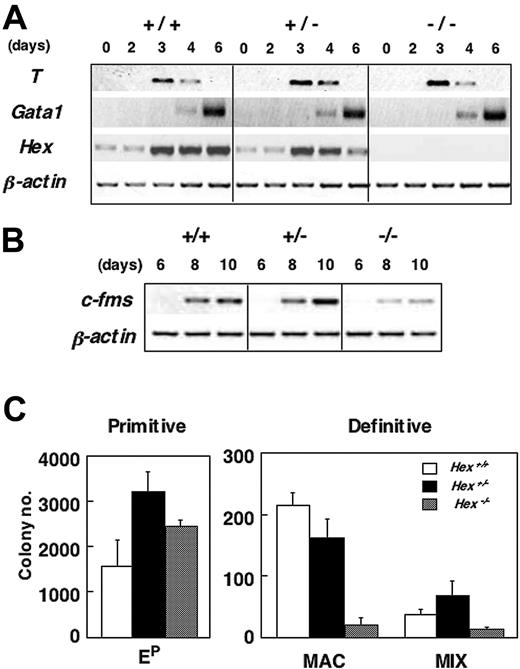
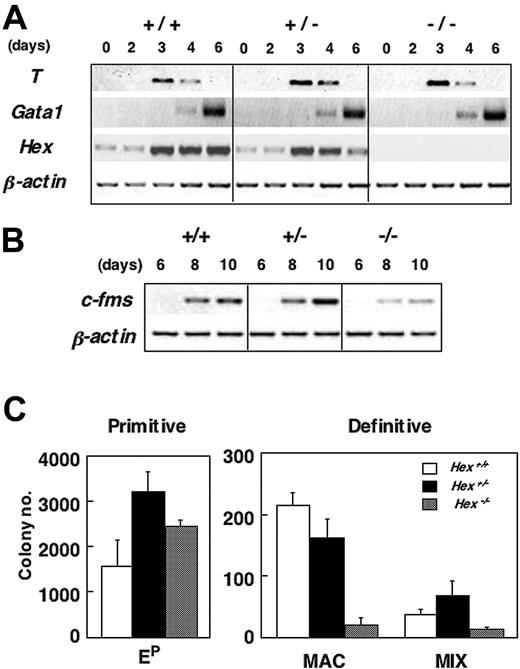
Following differentiation in the presence of serum, both Hex+/- and Hex-/- ES cells generated EBs that were indistinguishable in morphology and size from those produced by Hex+/+ cells (not shown). Molecular analysis revealed comparable levels of T38 and Gata139 expression in Hex+/+, Hex+/-, and Hex-/- EBs, indicating that Hex is not required for mesoderm induction and hematopoietic specification. Hex expression was up-regulated between days 2 and 3 of differentiation during this time course, at approximately the same stage as T, and persisted throughout the 6-day differentiation period (Figure 1A). c-fms expression levels were lower in the Hex-/- EBs than in wild-type counterparts at both 8 and 10 days of differentiation (Figure 1B), consistent with the observation from previous studies that this gene is essential for macrophage development.28,29 Hematopoietic progenitor analysis revealed a 10-fold reduction in the number of macrophage progenitors and a 3-fold reduction in the number of multipotential (Mix) progenitors in Hex-/- EBs compared with those generated from wild-type ES cells. Primitive erythroid progenitors were present at normal, if not slightly elevated, levels compared with controls (Figure 1C). Taken together, these findings indicate that Hex does not impact primitive erythroid development but is required for the establishment of normal numbers of macrophage and multipotential progenitors.
Hematopoietic potential of Hex-deficient ES cells. (A-B) RT-PCR expression analysis of different staged serum-stimulated EBs generated from Hex+/+ (+/+), Hex+/- (+/-), and Hex-/- (-/-) ES cells. (C) Hematopoietic progenitor analysis of Hex+/+,Hex+/-, and Hex-/- EBs differentiated for 6 days. Numbers represent colonies per 1 × 105 cells plated. Data represent means ± SEMs (n = 3). Ep indicates primitive erythroid colonies; MAC, macrophage colonies; MIX, large multilineage hematopoietic colonies consisting of macrophages, definitive erythroid cells, and neutrophils.
Hematopoietic potential of Hex-deficient ES cells. (A-B) RT-PCR expression analysis of different staged serum-stimulated EBs generated from Hex+/+ (+/+), Hex+/- (+/-), and Hex-/- (-/-) ES cells. (C) Hematopoietic progenitor analysis of Hex+/+,Hex+/-, and Hex-/- EBs differentiated for 6 days. Numbers represent colonies per 1 × 105 cells plated. Data represent means ± SEMs (n = 3). Ep indicates primitive erythroid colonies; MAC, macrophage colonies; MIX, large multilineage hematopoietic colonies consisting of macrophages, definitive erythroid cells, and neutrophils.
Expression of Hex in mesoderm and the hematopoietic and vascular lineages. The 3′ cDNA prepared by RT-PCR from the different populations and lineages was run on a 1.5% agarose gel, blotted, and probed separately with 3′ probes from the indicated genes. L32 was included as an internal control. Bry-Flk-1-, Bry+Flk-1-, and Bry+Flk-1+ representing premesoderm and mesodermal populations were isolated from day-3 serum-stimulated EBs. LiqTR (-) and LiqTR (+) represent pools of transitional colonies generated from day-2.5 EBs in the absence or presence of VEGF, respectively. Blast indicates pools of blast colonies from day-3 EBs, whereas Ep and Mac represent pools of primitive erythroid and macrophage colonies from day-6 EBs. D14FL is uncultured day-14 fetal liver cells, and d14FL/Epo (erythropoietin) and d14Fl/Mix represent fetal liver cells cultured for 7 days in erythropoietin or a mix of cytokines. D4T is an EB-derived endothelial cell line.
Expression of Hex in mesoderm and the hematopoietic and vascular lineages. The 3′ cDNA prepared by RT-PCR from the different populations and lineages was run on a 1.5% agarose gel, blotted, and probed separately with 3′ probes from the indicated genes. L32 was included as an internal control. Bry-Flk-1-, Bry+Flk-1-, and Bry+Flk-1+ representing premesoderm and mesodermal populations were isolated from day-3 serum-stimulated EBs. LiqTR (-) and LiqTR (+) represent pools of transitional colonies generated from day-2.5 EBs in the absence or presence of VEGF, respectively. Blast indicates pools of blast colonies from day-3 EBs, whereas Ep and Mac represent pools of primitive erythroid and macrophage colonies from day-6 EBs. D14FL is uncultured day-14 fetal liver cells, and d14FL/Epo (erythropoietin) and d14Fl/Mix represent fetal liver cells cultured for 7 days in erythropoietin or a mix of cytokines. D4T is an EB-derived endothelial cell line.
Hex is expressed in the hemangioblast
To further define the expression patterns of Hex in early development, we analyzed specific lineages and cell populations that span mesoderm induction, hemangioblast commitment, and hematopoietic and vascular differentiation. Bry-Flk1-, Bry+Flk-1-, and Bry+Flk-1+ populations from 3-day-old EBs generated from the green fluorescent protein (GFP)-Bry ES cell line40 were used to represent premesoderm, hemangioblast mesoderm, and the hemangioblast, respectively. In addition to these mesoderm populations, the analysis also included transitional colonies17 which encompass the prehemangioblast to hemangioblast stages of development (stages similar to the Bry+Flk-1- and Bry+Flk-1+ populations), blast colonies, primitive erythroid colonies, macrophage colonies, the endothelial cell line D4T, day-14 fetal liver and day-14 fetal liver populations expanded in erythropoietin, or a broad mix of cytokines to stimulate the growth of the definitive erythroid lineage and multiple hematopoietic lineages. Expression of Flk-1, T, Scl, and βH1-globin was used to verify the developmental stage of each population. Although Hex was broadly expressed across these populations and lineages, several aspects of the pattern are of interest (Figure 2). First, expression was up-regulated with the induction of mesoderm (compare Bry-Flk-1- and Bry+Flk-1-) and was detected in populations (Bry+Flk-1-) that do not yet express Flk-1 or Scl. Second, Hex was expressed in populations that contain BL-CFCs, including the Bry+Flk-1+ EB fraction and the transitional colonies, as well as in the blast colonies generated from the BL-CFC. Expression levels in the blast colonies were higher than in the various hematopoietic and endothelial populations, a pattern that suggests some role at the earliest stages of hematopoietic and vascular commitment.
Increased BL-CFC and endothelial potential in Hex-/- EBs
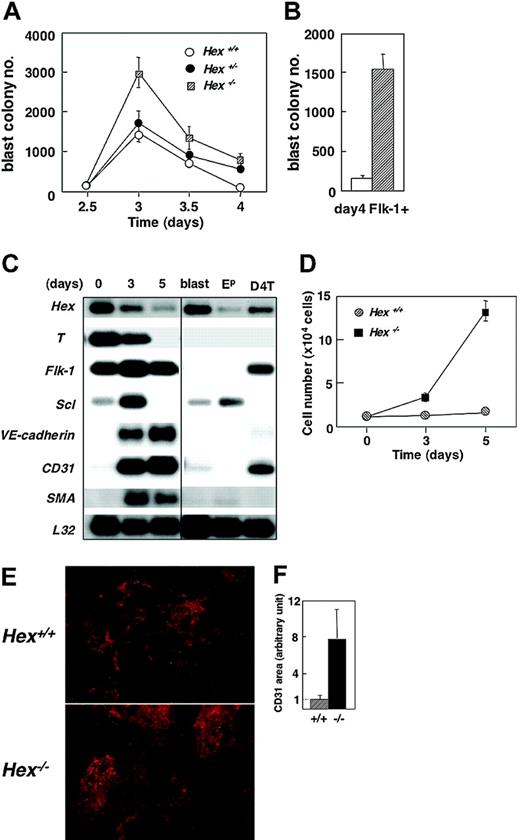
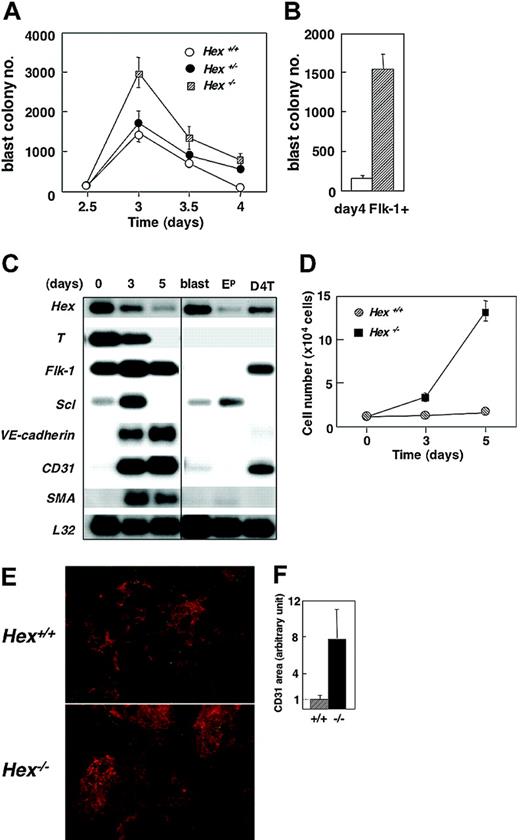
Analysis of day-4 Hex+/+, Hex+/-, and Hex-/- EBs revealed the presence of similar numbers of Flk-1+ cells, indicating that Hex is not required for this developmental step. While the levels of Flk-1+ cells were not affected, the BL-CFC numbers were. Hex-/- EBs consistently had approximately 2-fold higher numbers of BL-CFCs at day 3 of differentiation than were found in either the heterozygote or wild-type EBs (Figure 3A). This increase in blast colony numbers reflects a significant increase in the frequency of BL-CFCs within the Flk-1 population. When the potential of the day-4 sorted Flk-1 fractions was compared, the frequency of BL-CFCs in the Hex-/- population was 6-fold higher than in the wild-type population (Figure 3B). The increased number of BL-CFCs observed here suggests that Hex plays a role as a repressor at the level of the differentiation or expansion of these progenitors from Flk-1+ mesoderm.
BL-CFC and endothelial potential of Hex-deficient ES cells. (A) BL-CFC potential of Hex+/+, Hex+/-, and Hex-/- EBs at the indicated time points. Numbers represent colonies per 2.5 × 104 cells plated. Data represent means ± SEMs (n = 3). (B) BL-CFC potential of Hex+/+ (□) and Hex-/- (▨) Flk-1+ cells isolated from day-4 EBs. Data represent means ± SEMs (n = 3). (C) RT-PCR expression analysis of Hex+/+ day-4 Flk-1+ cells cultured in VEGF and bFGF for varying periods of time. The 3′ cDNA was prepared by RT-PCR from the Flk-1+ population at the time of isolation (0) or following 3 or 5 days of culture. The cDNA samples were run on a 1.5% agarose gel, blotted, and probed separately with 3′ probes from the indicated genes. L32 was included as an internal control. Control populations include a pool of blast colonies (blast), a pool of primitive erythroid colonies (Ep), and the D4T endothelial cell line. (D) Flk-1+ cells (1 × 104 cells) isolated from day 4 Hex+/+ and Hex-/- were cultured on matrigel-coated wells with VEGF and bFGF. Cells were harvested and counted at days 3 and 5 of culture. Data represent means ± SEMs (n = 3). (E) Immunohistochemistry showing CD31+ endothelial cell clusters (red) and SMA-positive smooth muscle cells (green) (× 50) at 5 days of culture. (F) The size of the CD31+ area in 8 separate fields of each culture was evaluated by image analyzer. The mean size of the CD31+ area in Hex+/+ cultures was adjusted to 1. Data represent means ± SEM.
BL-CFC and endothelial potential of Hex-deficient ES cells. (A) BL-CFC potential of Hex+/+, Hex+/-, and Hex-/- EBs at the indicated time points. Numbers represent colonies per 2.5 × 104 cells plated. Data represent means ± SEMs (n = 3). (B) BL-CFC potential of Hex+/+ (□) and Hex-/- (▨) Flk-1+ cells isolated from day-4 EBs. Data represent means ± SEMs (n = 3). (C) RT-PCR expression analysis of Hex+/+ day-4 Flk-1+ cells cultured in VEGF and bFGF for varying periods of time. The 3′ cDNA was prepared by RT-PCR from the Flk-1+ population at the time of isolation (0) or following 3 or 5 days of culture. The cDNA samples were run on a 1.5% agarose gel, blotted, and probed separately with 3′ probes from the indicated genes. L32 was included as an internal control. Control populations include a pool of blast colonies (blast), a pool of primitive erythroid colonies (Ep), and the D4T endothelial cell line. (D) Flk-1+ cells (1 × 104 cells) isolated from day 4 Hex+/+ and Hex-/- were cultured on matrigel-coated wells with VEGF and bFGF. Cells were harvested and counted at days 3 and 5 of culture. Data represent means ± SEMs (n = 3). (E) Immunohistochemistry showing CD31+ endothelial cell clusters (red) and SMA-positive smooth muscle cells (green) (× 50) at 5 days of culture. (F) The size of the CD31+ area in 8 separate fields of each culture was evaluated by image analyzer. The mean size of the CD31+ area in Hex+/+ cultures was adjusted to 1. Data represent means ± SEM.
To further investigate the role of Hex in vascular development, Flk-1+ cells were isolated from day-4 Hex-/- or wild-type EBs and cultured on matrigel in the presence of VEGF and bFGF, conditions that promote the proliferation and differentiation of endothelial and vascular smooth muscle cells. Differentiation progressed over a 5-day period within these cultures as demonstrated by the down-regulation of T, Hex, and Scl and by the up-regulation of VE-cadherin, CD31, and SMA, genes expressed in the endothelial and vascular smooth muscle lineages, respectively (Figure 3C). Analysis of cell numbers at day 5 revealed that the Hex-/- cultures contained 9 times more cells than found in the wild-type cultures (Figure 3D). These findings indicate that the mutant cells are proliferating significantly faster than the wild-type cells under these culture conditions. Immunostaining indicated that most of this proliferation was within the CD31+ endothelial cell lineage. Areas of CD31+ cell growth were up to 8 times larger in the Hex-/- cultures compared with those containing the Hex+/+ cells (Figure 3E-F). These findings strongly suggest that Hex plays a role in the establishment of the endothelial lineage and may function as a regulator to maintain an appropriate balance of cell types within the vascular system.
Forced expression of Hex suppresses EB growth, Flk-1, and BL-CFC development
As a complimentary approach to the analysis of the Hex-null EBs, we next evaluated the consequences of forced expression of Hex at different stages of differentiation of wild-type EBs. To enable inducible expression of Hex at specific time points, the tet-inducible ES model developed by Kyba et al33 was used (tet-Hex ES cells). Given that Hex is thought to be involved in the establishment of anterior identity, in part, by suppressing signals that promote dorsal mesoderm formation in the early embryo, we reasoned that it could function in a similar fashion in the earliest stages of EB differentiation. To determine the timing and regulation of germ layer induction in the unmanipulated cells, we first compared the mesoderm and neuroectoderm potential of EBs differentiated in the presence and absence of serum, the source of inducers in these cultures. T (GFP-Bry) expression together with the onset of expression of hematopoietic genes Gata1 and c-fms and the development of hematopoietic progenitors were used as an indication of mesoderm development and specification. We monitored the development of neuroectoderm by expression of Pax6,41 Wnt1,42 and NeuroD,43 and by neurite outgrowth from EBs.40
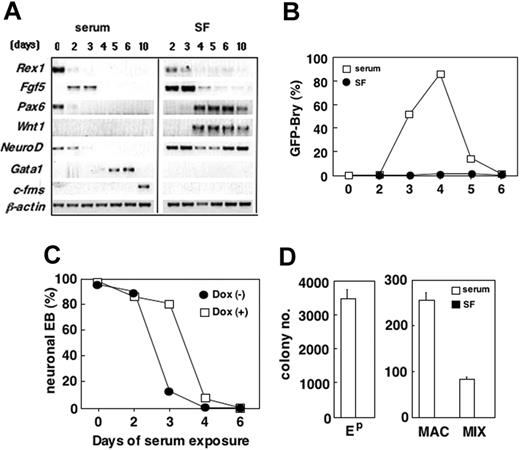
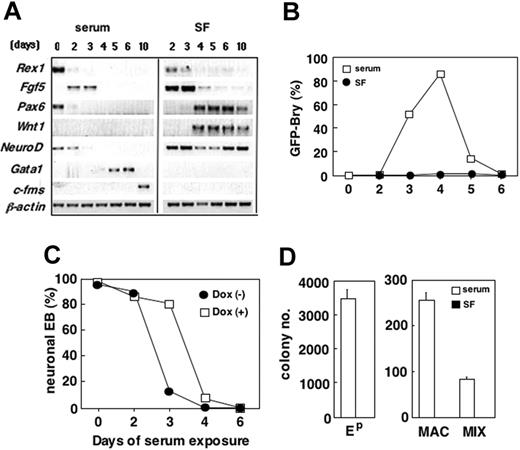
Prior to the onset of differentiation, ES cells expressed Rex1,44 Pax6, and NeuroD (Figure 4A). Within 2 days of differentiation, EBs generated in both conditions down-regulated Rex1 and Pax6 and up-regulated Fgf5, a marker of the primitive ectoderm of the epiblast.45-47 The patterns of Fgf5 expression indicate that the differentiation of epiblast-like cells takes place in both the presence and absence of serum, suggesting that the early differentiation events are not serum dependent.
Beyond 2 days of differentiation, the effects of serum were notable. Pax6 and Wnt1 were not expressed at any stage of EB development in the presence of serum. Expression of both genes was up-regulated within the serum-free EBs at day 4 of differentiation and maintained throughout the 10-day duration of the experiment (Figure 4A). NeuroD was undetectable beyond day 3 of differentiation in the presence of serum but was found in all stages of development in the serum-free EBs. The 2 hematopoietic genes, Gata1 and c-fms, were expressed at days 5 and 6 and at day 10, respectively, in the EBs grown in serum but were not detected at any time in the serum-free cultures. In EBs differentiated in serum, T expression as determined by GFP, was up-regulated between day 2 and 3 of differentiation, reached peak levels by day 4, and then declined to undetectable levels by day 6 (Figure 4B). GFP was not detected in the serum-free EBs at any stage of differentiation, indicating that these conditions do not support mesoderm induction.
The effect of serum on germ layer induction in EBs. (A) Bry-GFP ES cells were cultured in the presence (serum) and absence (SF) of serum. The resulting EBs were evaluated for expression of the indicated genes at the times (days) shown at the top of the panel. (B) FACS analysis of GFP-Bry expression in the serum and serum-free EBs. (C) tet-Hex ES cells were cultured for a total of 10 days. EBs were grown from 0 to 6 days in the presence of serum. The numbers refer to the number of days cultured in the presence of serum. At the indicated time point, the EBs were transferred to serum-free conditions and grown until a total of 6 days of culture. At 6 days of culture, EBs were replated, and neurite development was evaluated at day 10. Cells in each condition were cultured in the absence (-) or presence (+) of Dox (1 μg/mL). Day-6 EBs from each group were replated, and the number of EBs with neurite outgrowths was counted 4 days later. (D) Hematopoietic potential of day-6 EBs generated in the presence or absence of serum. Ep indicates primitive erythroid colonies; MAC, macrophage colonies; MIX, multilineage colonies. Data represent means ± SEMs (n = 3).
The effect of serum on germ layer induction in EBs. (A) Bry-GFP ES cells were cultured in the presence (serum) and absence (SF) of serum. The resulting EBs were evaluated for expression of the indicated genes at the times (days) shown at the top of the panel. (B) FACS analysis of GFP-Bry expression in the serum and serum-free EBs. (C) tet-Hex ES cells were cultured for a total of 10 days. EBs were grown from 0 to 6 days in the presence of serum. The numbers refer to the number of days cultured in the presence of serum. At the indicated time point, the EBs were transferred to serum-free conditions and grown until a total of 6 days of culture. At 6 days of culture, EBs were replated, and neurite development was evaluated at day 10. Cells in each condition were cultured in the absence (-) or presence (+) of Dox (1 μg/mL). Day-6 EBs from each group were replated, and the number of EBs with neurite outgrowths was counted 4 days later. (D) Hematopoietic potential of day-6 EBs generated in the presence or absence of serum. Ep indicates primitive erythroid colonies; MAC, macrophage colonies; MIX, multilineage colonies. Data represent means ± SEMs (n = 3).
Neurite formation and hematopoietic progenitor development reflected the expression patterns (Figure 4A). More than 90% of the EBs generated in the absence of serum differentiated toward a neuroectoderm fate and formed neurites (Figure 4C; day 0, doxycycline [Dox] [-]). None of the EBs differentiated in the serum for 6 days displayed this potential (Figure 4C; day-6 serum exposure, Dox [-]), indicating that serum inhibited development of neuroectoderm, most likely because of its strong mesoderm-inducing capacity. These inhibitory effects were not observed during the first 48 hours of differentiation, as significant numbers of EBs maintained in serum for this period of time were still able to generate neurites. (Figure 4C). Exposure to serum for a further 24 hours, however, significantly reduced the neuroectoderm potential of the EBs. The reduction in neuroectoderm potential during this time frame coincided with the onset of mesoderm development as reflected by GFP-Bry expression (Figure 4B), indicating that our conditions are incompatible with the simultaneous expansion and maturation of both germ layers. In contrast to the neuroectoderm potential, hematopoietic progenitors were detected in the serum-stimulated EBs, but not in those differentiated in the absence of serum (Figure 4D). Taken together, these findings suggest that the earliest stages of EB differentiation to a stage that approximates the epiblast are independent of serum-derived factors. Further differentiation is, however, significantly impacted by serum with mesoderm differentiation requiring serum and neuroectoderm development progressing in its absence.
Effects of forced Hex expression on EB growth, Flk-1 expression, and BL-CFC development. (A-B) The impact of Hex forced expression on EB cell number. ES cells carrying a tet-inducible Hex or GFP cDNA were differentiated in serum in the presence or absence of Dox (1 μg/mL). Dox was added at day 0 (A) or day 2 (B) of the differentiation. Cells were counted at the indicated time points. The cultures were initiated with 1 × 104 ES cells. The effect of forced Hex expression on the induction of Flk-1 (C) and BL-CFC development (D). tet-Hex ES cells were differentiated in the presence of serum with or without Dox (added time 0), and Flk-1 expression (C) and blast colonies formation (D) were examined at the indicated time points. Numbers for blast colony represent colonies per 2 × 104 cells plated. (E) The effect of Hex on blast colony growth. Cells from uninduced EBs were cultured in the presence and absence of Dox (0.3 μg/mL) in the blast colony assay. Numbers for blast colonies are per 2 × 104 cells plated. (F) tet-Hex ES cells were cultured with or without Dox (1 μg/mL) in serum-free conditions. Dox was added at day 2 of differentiation, and cells were counted at the indicated time points. The cultures were initiated with 1 × 104 cells.
Effects of forced Hex expression on EB growth, Flk-1 expression, and BL-CFC development. (A-B) The impact of Hex forced expression on EB cell number. ES cells carrying a tet-inducible Hex or GFP cDNA were differentiated in serum in the presence or absence of Dox (1 μg/mL). Dox was added at day 0 (A) or day 2 (B) of the differentiation. Cells were counted at the indicated time points. The cultures were initiated with 1 × 104 ES cells. The effect of forced Hex expression on the induction of Flk-1 (C) and BL-CFC development (D). tet-Hex ES cells were differentiated in the presence of serum with or without Dox (added time 0), and Flk-1 expression (C) and blast colonies formation (D) were examined at the indicated time points. Numbers for blast colony represent colonies per 2 × 104 cells plated. (E) The effect of Hex on blast colony growth. Cells from uninduced EBs were cultured in the presence and absence of Dox (0.3 μg/mL) in the blast colony assay. Numbers for blast colonies are per 2 × 104 cells plated. (F) tet-Hex ES cells were cultured with or without Dox (1 μg/mL) in serum-free conditions. Dox was added at day 2 of differentiation, and cells were counted at the indicated time points. The cultures were initiated with 1 × 104 cells.
To determine whether forced expression of Hex could affect these early developmental decisions, Dox was added at the onset of differentiation (day 0) to serum-induced cultures of the tet-Hex ES cells. Expression of Hex led to a 3-fold reduction in size of the resulting 6-day-old EBs compared with those in uninduced cultures (Figure 5A). The effect was not simply a result of the presence of Dox, as induced and uninduced control ES cells containing a GFP cDNA generated similar-sized EBs. A similar inhibition of EB growth was observed when Dox was added to the cultures at day 2 of differentiation (epiblast) rather than at the initiation (Figure 5B). Given that serum induces high levels of mesoderm in EBs (Figure 4B), these findings suggest that Hex expression inhibited the induction and specification of this germ layer. Analysis of Flk-1 and BL-CFC development support this interpretation. Induction of Hex at time 0 delayed the development of the Flk-1 population and reduced its size (Figure 5C). The impact of Hex expression on BL-CFC development was more pronounced, as induction at time 0 resulted in a more than 90% reduction of these progenitors (Figure 5D). The kinetics of development of the small number of BL-CFCs that were detected was delayed by 12 to 18 hours. When induced in the methylcellulose cultures during blast colony growth, Hex expression suppressed colony development by approximately 50% (Figure 5E). Together, these findings indicate that overexpression of Hex significantly inhibits hematopoietic and vascular development as demonstrated by the reduction in the number of Flk-1+ cells and BL-CFCs and the suppression of blast colony growth from the BL-CFCs.
Analysis of neurite formation revealed that Hex expression extended this potential by 24 hours in serum-stimulated EBs. In the presence of Dox, more than 80% of the day-3 EBs formed neurites, whereas less then 20% of those differentiated in the absence of Dox displayed this capacity (Figure 4C). These observations indicate that forced expression of Hex prolongs neuroectoderm development in serum-stimulated EBs. To further investigate the role of Hex in neuroectoderm development, we next induced its expression in EBs generated in the absence of serum, conditions that favor the development of this population. The addition of Dox did not affect EB growth under these serum-free conditions (Figure 5F). Neuroectoderm differentiation was also not affected, as demonstrated by the expression of Pax6 (data not shown) and the development of neurites (Figure 4C; day-0 serum exposure with or without Dox). These findings demonstrate that forced expression of Hex during serum-free EB differentiation does not significantly alter neuroectoderm development.
Discussion
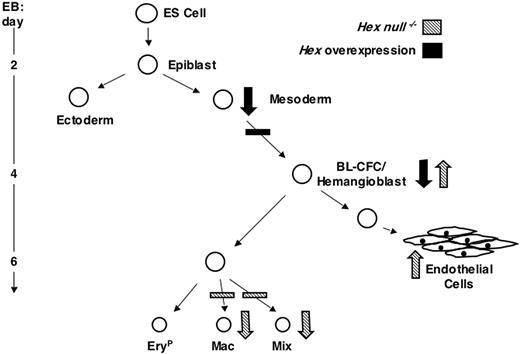
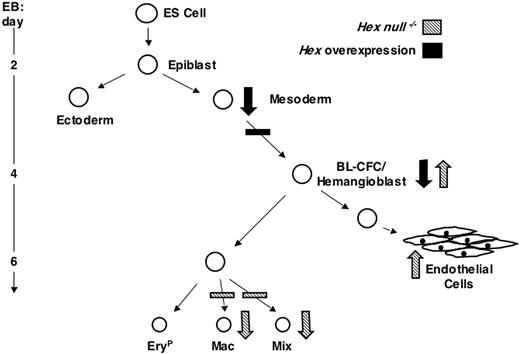
The findings from this study demonstrate that Hex can impact ES cell differentiation at multiple developmental stages and that its mode of action, varying between repressor and activator, is lineage and stage dependent as summarized in the model in Figure 6. Our expression analysis revealed an interesting early pattern for Hex, initiating in mesoderm prior to the onset of expression of Flk-1 and Scl and persisting through the hemangioblast stage and in the blast colonies derived from the BL-CFC/hemangioblast. Expression of Hex in the blast colonies identified here is in contrast with the findings of Guo et al29 that showed no expression in these colonies. These differences may reflect differences of the sensitivity of the PCR protocols used for the analysis. Alternatively, the types of colonies assayed in the 2 studies may differ, as we detect 7- to 10-fold more blast colonies in our conditions than that reported in their study. Our findings in this study are consistent with those in the mouse embryo that demonstrated transient Hex expression in the yolk sac blood islands26 as well as with those in the zebra fish that demonstrated expression in the intermediate cell mass that contains both hematopoietic and endothelial progenitors.27
Model depicting the role of Hex in hemangioblast, hematopoietic, and endothelial development during ES cell differentiation. Large arrows indicate the effect of Hex at different stages of development. Downward facing arrows represent a decrease in progenitor or cell numbers and upward facing arrows represent an increase.
Model depicting the role of Hex in hemangioblast, hematopoietic, and endothelial development during ES cell differentiation. Large arrows indicate the effect of Hex at different stages of development. Downward facing arrows represent a decrease in progenitor or cell numbers and upward facing arrows represent an increase.
The increased frequency of BL-CFCs in the Hex-/- EBs suggests that Hex functions as a negative regulator of hemangioblast development and may play an important role in maintaining the proper number of these progenitors in vivo. The fact that the size of the Flk-1 population is not affected in the Hex-/- EBs suggests that Hex functions specifically at the stage of BL-CFC commitment within this population. The findings from the Hex-null EBs are corroborated by those from the forced expression studies that demonstrated that increased levels of Hex in wild-type cells dramatically reduced BL-CFC numbers. Our findings demonstrating increased numbers of BL-CFCs in Hex-/- EBs differ from those of Guo et al29 that showed no apparent alteration in the number of these progenitors. Again, given the significant disparity in colony numbers in the 2 studies, it is possible that different populations are being evaluated.
In addition to increased levels of BL-CFCs, we also found increased proliferative potential in the endothelial cells generated from the Hex-/- EBs compared with those from wild-type cells. Other studies have provided evidence that Hex is important for development and maturation of the endothelial lineage, although its exact role in these processes remains somewhat controversial. In Xenopus, overexpression of Hex resulted in increased numbers of vascular progenitors and the development of a disorganized vasculature.48 Guo et al29 found that Hex-/- EBs generated lower numbers of vascular endothelial (VE)-cadherin-positive cells and formed fewer vascular sprouts compared with those from wild-type cells. When expressed in human umbilical vein endothelial cells, Hex completely abrogated the ability of these cells to proliferate and form vascular networks in response to VEGF.49 This diminished response is likely due to the fact that Hex significantly repressed expression of the VEGF receptors, Flt-1 (VEGFR1) and Kdr/Flk-1 (VEGFR2) in these cells. This repression of Flk-1 is consistent with the findings from our studies showing that Hex suppressed Flk-1 expression in EBs. A recent study by Hallaq et al50 demonstrated aberrant vascular development in Hex-/- embryos characterized by the presence of dilated large vessels with decreased numbers of vascular smooth muscle cells. This pattern of vasculogenesis could result from enhanced proliferative potential of the endothelial lineage, as observed in our study. The observed discrepancies in these various studies could reflect different functions of Hex at different stages in vascular development. Our findings together with those of Hallaq et al50 suggest that its initial role could be one of a repressor of endothelial development, proliferation, or both, possibly as a mechanism to regulate the proper balance of endothelial and vascular smooth muscle cells. At later stages, Hex may assume a role as a positive regulator of endothelial cell maturation, organization, and tube formation, as indicated by the studies of Guo et al.29
Our observations of increased BL-CFC and endothelial cell proliferation in the absence of Hex and suppression of EB cell growth and BL-CFC formation and growth following forced Hex expression are consistent with other studies which suggest that this transcription factor can function as an inhibitor of proliferation, by directly altering cell-cycle proteins or growth factors and receptors associated with cell proliferation.30,51,52 The ability to impact the cell cycle may reflect a role for Hex in maintaining the appropriate balance between proliferation and differentiation in specific lineages. Within the endothelial lineage, removal of Hex resulted in increased proliferative potential, possibly at the expense of differentiation. Such cells may not be able to participate in normal vascular development.
In addition to demonstrating a role in BL-CFC development and endothelial cell growth, our studies suggest that forced expression of Hex can also alter primary germ layer development in EBs. When induced early during the differentiation of serum-induced EBs, Hex suppressed cell proliferation and development of mesoderm derivatives. The apparent specificity for mesoderm-derived populations was confirmed by demonstrating that Hex expression had no effect on EB development in serum-free conditions that specifically support neuroectoderm development. These findings are consistent with observations in the early mouse embryo which indicate that Hex expression in anterior visceral endoderm suppresses Goosecoid and Chordin, 2 factors involved in dorsal mesoderm specification.31 Taken together, these findings indicate that one of the earliest functions of Hex is to repress mesoderm induction and specification and, in doing so, promote the development of neuroectoderm.
In summary, the findings in this study have revealed a unique role for Hex as a negative regulator of hemangioblast commitment and endothelial differentiation and expansion within the ES/EB model. These observations highlight the complexity of transcriptional regulation in lineage commitment and point to a requirement of both negative and positive regulators to achieve the appropriate balance of cell types during the establishment of the hematopoietic and vascular systems. Access to the early stages of development in the ES/EB system provides a unique opportunity to define the mechanism(s) by which Hex regulates BL-CFC commitment and proliferation.
Prepublished online as Blood First Edition Paper, February 22, 2005; DOI 10.1182/blood-2004-10-4137.
Supported by the National Institutes of Health (grants HL 48834, R01 HL65169, and R01 HL 71800-01) and Human Frontiers in Science (grant RG0345/1999-M 103). A.K. was supported by Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellowships for Research Abroad (Japan).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The Hex-/- ES cells were a gift from the late Dr Rosa Beddington. We thank members of the Keller lab for critically reading the manuscript.